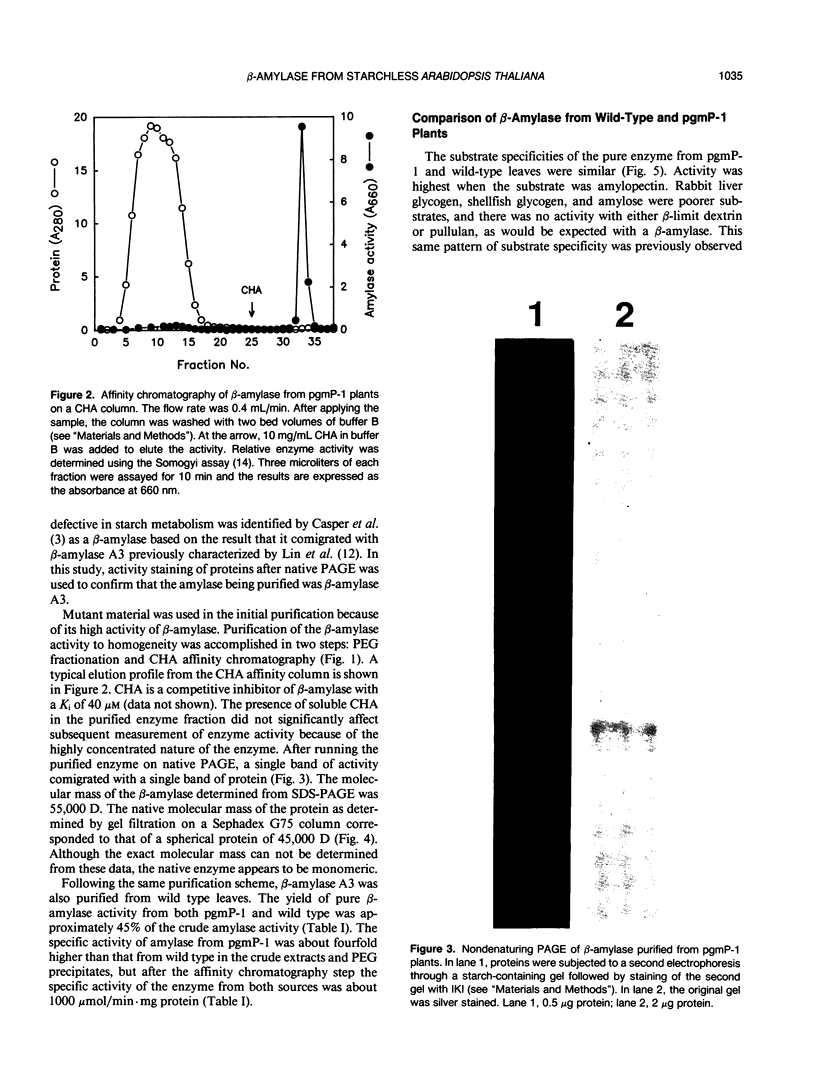
Abstract
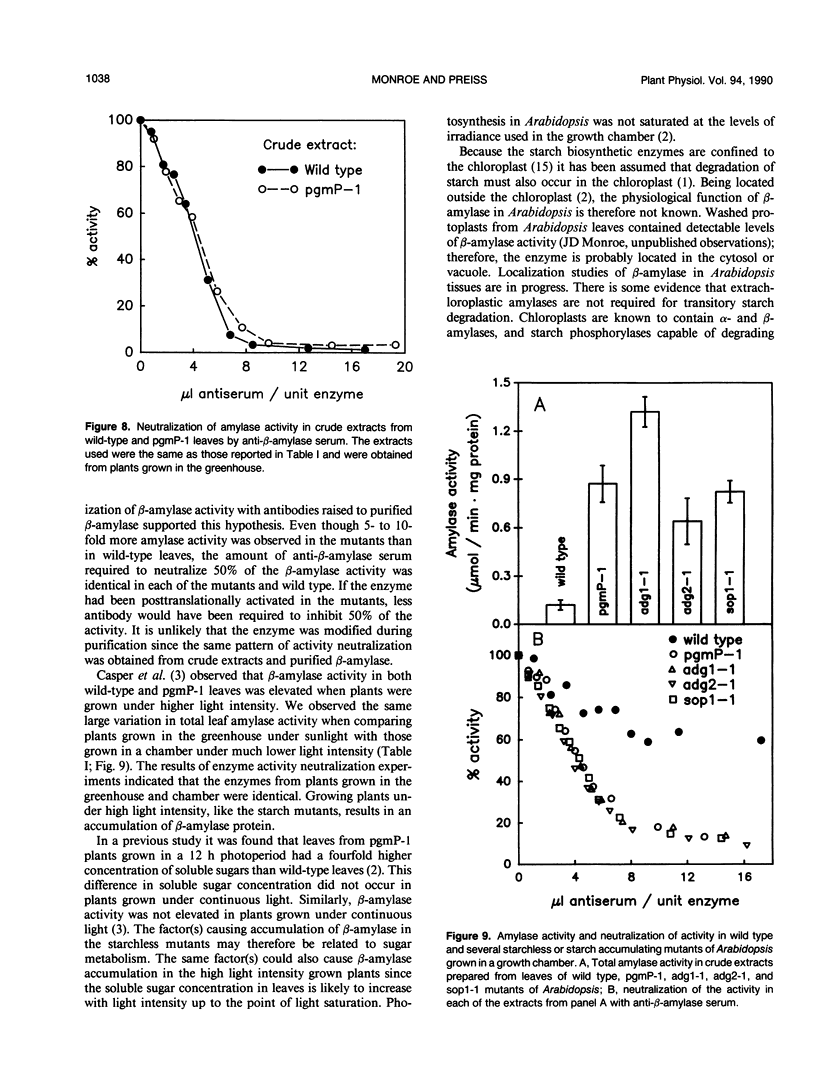
Amylase activity is elevated 5- to 10-fold in leaves of several different Arabidopsis thaliana mutants defective in starch metabolism when they are grown under a 12-hour photoperiod. Activity is also increased when plants are grown under higher light intensity. It was previously determined that the elevated activity was an extrachloroplastic β-(exo)amylase. Due to the location of this enzyme outside the chloroplast, its function is not known. The enzyme was purified to homogeneity from leaves of both a starchless mutant deficient in plastid phosphoglucomutase and from the wild type using polyethylene glycol fractionation and cyclohexaamylose affinity chromatography. The molecular mass of the β-amylase from both sources was 55,000 daltons as determined by denaturing gel electrophoresis. Gel filtration studies indicated that the enzyme was a monomer. The specific activities of the purified protein from mutant and wild-type sources, their substrate specificities, and Km for amylopectin were identical. Based on these results it was concluded that the mutant contained an increased level of β-amylase protein. Enzyme neutralization studies using a polyclonal antiserum raised to purified β-amylase showed that in each of two starchless mutants, one starch deficient mutant and one starch overproducing mutant, the elevated amylase activity was due to elevated β-amylase protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspar T., Huber S. C., Somerville C. Alterations in Growth, Photosynthesis, and Respiration in a Starchless Mutant of Arabidopsis thaliana (L.) Deficient in Chloroplast Phosphoglucomutase Activity. Plant Physiol. 1985 Sep;79(1):11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T., Lin T. P., Monroe J., Bernhard W., Spilatro S., Preiss J., Somerville C. Altered regulation of beta-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5830–5833. doi: 10.1073/pnas.86.15.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G. W., Jr, Pallas J. E., Jr, Mendicino J. The hydrolysis of maltodextrins by a -amylase isolated from leaves of Vicia faba. Biochim Biophys Acta. 1972 Aug 28;276(2):491–507. doi: 10.1016/0005-2744(72)91010-8. [DOI] [PubMed] [Google Scholar]
