Abstract
Background
Cytomegalovirus (CMV) reactivation after unmanipulated haploidentical stem cell transplantation (SCT) frequently occurs, causing life-threatening morbidities and transplantation failure. Pre-emptive therapy upon the detection of CMV viremia using antiviral agents is currently the standard of care but it was associated with significant toxicity. The CMV antigen-specific cytotoxic T lymphocyte therapy was limited by the time-consuming manufacture process and relatively low success rate. More effective and safer approaches for the treatment of CMV reactivation after haploidentical SCT are in urgent need.
Methods
A single-arm, open-label, phase I clinical trial evaluating the safety and efficacy of CMV-targeting T cell receptor-engineered T (CMV-TCR-T) cell therapy as the first-line pre-emptive therapy for patients with CMV reactivation after haploidentical peripheral blood SCT (PBSCT) was conducted in the Chinese PLA General Hospital. Six patients with CMV reactivation after haploidentical SCT were adoptively transferred by one to three doses of SCT donors-derived CMV-TCR-T cells. This trial was a dose-escalation study with doses ranging from 1×103 CMV-TCR-T cells/kg body weight per dose to 5×105 CMV-TCR-T cells/kg per dose.
Results
Except for the grade 1 cytokine release syndrome observed in one patient and mild fever in two patients, no other adverse events were observed. Four patients had response within a month after CMV-TCR-T cell infusion without the administration of any antiviral agents. The other two patients who initially did not respond to CMV-TCR-T cell therapy had salvage ganciclovir and foscarnet administration and then had rapid CMV clearance. The CMV-TCR-T cells displayed overall robust expansion and persistence in the peripheral blood after infusion. The CMV-TCR-T cells were first detected in the peripheral blood of these patients 3–7 days after the first dose of CMV-TCR-T infusion, rapidly expanded and persisted for at least 1–4 months, providing long-term protection against CMV reactivation. In one patient, the CMV-TCR-T cells started to expand even when the anti-graft-versus-host disease reagents were still being used, further indicating the proliferation potential of CMV-TCR-T cells.
Conclusions
Our study first showed CMV-TCR-T cell as a highly feasible, safe and effective first-line pre-emptive treatment for CMV reactivation after haploidentical PBSCT.
Trial registration number
ClinicalTrials.gov Registry (NCT05140187).
Keywords: CD8-Positive T-Lymphocytes; Cell Engineering; Clinical Trials as Topic; Immunotherapy, Adoptive
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current first-line pre-emptive therapy for the treatment of cytomegalovirus (CMV) reactivation after haploidentical stem cell transplantation (SCT) is associated with toxicity. More effective and safer approaches are in urgent need.
WHAT THIS STUDY ADDS
SCT donors-derived CMV-targeting T cell receptor-engineered T (TCR-T) cell is a highly feasible, safe and effective first-line pre-emptive treatment for CMV reactivation after haploidentical SCT.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study first showed the potential of CMV-targeting TCR-T cells as a new modality of pre-emptive therapy for CMV reactivation after SCT.
Background
Cytomegalovirus (CMV) is a β-herpesvirus that causes lifelong infection in immunocompromised patients, with a seropositivity of ~60% of adults in developed countries and >90% in developing countries.1 The primary CMV infection is controlled by robust antigen-specific T cell responses to immunodominant CMV epitopes, with CD8+ T cells responsible for the direct eradication of the CMV-infected cells to clear the infection. After primary infection, CMV becomes latent in monocytes, tissue macrophages and other cells. The persistence of CMV antigen-specific memory T cells provides a long-term surveillance to prevent the reactivation of latent CMV.2–5 However, in patients with suppressed immune system, such as those who underwent allogenic stem cell transplantation (SCT), the T cell-mediated protection against CMV is severely compromised, leading to the reactivation of CMV.5 6
The risk of CMV reactivation increases in patients receiving SCT, which may cause failure of SCT and life-threatening end-organ disease. CMV reactivation with viremia occurs in up to 80% of seropositive recipients after SCT with progression to CMV disease in 20–30% of them without intervention.7 Reactivation usually occurs in the first 100 days post-SCT. CMV end-organ disease has a variety of clinical manifestations post-SCT, including pneumonitis, colitis, gastritis, hepatitis, retinitis, and bone marrow suppression.2 4 8 Symptoms of CMV pneumonia include cough, shortness of breath, fever and fatigue. The mortality rate of CMV pneumonia with delayed treatment or ineffective prophylaxis is over 85%.9 Symptoms of CMV colitis usually include diarrhea, hematochezia, fever, abdominal pain, and even colonic perforation.10 CMV ulcerative colitis can be life-threatening.
