Abstract
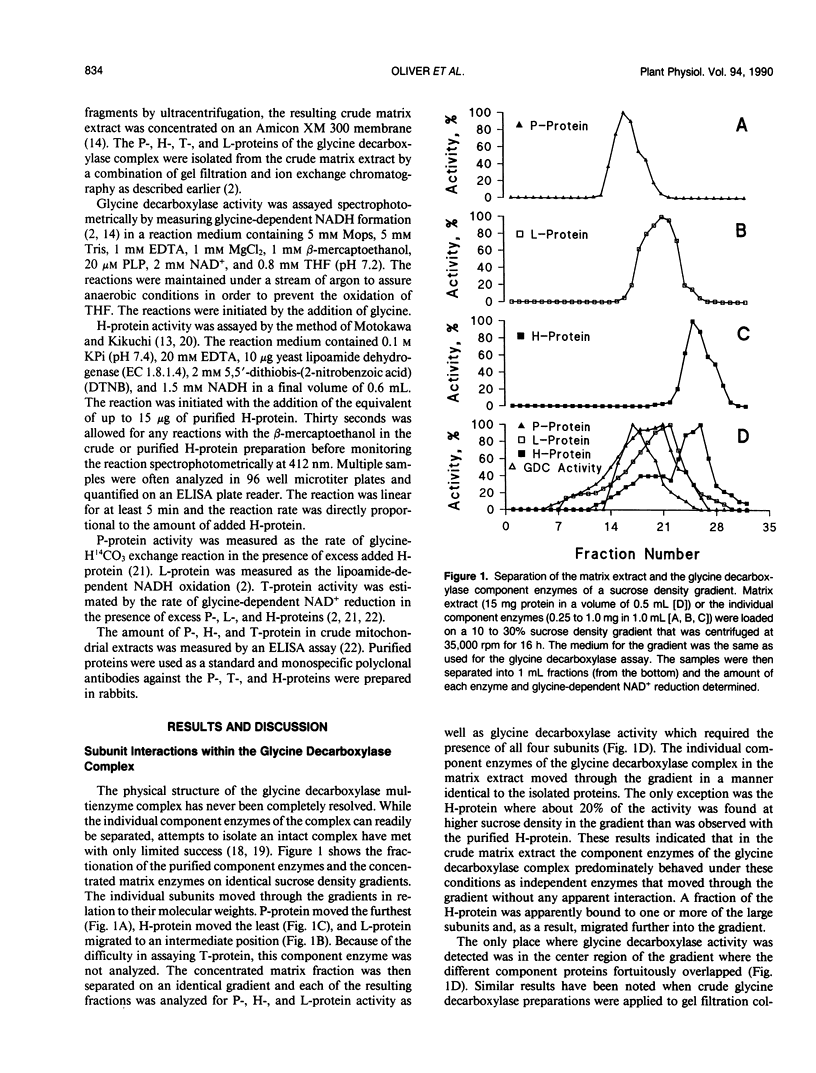
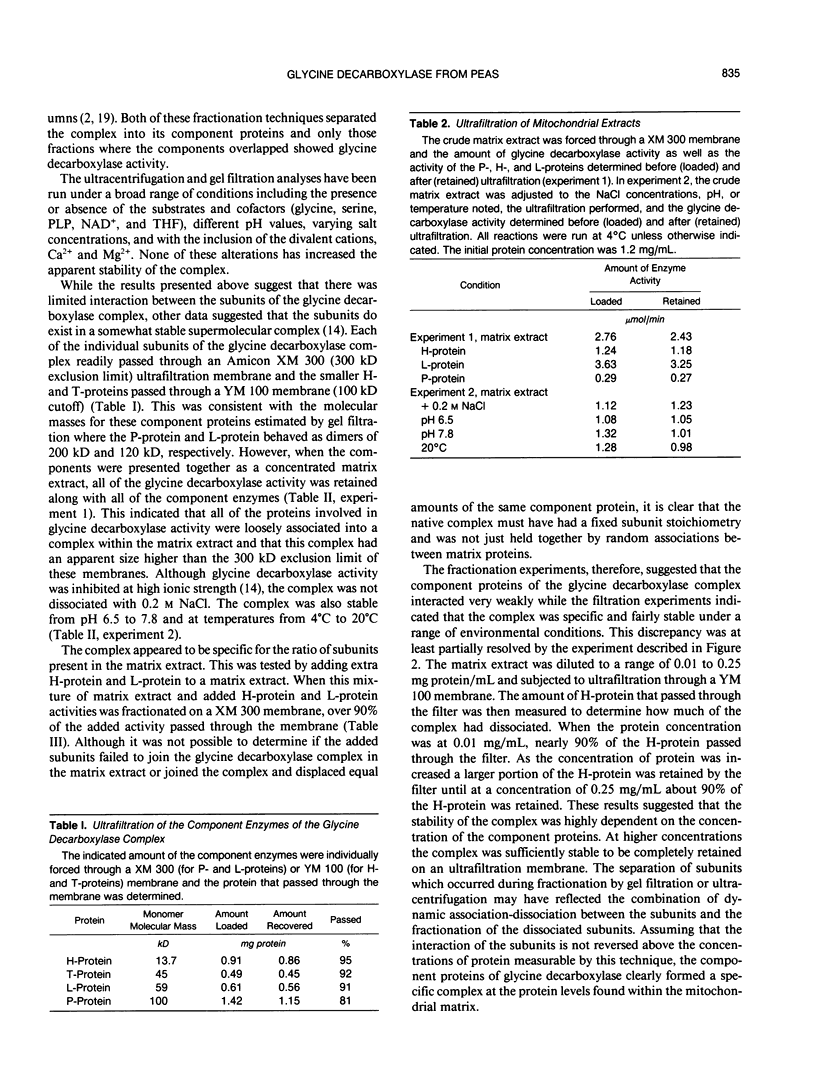
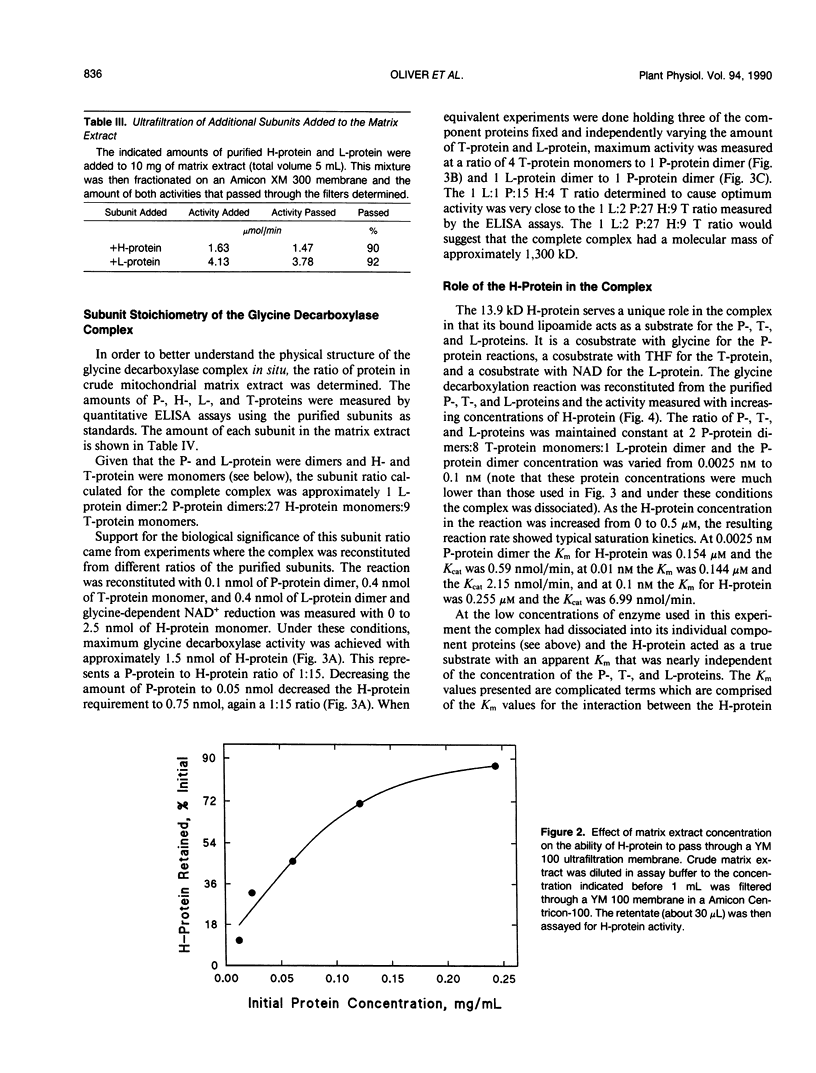
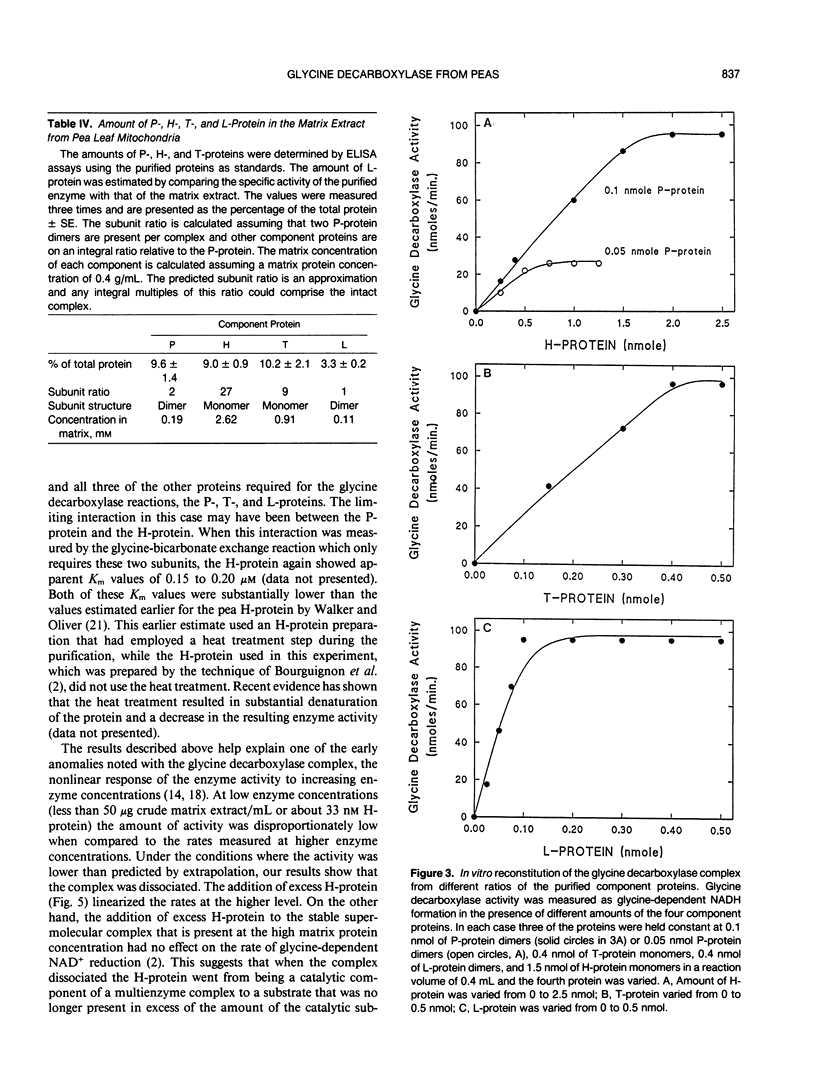
The glycine decarboxylase multienzyme complex comprises about one-third of the soluble protein of the matrix of pea (Pisum sativum) leaf mitochondria where it exists at a concentration of approximately 130 milligrams protein/milliliter. Under these conditions the complex is stable with an approximate subunit ratio of 2 P-protein dimers:27 H-protein monomers:9 T-protein monomers:1 L-protein dimer. When the complex is diluted it tends to dissociate into its component enzymes. This prevents the purification of the intact complex by gel filtration or ultracentrifugation. In the dissociated state the H-protein acts as a mobile cosubstrate that commutes between the other three enzymes and shows typical substrate kinetics. When the complex is reformed, the H-protein no longer acts as a substrate but as an integrated part of the enzyme complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon J., Neuburger M., Douce R. Resolution and characterization of the glycine-cleavage reaction in pea leaf mitochondria. Properties of the forward reaction catalysed by glycine