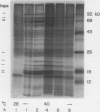
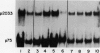
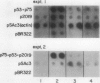
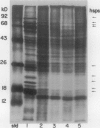
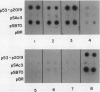
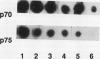
Abstract
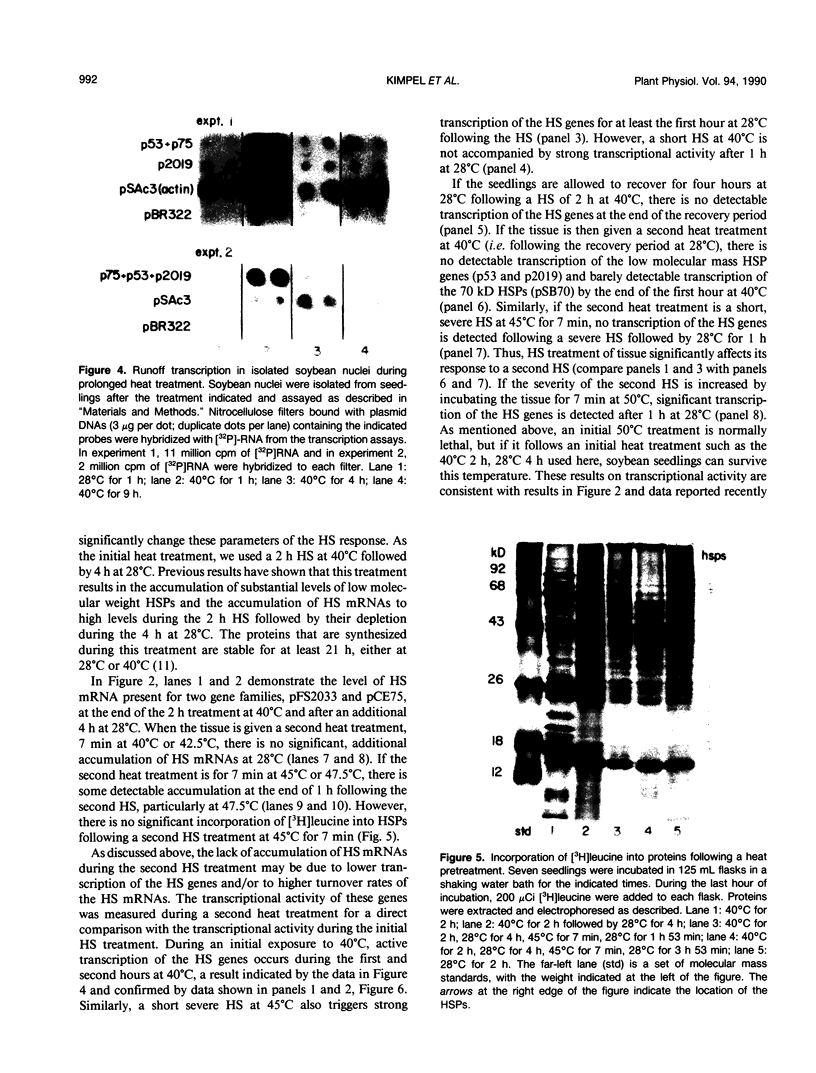
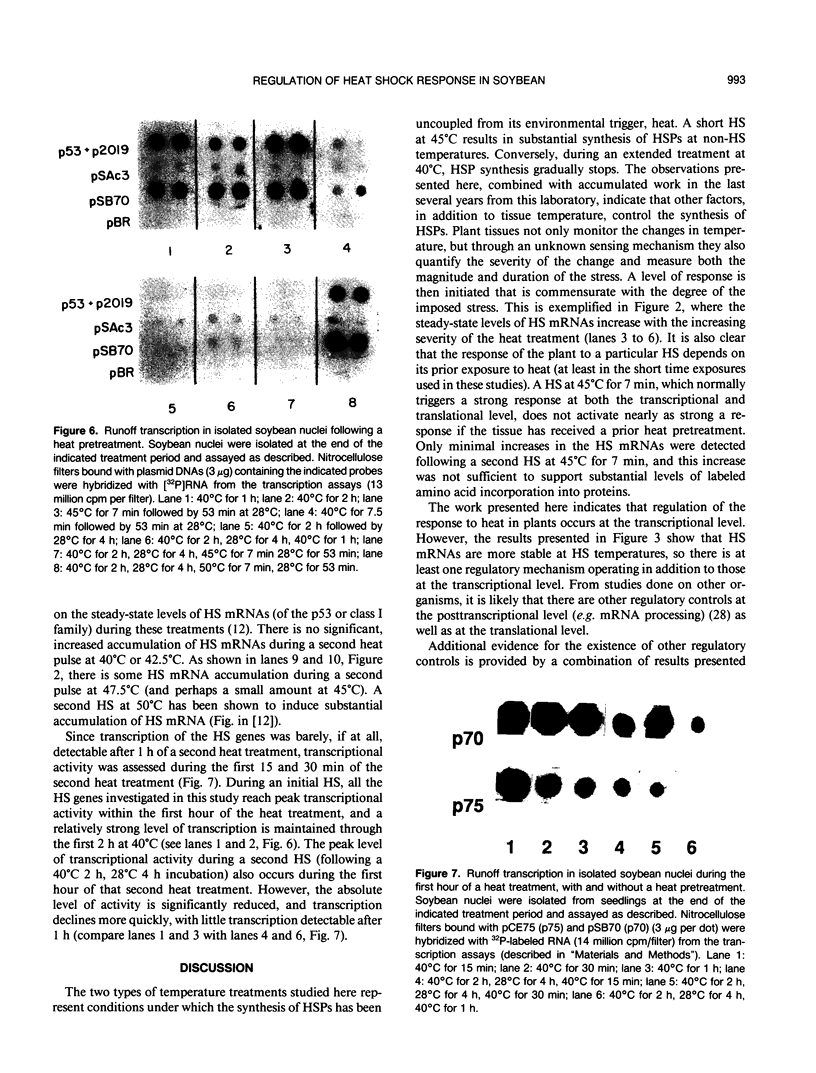
The transcriptional response of soybean (Glycine max) seedlings during heat shock (HS) was investigated under two different treatment regimes. During prolonged heat treatment at 40°C, active transcription of the HS genes (as measured by “runoff” transcription assays) occurs only during the first few hours. Nonetheless, mRNAs for these genes are present at relatively high abundance even after 9 hours of exposure to 40°C. Because HS mRNAs have a fairly short half-life (less than 3 hours) at 28°C, these results indicate that HS mRNAs are inherently more stable at 40°C. During a second type of heat treatment regime—short pulses of high (45°C) heat followed by 1 to 2 hours at 28°C—transcription of HS genes is comparable to that achieved at 40°C for the first few hours, even though the tissue is maintained at non-HS temperatures. The transcriptional responses to these two different heat treatments indicate that regulatory controls for the transcription of the HS genes must involve more than a simple sensing of ambient