Prophylaxis for CMV infection after SCT has not been recommended by the Chinese Society of Clinical Oncology Guidelines in China. Pre-emptive therapy upon the detection of CMV viremia using antiviral agents such as ganciclovir and foscarnet is currently the standard of care.11 12 However, these agents are associated with significant toxicity. For example, ganciclovir is associated with myelosuppression,13 14 while foscarnet is limited by nephrotoxicity.15 Treatment of CMV reactivation and disease after SCT has been a large burden of both the patients and the healthcare system; thus, more effective and safer therapeutic modalities are in urgent need.
In recent years, adoptive immunotherapy with CMV antigen-specific cytotoxic T lymphocytes (CTLs) has been developed as an important alternative option for treating CMV infection post-SCT.6 15–20 Rapid reconstitution of T cell immunity by adoptive infusion of CTLs against CMV is crucial to the control of CMV reactivation post-SCT. Though CTL provided some clinical benefit in decreasing the rate and severity of CMV infection post-SCT15 17 with low toxicity,16 the time-consuming manufacture process with relatively low success rate largely limited its clinical application. In addition, CTLs may become exhausted after repeated antigen stimulation and expansion in vitro, which reduces their efficacy. T cell receptor-engineered T (TCR-T) cell therapy is a modality of immunotherapy that involves the transfer of gene constructs encoding TCR α and β chains which specifically recognize viral or tumor antigens presented by certain human leukocyte antigen (HLA) molecules into T cells derived from the peripheral blood. Compared with CTL therapy, TCR-T cells allow the rapid manufacture of a large number of T cells redirected to specifically target and lyse virus-infected or tumor cells, which showed potent and specific activity against target cells expressing specific antigens in vitro and in vivo. Furthermore, TCR-T cells employ physiological signaling pathways which may provide a safety advantage compared with chimeric antigen receptor-T cell therapy. The in vitro activity of CMV antigen-specific TCR-T cells has been reported in 2009.21 In this study, HLA-A*01:01-restricted, HLA-A*02:01-restricted, and HLA-B*35:01-restricted CMV pp65-specific TCR-T cells were established, respectively. These CMV-TCR-T cells displayed specific and efficacious effector functions and cytolytic activity against target cells expressing pp65 and showed robust expansion upon antigen-specific stimulation. In 2022, Liu and colleagues reported the first clinical trial of CMV-targeting TCR-T (CMV-TCR-T) cell therapy in treating patients with refractory CMV viremia or disease after allo-hematopoietic SCT, which demonstrated the safety and efficacy of this new therapeutic modality.22 However, TCR-T cell was used as a salvage therapy in their study. There was no published clinical study of the CMV-TCR-T cells as a first-line pre-emptive therapeutic approach for patients with CMV infection after haploidentical SCT until now.
In this study, we conducted the first phase I clinical trial evaluating the safety and efficacy of CMV-TCR-T cell therapy as the first-line pre-emptive therapy for patients with CMV infection post anti-thymocyte globulin (ATG)-based adult unmanipulated haploidentical peripheral blood SCT (PBSCT). Our study represented the first clinical application of CMV-TCR-T cell therapy as a first-line pre-emptive treatment for CMV reactivation after haploidentical SCT.
Methods
Patients and study design
From January 2022 to March 2023, patients who received ATG-based adult unmanipulated haploidentical PBSCT in the Chinese PLA General Hospital were recruited in this study. The inclusion criteria were as follows: (1) 18–60 years old; (2) patients with hematologic malignancies and have undergone haploidentical SCT; (3) Karnofsky Score ≥70; (4) related haploidentical donor; (5) both donor and the patient had HLA-A*02:01, HLA-A*11:01 or HLA-A*24:02. The exclusion criteria were as follows: (1) patients with active acute graft-versus-host disease (aGvHD) 1 day before TCR-T cell infusion, (2) patients with serious organ dysfunction within 1 week before TCR-T cell infusion, (3) patients already received or was anticipated to receive other adoptive cellular therapies in 1 month post TCR-T infusion, (4) had other malignancies or relapsed and uncontrolled hematologic malignancies. The inclusion criteria for CMV-TCR-T cell donors were as follows: (1) provided haploidentical SCT for the patient; (2) both donor and the patient had HLA-A*02:01, HLA-A*11:01 or HLA-A*24:02. The exclusion criteria for CMV-TCR-T cell donors were (1) positive for any of the following: hepatitis B surface antigen, hepatitis B e antigen, hepatitis B virus-DNA, hepatitis C virus antibody, hepatitis C virus-RNA, HIV antibody, Treponema pallidum antibody, Epstein-Barr virus-DNA or CMV-DNA; (2) had taken immunosuppressive agents 1 week before peripheral blood mononuclear cell (PBMC) collection.