decarboxylase and serine hydroxymethyltransferase. Biochem J. 1988 Oct 1;255(1):169–178. doi: 10.1042/bj2550169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Functional association of glycine decarboxylase and aminomethyl carrier protein. J Biol Chem. 1980 Dec 25;255(24):11671–11676. [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kim Y., Oliver D. J. Molecular cloning, transcriptional characterization, and sequencing of cDNA encoding the H-protein of the mitochondrial glycine decarboxylase complex in peas. J Biol Chem. 1990 Jan 15;265(2):848–853. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Reactions of glycine synthesis and glycine cleavage catalyzed by extracts of Arthrobacter globiformis grown on glycine. Arch Biochem Biophys. 1969 Jul;132(2):359–369. doi: 10.1016/0003-9861(69)90377-4. [DOI] [PubMed] [Google Scholar]
- Macherel D., Lebrun M., Gagnon J., Neuburger M., Douce R. cDNA cloning, primary structure and gene expression for H-protein, a component of the glycine-cleavage system (glycine decarboxylase) of pea (Pisum sativum) leaf mitochondria. Biochem J. 1990 Jun 15;268(3):783–789. doi: 10.1042/bj2680783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. IV. Isolation and characterization of hydrogen carrier protein, an essential factor for glycine metabolism. Arch Biochem Biophys. 1969 Dec;135(1):402–409. doi: 10.1016/0003-9861(69)90556-6. [DOI] [PubMed] [Google Scholar]
- Okamura-Ikeda K., Fujiwara K., Motokawa Y. Purification and characterization of chicken liver T-protein, a component of the glycine cleavage system. J Biol Chem. 1982 Jan 10;257(1):135–139. [PubMed] [Google Scholar]
- Sarojini G., Oliver D. J. Extraction and partial characterization of the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Plant Physiol. 1983 May;72(1):194–199. doi: 10.1104/pp.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Kochi H., Motokawa Y., Kawasaki H., Kikuchi G. Glycin metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J Biochem. 1969 Jan;65(1):63–70. [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Glycine decarboxylase multienzyme complex. Purification and partial characterization from pea leaf mitochondria. J Biol Chem. 1986 Feb 15;261(5):2214–2221. [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Light-induced increases in the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Arch Biochem Biophys. 1986 Aug 1;248(2):626–638. doi: 10.1016/0003-9861(86)90517-5. [DOI] [PubMed] [Google Scholar]


