Abstract
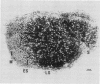
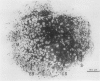
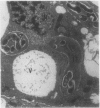
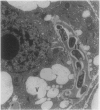
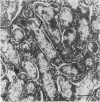
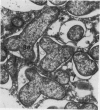
Treatment of Pisum sativum (L.) cv. `Sparkle' with ethyl methanesulfonic acid (EMS) produced a stable mutant, E135F, which forms small, white, ineffective nodules. These nodules exhibit histological zonation typical of an indeterminant nodule, e.g. meristematic, early symbiotic, late symbiotic, and senescent zones. Compared with the nitrogen fixing nodules of the parent, the zones are smaller and the nodules senesce prematurely. Bacteroids in E135F are less elongated and less differentiated than those in `Sparkle.' The E135F mutant forms ineffective nodules when inoculated with nine different effective strains of Rhizobium leguminosarum and also when grown in a soil containing effective strains. The ineffective phenotype of E135F is under monogenic recessive control; the gene is designated sym 13. sym 13 was located on chromosome 2 by linkage with genes for shikimic dehydrogenase and esterase-2. The original selection E135F carried another mutation in heterozygous form at a separate locus, yielding some homozygous recessive nonnodulating progeny, E135N, in later generations. This indicates that EMS treatments may cause mutations at more than one sym gene. The gene conditioning non-nodulation in E135N was designated sym 14. It mapped to a locus on a different part of chromosome 2 by linkage to the gene for fumarase. The data demonstrate that sym genes are not necessarily closely linked.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Egli M. A., Griffith S. M., Miller S. S., Anderson M. P., Vance C. P. Nitrogen Assimilating Enzyme Activities and Enzyme Protein during Development and Senescence of Effective and Plant Gene-Controlled Ineffective Alfalfa Nodules. Plant Physiol. 1989 Nov;91(3):898–904. doi: 10.1104/pp.91.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Bang M., Ausubel F. M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Smith C. A. Effects of Rhizobium meliloti nif and fix mutants on alfalfa root nodule development. J Bacteriol. 1987 Mar;169(3):1137–1146. doi: 10.1128/jb.169.3.1137-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Norris J. H., Macol L. A., Hirsch A. M. Nodulin gene expression in effective alfalfa nodules and in nodules arrested at three different stages of development. Plant Physiol. 1988 Oct;88(2):321–328. doi: 10.1104/pp.88.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suiter K. A., Wendel J. F., Case J. S. LINKAGE-1: a PASCAL computer program for the detection and analysis of genetic linkage. J Hered. 1983 May-Jun;74(3):203–204. doi: 10.1093/oxfordjournals.jhered.a109766. [DOI] [PubMed] [Google Scholar]