CMV reactivation was monitored based on blood CMV-DNA levels using a real-time quantitative PCR (qPCR) kit two times per week after SCT. Blood 2 mL was used for this assay. CMV reactivation occurred when blood CMV-DNA level ≥1×104 copies/mL was detected once or ≥1×103 copies/mL was detected twice consecutively. The endpoints were safety, clinical responses and CMV-TCR-T cell persistence. Blood CMV-DNA level was monitored two times per week after TCR-T cell infusion. The efficacy of the TCR-T cell therapy was documented when CMV-DNA level <1×103 copies/mL was detected twice consecutively. Response to the therapy was defined as that the blood CMV-DNA level remains <1×103 copies/mL for at least 2 weeks. The in vivo expansion and persistence of CMV-TCR-T were documented by the change of gene copies of transgenic TCR in peripheral blood. This trial is registered at www.clinicaltrials.gov as NCT05140187.
CMV-specific TCR discovery
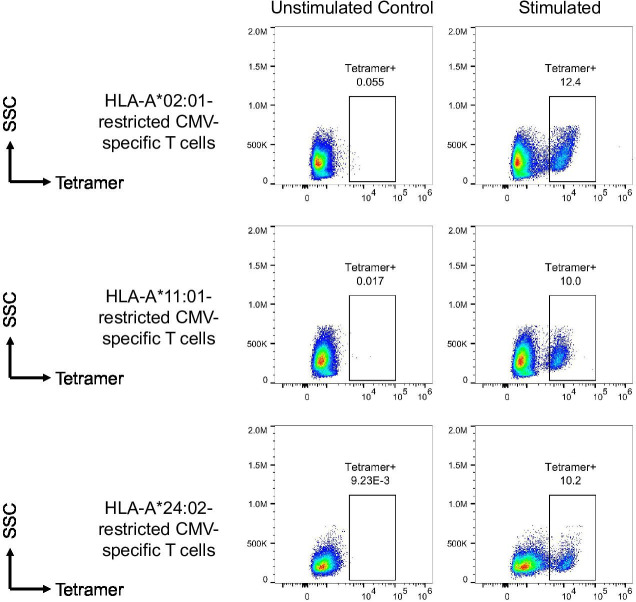
To obtain the sequences of TCR specifically recognizing CMV antigens, the PBMCs from CMV-seropositive healthy donors were stimulated by CMV antigen peptides derived from CMV pp65 protein (online supplemental figure 1), including NLVPMVATV (HLA-A*02:01-restricted), ATVQGQNLK (HLA-A*11:01-restricted), and QYDPVAALF (HLA-A*24:02-restricted). PBMCs from two HLA-matched donors were used for each CMV epitope. For each peptide, totally, 2.4×107 PBMCs were suspended by CMX media (45% RPMI-1640, 45% X-VIVO15 and 10% FBS) at 8×105 cells/mL and added to one 6-well plate at 5 mL/well. Peptide 20 µg/mL was added to each well. The plate was cultured at 37°C, 5% CO2. After 7 days, interleukin (IL)-2 was added to cells. Half volume of media was replaced every 3–4 days. After another 2 weeks, cells were harvested, washed by phosphate buffered saline and stained by corresponding phycoerythin-labeled tetramers (MBL TS-0010-1C, TS-0020-1C and TS-M012-1). Tetramer+ T cells were sorted for single-cell sequencing. The TCR sequences of top three most prevalent T cell clones were cloned, re-expressed in T cells and tested for their cytolytic activity. For each epitope, the TCR with the highest cytolytic activity among the three was regarded as the top candidate TCR, and was used to manufacture CMV-TCR-T cells in the clinical study.
jitc-2023-007735supp001.pdf (111.3KB, pdf)
jitc-2023-007735supp002.pdf (1.9MB, pdf)
CMV-specific TCR cloning
Full-length cDNA sequences of TCRα and β chain linked by the 2A peptide of porcine teschovirus-1 were synthesized by Beijing Tsingke Biotech Company and cloned into pLV4 lentiviral vector using XbaI and XhoI restriction sites, for lentivirus-mediated TCR gene transduction.
Generation of CMV-TCR-T cells
CMV-TCR-T cells were manufactured by Beijing Yongtai Ruike Biotechnology Company. T cells were isolated from the PBMCs of SCT donors and activated by the α-CD3/α-CD28 Dynabeads (Gibco 40203D) for 2 days. Activated T cells were transduced with pLV4 lentiviral vector containing the encoding genes of CMV-specific TCR. Both CD4+ and CD8+ T cells were transduced. After lentiviral transduction, the T cells were cultured in X-VIVO 15 media (Lonza 02-060Q) supplemented with 200 IU/mL IL-2 at 37°C and 5% CO2 for approximately 5–11 days to obtain sufficient cells and then cryopreserved. The number of CMV-TCR-T cells manufactured was at least twofold of the maximum required doses of a certain patient before cryopreservation to ensure this patient get sufficient number of CMV-TCR-T cells for infusion after thawing. Cell staining with peptide–major histocompatibility complex (MHC) tetramer and CD3/CD4/CD8 antibodies and flow cytometry analysis were performed to determine the transduction efficiency.