temperature, since transcription of these genes can be turned off at 40°C (in the case of prolonged exposure) and can continue at 28°C (following a short, severe heat treatment). Additional results demonstrate that the response of soybean seedlings to a particular HS depends on their prior exposure to heat; seedlings given a preheat treatment (that is known to induce thermotolerance) respond more moderately to a short heat pulse at 45°C. Overall, this research indicates that plants have mechanisms for both monitoring the severity of changes in temperature and for measuring the magnitude and duration of the stress. Such information is then used to regulate the plant's response to heat both transcriptionally and posttranscriptionally.
Full text
PDF

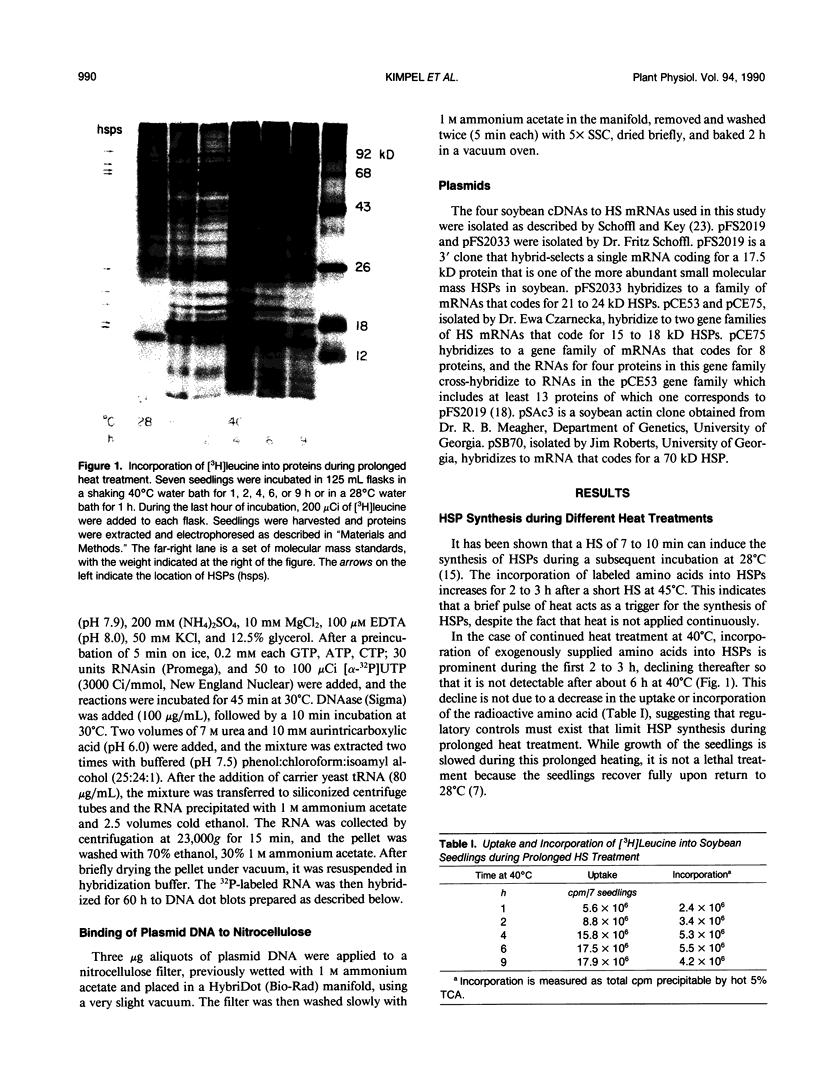
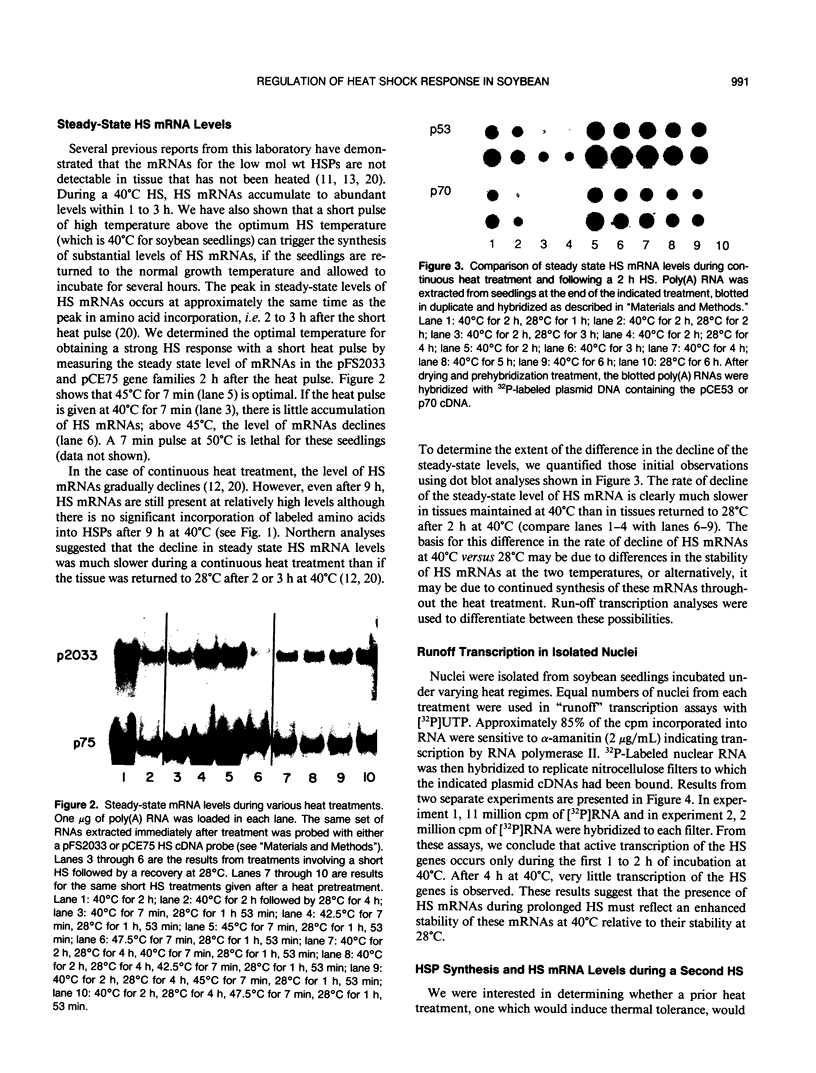


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Chou M., Chen Y. M., Lin C. Y. Thermotolerance of isolated mitochondria associated with heat shock proteins. Plant Physiol. 1989 Feb;89(2):617–621. doi: 10.1104/pp.89.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Hamilton R. H., Künsch U., Temperli A. Simple rapid procedures for isolation of tobacco leaf nuclei. Anal Biochem. 1972 Sep;49(1):48–57. doi: 10.1016/0003-2697(72)90241-2. [DOI] [PubMed] [Google Scholar]
- Kimpel J. A., Key J. L. Presence of Heat Shock mRNAs in Field Crown Soybeans. Plant Physiol. 1985 Nov;79(3):672–678. doi: 10.1104/pp.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton K. W., Karmin P., Hahn G. M., Minton A. P. Nonspecific stabilization of stress-susceptible proteins by stress-resistant proteins: a model for the biological role of heat shock proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7107–7111. doi: 10.1073/pnas.79.23.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. Heat-shock proteins. Coming in from the cold. Nature. 1988 Apr 28;332(6167):776–777. doi: 10.1038/332776a0. [DOI] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J Bacteriol. 1985 Jun;162(3):1083–1091. doi: 10.1128/jb.162.3.1083-1091.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Key J. L. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Genet. 1982;1(4):301–314. [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Stone D. E., Craig E. A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jul;7(7):2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood C. E. Acquired Tolerance of Leaves to Heat. Science. 1961 Sep 29;134(3483):941–942. doi: 10.1126/science.134.3483.941. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. Translation of unspliced transcripts after heat shock. Science. 1988 Dec 16;242(4885):1544–1548. doi: 10.1126/science.3201243. [DOI] [PubMed] [Google Scholar]