Abstract
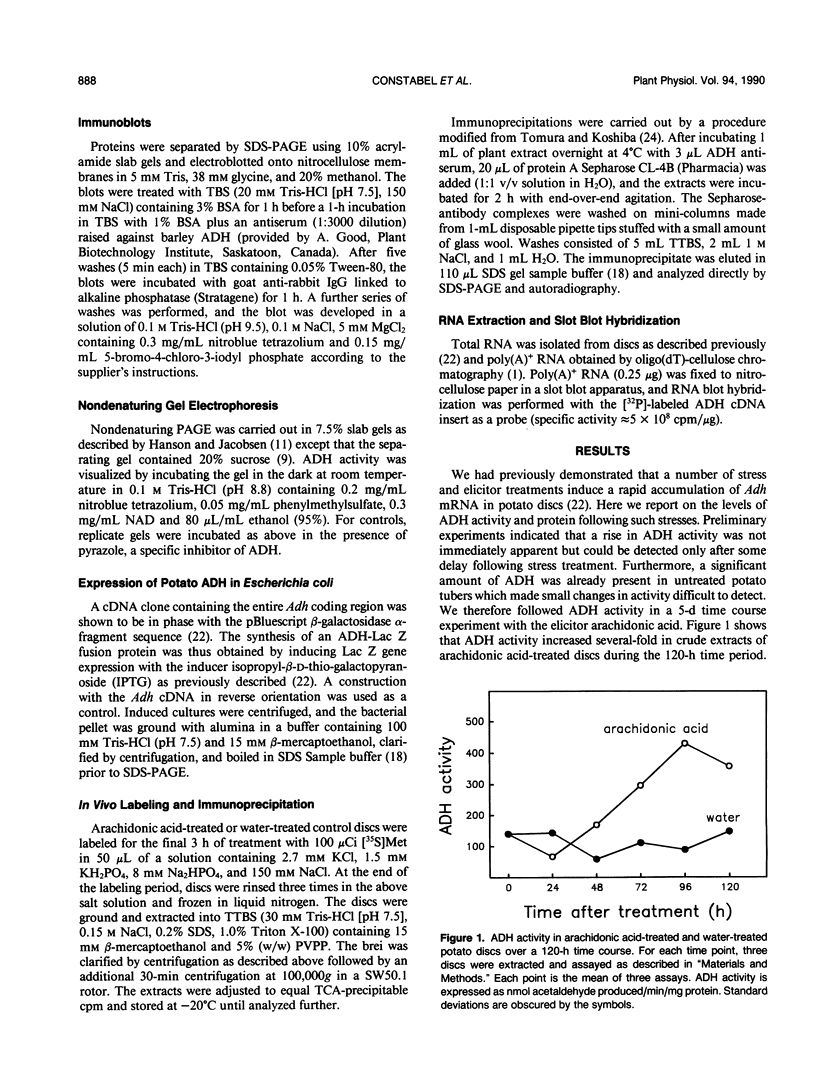
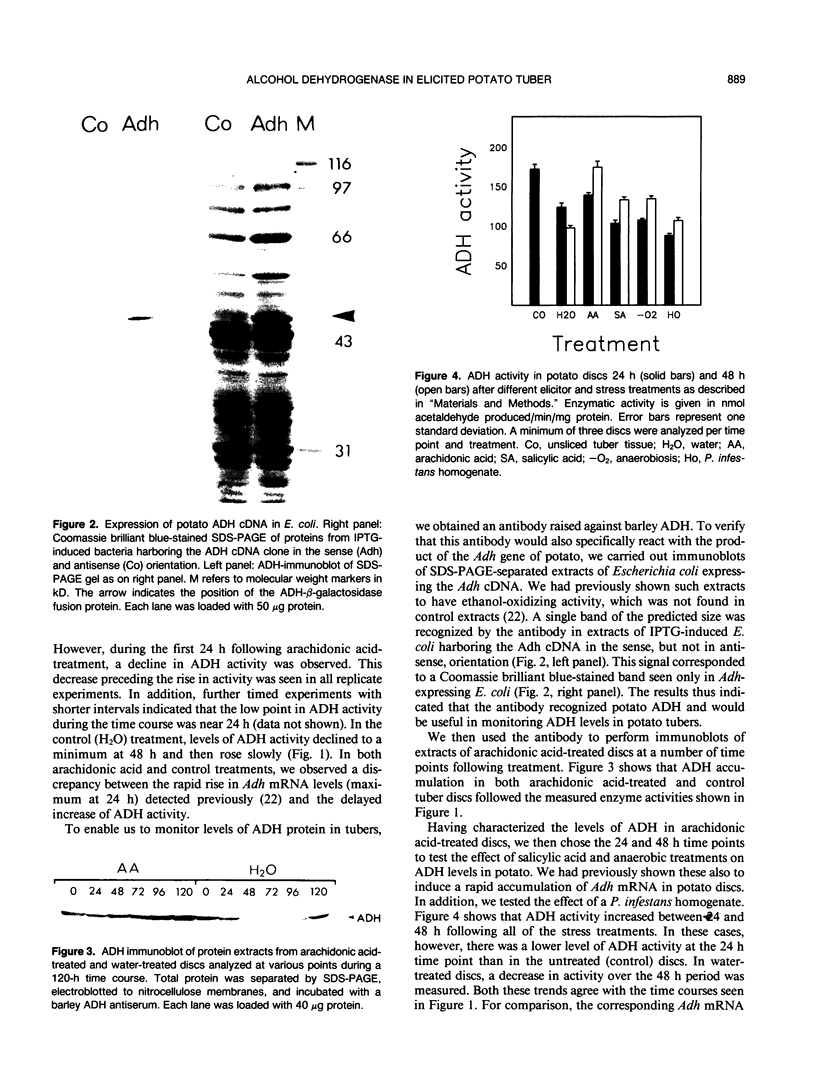
The accumulation of alcohol dehydrogenase (ADH) in arachidonic acid-elicited potato (Solanum tuberosum L.) tuber discs was studied. In accordance with our previous report of the accumulation of Adh mRNA beginning 2 hours after elicitor treatment (DP Matton, CP Constabel, N Brisson [1990] Plant Mol Biol 14: 775-783), immunoprecipitation of ADH from in vivo labeled discs indicated that ADH synthesis occurred as early as 12 hours after treatment. However, levels of ADH activity and protein, as shown by enzyme assay and immunoblot, did not rise in parallel but decreased during the first 24 hours of treatment. After 24 hours, ADH activity and protein began to increase, reaching a several-fold increase at 96 hours after elicitation. Water-treated control discs showed a similar though delayed and less pronounced pattern. These results imply a turnover of ADH following elicitor treatment of potato tuber discs. As shown by nondenaturing gel electrophoresis, the synthesis and degradation involved the same ADH isozyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Huang J. S., Knopp J. A. The Hypersensitive Reaction of Tobacco to Pseudomonas syringae pv. pisi: Activation of a Plasmalemma K/H Exchange Mechanism. Plant Physiol. 1985 Nov;79(3):843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicsak T. A., Kann L. R., Reiter A., Chase T., Jr Tomato alcohol dehydrogenase: purification and substrate specificity. Arch Biochem Biophys. 1982 Jul;216(2):605–615. doi: 10.1016/0003-9861(82)90250-8. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Kuc J. A., Laine R. A. Eicosapentaenoic and Arachidonic Acids from Phytophthora infestans Elicit Fungitoxic Sesquiterpenes in the Potato. Science. 1981 Apr 3;212(4490):67–69. doi: 10.1126/science.212.4490.67. [DOI] [PubMed] [Google Scholar]