In vitro cytolysis assay of CMV-TCR-T cells
For each cytolysis assay, 1×105 carboxyfluorescein succinimidyl ester (CFSE)-labeled lymphoblastoid cell lines (LCLs) stably expressing CMV pp65 protein at the density of 5×105/mL were co-cultured with 3×105 (E/T=3), 6×105 (E/T=6), or 1.2×106 CMV-TCR-T cells (E/T=12), respectively, in 96-well plate. On the next day, the co-cultured cells were harvested and stained with 7-AAD and analyzed by flow cytometry. Percentage of 7-AAD+ cells among CFSE+ population was calculated and represented as cytolytic lysis (%) after the value of non-effector control was subtracted. The assay was in triplicate (n=3). Data were analyzed using GraphPad Prism software (GraphPad Software). Statistical significance was determined by paired Student’s t-tests between groups (*p<0.05).
Persistence of CMV-TCR-T cells in patients
To monitor the expansion and persistence of CMV-TCR-T cells (both CD4+ and CD8+) after infusion, DNA was extracted from the peripheral blood of patients collected at the following time points after TCR-T cell infusion: day 0, day 1, day 2, day 3, day 4, day 5, day 6, day 7, day 14, day 21±2, day 28±2, week 8 day 1±4, week 10 day 1±4, week 12 day 1±4, week 26 day 1±7, week 39 day 1±7, and week 52 day 1±7. Blood 2 mL was used for each time point. DNA was extracted by QIAamp DNA Mini Kit (QIAGEN 51306). Real-time qPCR was conducted to quantify the level of the CMV-TCR gene (<100 copies/μg DNA was defined as negative). The qPCR primers specific for transgenic TCR construct were as follows:
Forward: 5’-AGTAAGACCACCGCACAGCA-3’
Reverse: 5’-CCTTGGTGGGTGCTACTCCT-3’
Probe: 5’-FAM-CCTCCAGGTCTGAAGATCAGCGGCCGC-TAMRA-3’
TCRβ chain sequencing
To monitor the persistence of CMV-TCR-T cells and the diversity of TCR repertoire, genomic DNA was extracted from the PBMCs of patients collected. TCRβ chain sequencing was performed at MyGenostics (Beijing, China) using the ImmunoSEQ platform with primers specific for 53 human Vβ regions and 13 Jβ regions.
Results
In vitro potency of CMV-TCR-T cells
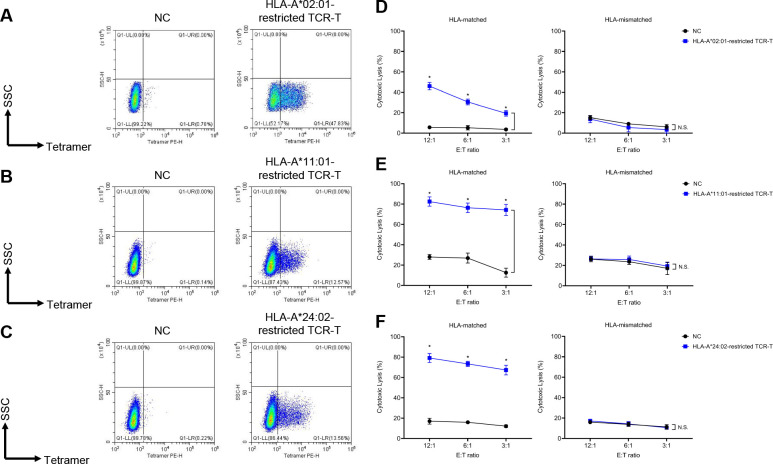
To obtain the sequences of TCR specifically recognizing CMV antigens, the PBMCs from healthy donors were stimulated by CMV antigen peptides that strongly bind to most prevalent HLA-A haplotypes in Beijing Yongtai Ruike Biotechnology Company (online supplemental figure 1). Peptides used in this study included NLVPMVATV (HLA-A*02:01-restricted), ATVQGQNLK (HLA-A*11:01-restricted), and QYDPVAALF (HLA-A*24:02-restricted). After stimulation, cells were stained by specific peptide–MHC tetramers and tetramer+ cells were sorted for single-cell TCR sequencing (figure 1). Top candidate TCR sequence for each peptide was cloned into lentiviral vector and transduced into the T cells (both CD4+ and CD8+) derived from healthy donors’ PBMCs to make TCR-T cells. The transduction efficiencies ranged from 12% to 47% for different TCRs (figure 2A–C). In vitro cytolysis assay was performed to validate the specificity and potency of these fully human TCRs. These TCR-T cells of various HLA restrictions showed strong cytolytic activity against HLA-matched target cells expressing CMV antigens but not the HLA-unmatched target cells (figure 2D–F). The cytolysis percentages of HLA-A*02:01-restricted, HLA-A*11:01-restricted, and HLA-A*24:02-restricted CMV-TCR-T cells were 20–50%, 70–80%, and 70–80%, respectively, when co-cultured with HLA-matched target cells expressing CMV antigens, which were significantly higher than their corresponding non-transduced negative control T cells and than those when co-cultured with HLA-unmatched target cells. These data suggested that T cells equipped with these CMV antigen-recognizing TCRs can specifically and potently eradicate target cells mimicking CMV-infected cells.
