Abstract
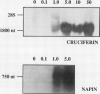
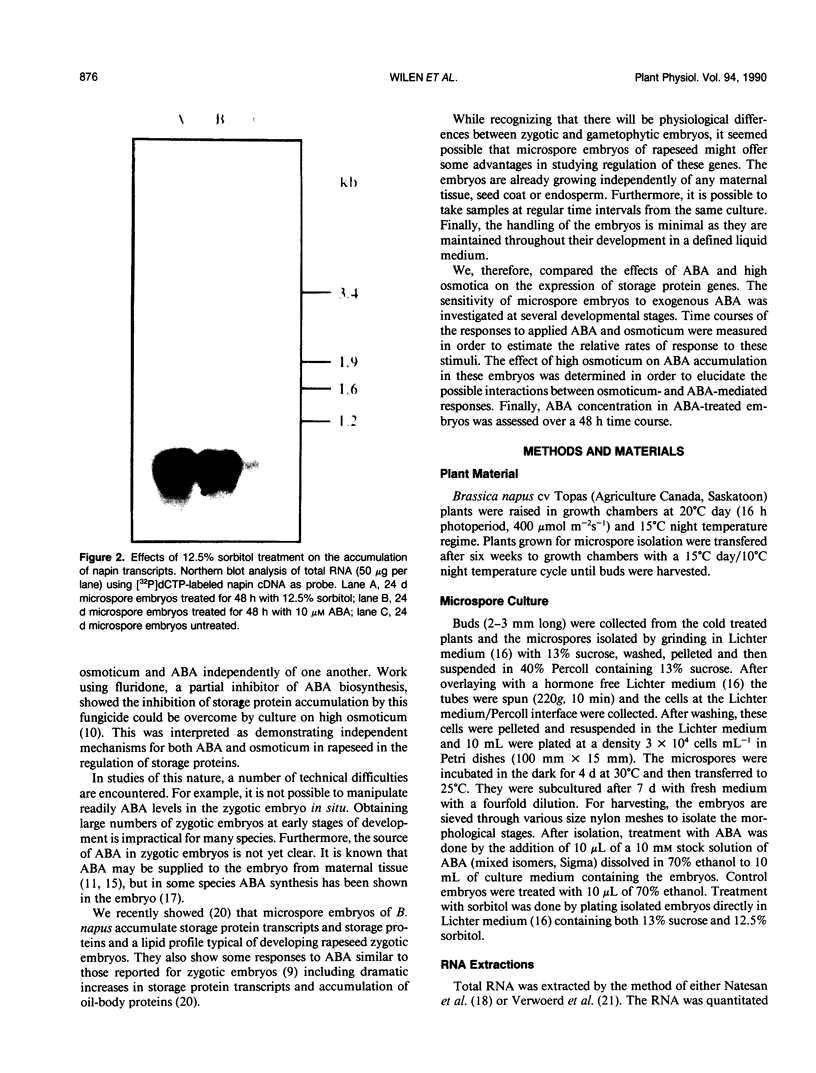
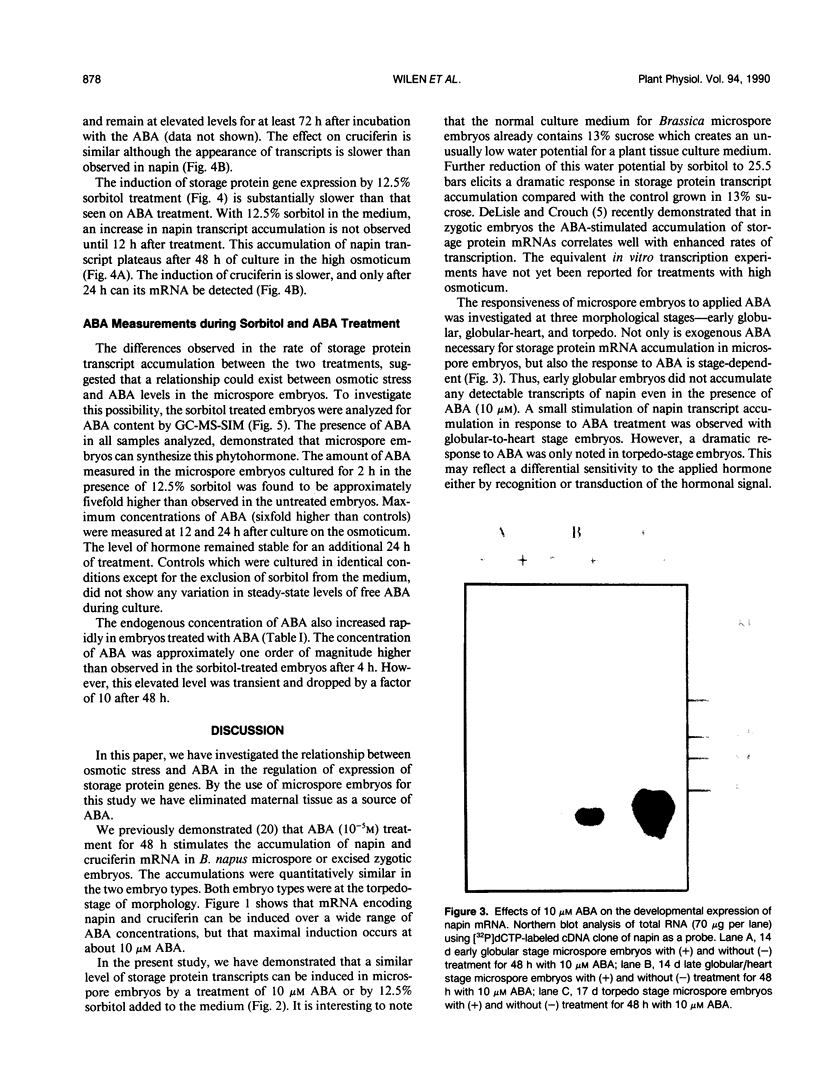
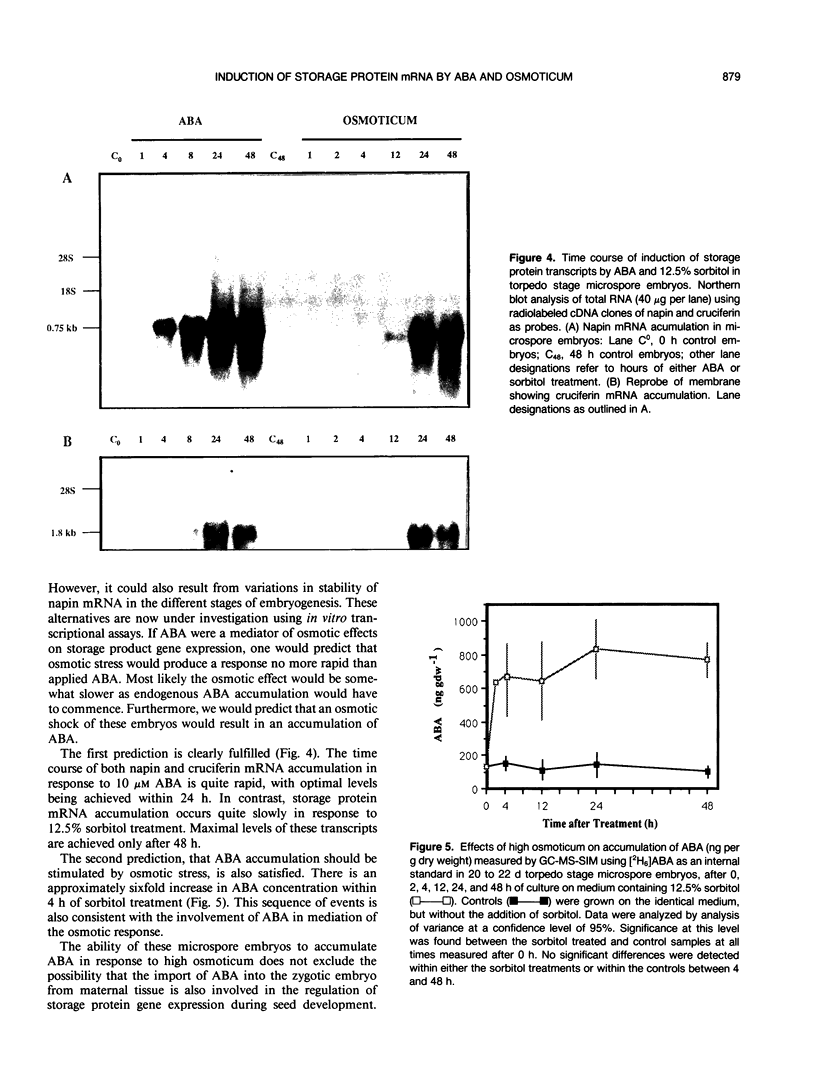
Storage protein gene expression, characteristic of mid- to late embryogenesis, was investigated in microspore embryos of rapeseed (Brassica napus). These embryos, derived from the immature male gametophyte, accumulate little or no detectable napin or cruciferin mRNA when cultured on hormone-free medium containing 13% sucrose. The addition of abscisic acid (ABA) to the medium results in an increase in detectable transcripts encoding both these polypeptides. Storage protein mRNA is induced at 1 micromolar ABA with maximum stimulation occurring between 5 and 50 micromolar. This hormone induction results in a level of storage protein mRNA that is comparable to that observed in zygotic embryos of an equivalent morphological stage. Effects similar to that of ABA are noted when 12.5% sorbitol is added to the microspore embryo medium (osmotic potential = 25.5 bars). Time course experiments, to study the induction of napin and cruciferin gene expression demonstrated that the ABA effect occurred much more rapidly than the high osmoticum effect, although after 48 hours, the levels of napin or cruciferin mRNA detected were similar in both treatments. This difference in the rates of induction is consistent with the idea that the osmotic effect may be mediated by ABA which is synthesized in response to the reduced water potential. Measurements of ABA (by gas chromatography-mass spectrometry using [2H6]ABA as an internal standard) present in microspore embryos during sorbitol treatment and in embryos treated with 10 micromolar ABA were performed to investigate this possibility. Within 2 hours of culture on high osmoticum the level of ABA increased substantially and significantly above control and reached a maximum concentration within 24 hours. This elevated concentration was maintained for 48 hours after culturing and represents a sixfold increase over control embryos. The ABA-treated embryos accumulated the hormone very quickly, but ABA concentrations returned to basal levels within 72 hours after treatment. The possibility that embryo-synthesized ABA may be a mediator of effects of osmotic stress on gene expression in Brassica embryos is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray E. A., Beachy R. N. Regulation by ABA of beta-Conglycinin Expression in Cultured Developing Soybean Cotyledons. Plant Physiol. 1985 Nov;79(3):746–750. doi: 10.1104/pp.79.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle A. J., Crouch M. L. Seed Storage Protein Transcription and mRNA Levels in Brassica napus during Development and in Response to Exogenous Abscisic Acid. Plant Physiol. 1989 Oct;91(2):617–623. doi: 10.1104/pp.91.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Crouch M. L. Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol. 1986 Jul;81(3):907–912. doi: 10.1104/pp.81.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. R., Tenbarge K. M., Shumway J. E., Crouch M. L. Role of ABA in Maturation of Rapeseed Embryos. Plant Physiol. 