- Freeling M., Bennett D. C. Maize Adh1. Annu Rev Genet. 1985;19:297–323. doi: 10.1146/annurev.ge.19.120185.001501. [DOI] [PubMed] [Google Scholar]
- Good A. G., Crosby W. L. Induction of alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiol. 1989 Jul;90(3):860–866. doi: 10.1104/pp.90.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V., Zwar J. A. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Kozlowski T. T. Ethylene, Ethane, Acetaldehyde, and Ethanol Production By Plants under Stress. Plant Physiol. 1982 Apr;69(4):840–847. doi: 10.1104/pp.69.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Macdonald R. C. Acetaldehyde and ethanol biosynthesis in leaves of plants. Plant Physiol. 1987 Aug;84(4):1204–1209. doi: 10.1104/pp.84.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Stringer M. A. Alcohol dehydrogenase and ethanol in the stems of trees : evidence for anaerobic metabolism in the vascular cambium. Plant Physiol. 1988 Jul;87(3):693–697. doi: 10.1104/pp.87.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneusel R. E., Matern U., Nicolay K. Formation of trans-caffeoyl-CoA from trans-4-coumaroyl-CoA by Zn2+-dependent enzymes in cultured plant cells and its activation by an elicitor-induced pH shift. Arch Biochem Biophys. 1989 Mar;269(2):455–462. doi: 10.1016/0003-9861(89)90129-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matton D. P., Constabel P., Brisson N. Alcohol dehydrogenase gene expression in potato following elicitor and stress treatment. Plant Mol Biol. 1990 May;14(5):775–783. doi: 10.1007/BF00016510. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Tomura H., Koshiba T. Biosynthesis of alpha-Amylase in Vigna mungo Cotyledon. Plant Physiol. 1985 Dec;79(4):939–942. doi: 10.1104/pp.79.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]