Figure 1.
Isolation of CMV-specific T cells. The peripheral blood mononuclear cells from healthy donors were stimulated by CMV antigen peptides including NLVPMVATV (HLA-A*02:01-restricted), ATVQGQNLK (HLA-A*11:01-restricted), and QYDPVAALF (HLA-A*24:02-restricted). After stimulation, cells were stained by specific peptide–MHC tetramers. Stimulated T cells were compared with their unstimulated controls to define the tetramer+ populations which were then sorted for single-cell TCR sequencing. CMV, cytomegalovirus; HLA, human leukocyte antigen; MHC, major histocompatibility complex; TCR, T cell receptor; SSC, side scatter.
Figure 2.
In vitro efficacy of CMV-TCR-T cells. (A–C) Transduction rate of CMV-specific TCRs with different HLA restrictions was represented by the percentage of tetramer+ population. Non-transduced T cells corresponding to each group of TCR-T cells were used as negative controls (NC). (D–F) In vitro cytolytic activity against HLA-matched/unmatched target cells of CMV-TCR-T cells with different HLA restrictions, compared with corresponding NC. Asterisks indicated statistical significance determined by paired Student’s t-tests between groups (*p<0.05). CMV, cytomegalovirus; HLA, human leukocyte antigen; N.S., not significant; TCRs, T cell receptors; SSC, side scatter; SSC-H, side scatter height; PE-H, phycoerythrin height.
Characteristics of patients and treatment of TCR-T cells
We conducted a single-arm, open-label, phase I clinical trial in the Chinese PLA General Hospital to assess the safety and efficacy of CMV-TCR-T cell therapy in patients with CMV reactivation after ATG-based adult unmanipulated haploidentical PBSCT. From January 2022 to March 2023, 13 patients who received haploidentical SCT were enrolled in this study. The CMV-TCR-T cells were successfully manufactured and cryopreserved for all of them. The choice of HLA-A*02:01-restricted, HLA-A*11:01-restricted, and HLA-A*24:02-restricted TCRs for transduction was based on the HLA-A haplotypes of both SCT recipient and donor (table 1). For example, only when the recipient/patient and his/her donor simultaneously have HLA-A*02:01 haplotype, the HLA-A*02:01-restricted CMV-TCR-T cells were manufactured. This ensures the capability of CMV-TCR-T cells to lyse CMV-infected blood cells (derived from the donor) and non-blood cells of the patient. Of the 13 patients, 6 were excluded because CMV was not reactivated and 1 withdrew before receiving CMV-TCR-T cell infusion. Three patients with CMV viremia and three patients with CMV disease received one to three doses of CMV-TCR-T cell infusion as the first-line pre-emptive therapy after CMV reactivation was detected (table 1). The median time until the first detection of CMV reactivation was 31 days (28–120 days) after SCT. Four patients (patients 01–04) experienced aGvHD after SCT. Glucocorticoid steroids, anti-CD25 monoclonal antibody and ruxolitinib were administered to control aGvHD in these patients (table 1). Prior to TCR-T cell infusion, the aGvHD of all four patients already achieved complete response to the treatment. The detailed information of aGvHD occurrence, use of steroids and immunosuppressive reagents to treat GvHD was listed in table 1.
Table 1.