1985 Jul;78(3):630–636. doi: 10.1104/pp.78.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Radin J. W., Nieman R. A. Intracellular pH of Cotton Embryos and Seed Coats during Fruit Development Determined by P Nuclear Magnetic Resonance Spectroscopy. Plant Physiol. 1987 Oct;85(2):588–591. doi: 10.1104/pp.85.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C. J., Hilhorst H. W., Karssen C. M. In Vivo Inhibition of Seed Development and Reserve Protein Accumulation in Recombinants of Abscisic Acid Biosynthesis and Responsiveness Mutants in Arabidopsis thaliana. Plant Physiol. 1989 Jun;90(2):463–469. doi: 10.1104/pp.90.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S., Quinn E. M., Bentley M. M. The expression of sequences similar to the human c-erb-A oncogene are regulated in a tissue and stage specific manner in Drosophila melanogaster. Oncogene. 1989 Nov;4(11):1397–1401. [PubMed] [Google Scholar]
- Ross G. S., Elder P. A., McWha J. A., Pearce D., Pharis R. P. The development of an indirect enzyme linked immunoassay for abscisic Acid. Plant Physiol. 1987 Sep;85(1):46–50. doi: 10.1104/pp.85.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzicki T. J., Abe H., Wodzicki A. B., Pharis R. P., Cohen J. D. Investigations on the Nature of the Auxin-Wave in the Cambial Region of Pine Stems : Validation of IAA as the Auxin Component by the Avena Coleoptile Curvature Assay and by Gas Chromatography-Mass Spectrometry-Selected Ion Monitoring. Plant Physiol. 1987 May;84(1):135–143. doi: 10.1104/pp.84.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]