- Doehlert D. C., Duke S. H., Anderson L. Beta-Amylases from Alfalfa (Medicago sativa L.) Roots. Plant Physiol. 1982 May;69(5):1096–1102. doi: 10.1104/pp.69.5.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E., Boyer C., Preiss J. Immunological characterization of Escherichia coli B glycogen synthase and branching enzyme and comparison with enzymes from other bacteria. J Bacteriol. 1982 Sep;151(3):1444–1453. doi: 10.1128/jb.151.3.1444-1453.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C. R., Preiss J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988 Dec;88(4):1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Spilatro S. R., Preiss J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988 Jan;86(1):251–259. doi: 10.1104/pp.86.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P. A., Henson C. A., Duke S. H. Purification and Characterization of Pea Epicotyl beta-Amylase. Plant Physiol. 1990 Mar;92(3):615–621. doi: 10.1104/pp.92.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Stitt M., Heldt H. W. Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol. 1981 Sep;68(3):755–761. doi: 10.1104/pp.68.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K., Sharma R. Affinity chromatography of mustard beta-amylase on starch columns. J Biochem Biophys Methods. 1985 Mar;10(5-6):315–320. doi: 10.1016/0165-022x(85)90066-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretblad P. Immobilization of ligands for biospecific affinity chromatography via their hydroxyl groups. The cyclohexaamylose-beta-amylase system. FEBS Lett. 1974 Oct 1;47(1):86–89. doi: 10.1016/0014-5793(74)80431-x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Ziegler P., Beck E. Exoamylase activity in vacuoles isolated from pea and wheat leaf protoplasts. Plant Physiol. 1986 Dec;82(4):1119–1121. doi: 10.1104/pp.82.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]