Patient characteristics and CMV disease/infection after SCT
| Patient no | Diagnosis | Patient’s HLA-A | Donor | Donor’s HLA-A | Donor’s CMV serostatus | Conditioning regimen | aGvHD post-SCT | Day of aGvHD post-SCT | Day of aGvHD treatment post-SCT | Day of CMV reactivation post-SCT | CMV reactivation symptom | CMV load upon 1st dose of TCR-T cell | Day of TCR-T cell infusion post-SCT |
| 01 | AML | 02:01, 02:07 | Daughter | 02:01, 02:07 | Positive | DAC+BU/CY+ATG | Yes | 20 | Steroids (1 mg/kg, D20–27, 0.5 mg/kg, D28–32) and ruxolitinib (5 mg, D20–32) | 37 | Viremia | 16 520 copies/mL | 41, 47 |
| 02 | MDS-EB2 | 02:01, 33:03 | Sibling | 02:01, 33:03 | Positive | DAC+BU/CY+ATG | Yes | 111 | Steroids (1 mg/kg, D111–119, 0.5 mg/kg, D120–125), anti-CD25 monoclonal antibody (D111, 113, 118, 125 and 133) and ruxolitinib (5 mg, D112–127) | 118 | Colitis | 2368 copies/mL | 120 |
| 03 | AML | 24:02, 02:01 | Son | 24:02, 02:01 | Positive | DAC+BU/CY+ATG | Yes | 17 | Steroids (1 mg/kg, D17–21, 0.5 mg/kg, D22–23), anti-CD25 monoclonal antibody (D24, 27 and 32) and ruxolitinib (5 mg, D17–37) | 30 | Colitis | <1000 copies/mL* | 32, 37, 55 |
| 04 | AML | 24:02, 02:06 | Father | 24:02, 33:03 | Positive | DAC+BU/CY+ATG | Yes | 30 | Steroids (1 mg/kg, D30–35, then 0.5 mg/kg, D36–38), anti-CD25 monoclonal antibody (D31, 33, 38, 45, 53) and ruxolitinib (5 mg, D30–44) | 47 | Pneumonia | 3710 copies/mL | 50, 57, 68 |
| 05 | ALL | 02:03, 24:02 | Son | 02:03, 24:02 | Positive | DAC+BU/CY+ATG | No | N/A | N/A | 43 | Viremia | 10 650 copies/mL | 61, 65 |
| 06 | AML | 11:01, 02:07 | Son | 11:01, 11:01 | Positive | DAC+BU/CY+ATG | No | N/A | N/A | 45 | Viremia | 1100 copies/mL | 45 |
*Patient 03 developed CMV colitis on day 30 post-SCT, 9 days before blood CMV load reached ≥1×103 copies/mL. To treat CMV colitis on time, the first dose of CMV-TCR-T cells was infused on day 32 post-SCT. On that day, blood CMV-DNA level was <1000 copies/mL.
aGvHD, acute graft-versus-host disease; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; BU, busulfan; CMV, cytomegalovirus; CY, cyclophosphamide; DAC, decitabine; HLA, human leukocyte antigen; MDS-EB2, myelodysplastic syndromes with excess blasts type 2;N/A, not applicable; SCT, stem cell transplantation; TCR-T cell, T cell receptor-engineered T cell;
This trial was a dose-escalation study with the first patient receiving 1×103 CMV-TCR-T cells/kg body weight per dose for two doses, followed by three patients receiving 1×105 CMV-TCR-T cells/kg per dose for one (one patient) or three doses (two patients), and then two patients of 5×105 CMV-TCR-T cells/kg per dose for one (one patient) or two doses (one patient). The number of doses was determined by whether dose-dependent toxicity (DLT) occurred and whether CMV infection was cleared by the first dose. If the previous dose failed to clear the CMV infection and did not cause any DLT, the patient will receive the next dose, until this patient received three doses in total. The median follow-up time for the entire cohort after haploidentical SCT was 373.5 days (135–463 days) (table 2).
Table 2.
TCR-T cell infusion and follow-up information
| Patient no | Day of TCR-T cell infusion post-SCT | TCR-T HLA restriction | CMV-TCR-T cell dose | % CD8+ CMV-TCR+ T cells | Day of follow-up | GvHD post-TCR-T | Any AEs or SAEs | Salvage anti-viral drugs | Day of CMV clearance post-TCR-T infusion | Day of 1st TCR-T detection post-infusion | Day of TCR-T persistence |
| 01 | 41, 47 | HLA-A*02:01 | 1×103/kg per dose, 2 doses | 21.06 | 463 | No | No | Ganciclovir | 28 | N/A | 0 |
| 02 | 120 | HLA-A*02:01 | 1×105/kg per dose, 1 dose | 28.27 | 401 | No | No | No | 9 | 3 | 12 |
| 03 | 32, 37, 55 | HLA-A*24:02 | 1×105/kg per dose, 3 doses | 29.81 | 386 | No | Fever | No | 19 | 7 | ≥101 |
| 04 | 50, 57, 68 | HLA-A*24:02 | 1×105/kg per dose, 3 doses | 22.14 | 361 | No | Grade 1 CRS (fever) | Ganciclovir, foscarnet | 22 | 5 | >74 |
| 05 | 61, 65 | HLA-A*24:02 | 5×105/kg per dose, 2 doses | 29.06 | 273 | No | Fever | No | 28 | 3 | ≥125 |
| 06 | 45 | HLA-A*11:01 | 5×105/kg per dose, 1 dose | 52.34 | 135 | No | No | No | 4 | 4 | >61 |
AEs, adverse effects; CMV, cytomegalovirus; CRS, cytokine release syndrome; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; N/A, not applicable; SAEs, serious adverse effects; SCT, stem cell transplantation; TCR-T cell, T cell receptor-engineered T cell.
TCR-T cell therapy responses
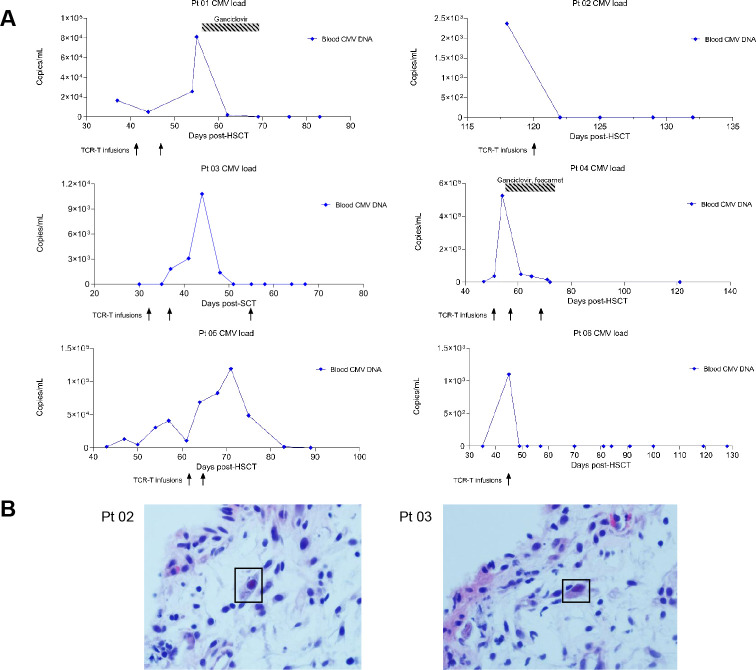
The median time from CMV-TCR-T cell infusion to the first CMV clearance among all the six patients was 20.5 days (4–28 days) (table 2). Four patients (66.7%) showed response (blood CMV-DNA level remains <103 copies/mL for at least 2 weeks) after CMV-TCR-T cell infusion, without any salvage treatment (table 2 and figure 3A).
Figure 3.
Clinical responses of patients. (A) Dynamics of viral load of each individual patient after SCT. The time of TCR-T cell infusion and salvage treatment was also indicated. Day 0 is the day of SCT. (B) Pathological presentations of CMV colitis in patients 02 (left) and 03 (right). The CMV-infected cells were emphasized by the rectangle. CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; SCT, stem cell transplantation; TCR-T cell, T cell receptor-engineered T cell.
Among the four patients, two (patients 02 and 03) were from the 1×105 CMV-TCR-T cells/kg group and the other two (patients 05 and 06) were from the 5×105 CMV-TCR-T cells/kg group, indicating both doses were effective. Patient 02 started to experience CMV reactivation 118 days after SCT (figure 3A) and suffered from CMV colitis. This patient had watery diarrhea (8–11 times, 800–1500 mL/day) with umbilical pain and pathological presentations of CMV colitis were observed in patient 02 (figure 3B). Nine days after CMV-TCR-T cell infusion, the blood CMV viral load turned negative and remained undetectable (figure 3A). In addition, the defecation frequency was reduced with paste stool 300–400 mL/day and abdominal pain was significantly relieved in patient 02 after TCR-T cell treatment. Patient 03 also developed CMV colitis on day 30 post-SCT with diarrhea (9–10 times, 1000–1200 mL/day) and pathological presentations of CMV colitis (figure 3B). Blood CMV-DNA level of this patient started to be higher than 1×103 copies/mL on day 37 post-SCT. Nineteen days after first dose of TCR-T, blood viral load was cleared and remained negative (figure 3A). The defecation frequency was reduced to four times/day with paste stool 200–300 mL/day in patient 03.
Of the four responders, patients 05 and 06 had CMV viremia, without any CMV end-organ diseases. Blood viral load was cleared on day 28 after first dose of TCR-T and remained negative in patient 05. In patient 06, CMV viremia was cleared within only 4 days after TCR-T cell infusion (figure 3A).
Two patients (patients 01 and 04) who initially did not respond to CMV-TCR-T infusion received antiviral agents (ganciclovir and/or foscarnet) as a salvage treatment (table 2 and figure 3A). In patient 01, CMV viremia occurred on day 37 post-SCT and two doses of 1×103 CMV-TCR-T cells/kg were infused on days 41 and 47, respectively. On the third day after the first dose of TCR-T cells was infused, the blood viral load had a mild reduction but soon started to increase rapidly after that. On day 55 post-SCT, the blood CMV load was as high as 8.118×103 copies/mL, indicating that 1×103 CMV-TCR-T cells/kg body weight per dose for two doses alone might not be effective enough to control CMV viremia. As a salvage measure, ganciclovir was administered during days 55–69 post-SCT. Blood viral load was cleared on day 69 post-SCT (28 days after first dose of TCR-T) and remained negative since then in patient 01 (table 2 and figure 3A). Patient 04 started to have CMV viremia on day 47 post-SCT and on day 50, the first dose of 1×105 CMV-TCR-T cells/kg was infused. However, the CMV load in blood kept increasing after the first infusion. On day 53, 3 days after the first dose of TCR-T, the CMV load exceeded 5×105 copies/mL. In addition to viremia, patient 04 developed CMV pneumonia, experiencing cough, expectoration, and rapidly progressed to type I respiratory failure. Pulmonary CT showed interstitial pneumonia with obvious diffuse ground-glass opacity in both lungs. After noticing that the first dose of CMV-TCR-T cells failed to control CMV infection in this patient, ganciclovir and foscarnet were administrated during days 54–76 post-SCT as a salvage measure, in combination with the next two doses of CMV-TCR-T cells (on day 57 and day 68 post-SCT), in order to ensure the clinical benefit of this patient as the mortality rate of CMV pneumonia with delayed treatment or ineffective prophylaxis was over 85%.9 After 5 days of salvage treatment, cough and expectoration were significantly relieved and the respiratory failure was corrected. The CMV viremia was also cleared on day 72 and remained negative since then in this patient (figure 3A).
TCR-T cell persistence without discontinuation of immunosuppressive drugs for aGvHD treatment
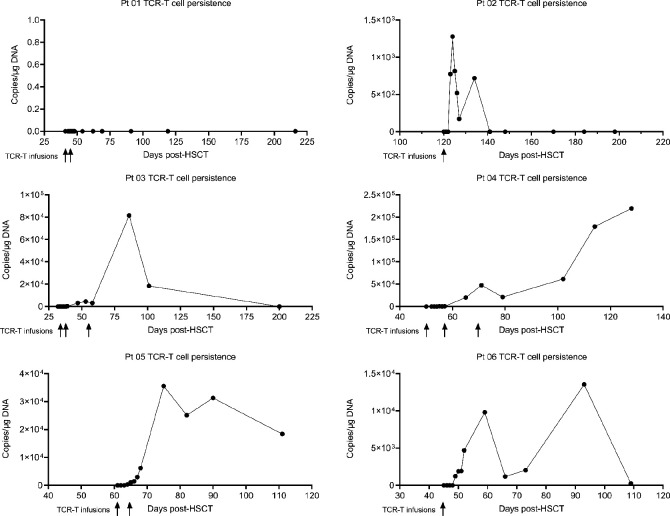
The CMV-TCR-T cells displayed overall robust expansion and persistence in the peripheral blood after infusion. Except for patient 01 who received the lowest dose of TCR-T cells, circulating CMV-TCR-T cells were detected by qPCR method in all other patients. The CMV-TCR-T cells were first detected in blood of these patients 3–7 days after the first dose of CMV-TCR-T infusion and can persist for more than 4 months (table 2, figure 4 and online supplemental figure 2). In patient 02, the expansion of TCR-T cells was very rapid. It took only 1 day for the TCR-T cells to reach the peak but the peak value was relatively lower (1.2×103 copies/μg DNA) and their persistence lasted for 12 days (days 123–134 post-SCT). However, in patients 03–06, CMV-TCR-T cells expanded to a very high level and persisted for more than 1.5 months with detectable copy number. During the time of TCR-T persistence, CMV reactivation did not reoccur, implying that CMV-TCR-T cells can provide a relatively long-term protection against CMV infection before the reconstitution of cellular immunity mediated by the endogenous CTLs in these patients.
Figure 4.
TCR-T cell expansion and persistence. Expansion and persistence of CMV-TCR-T cells in the peripheral blood of each individual patient were measured by real-time qPCR and represented by the copy number of CMV-TCR transgenes. The time of TCR-T cell infusion was also indicated. CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; qPCR, quantitative PCR; TCR-T cell, T cell receptor-engineered T cell.
It is worth noticing that the CMV-TCR-T cells still can expand in patients even when immunosuppressive drugs for treating GvHD (steroids, anti-CD25 antibody and ruxolitinib) were administered. For example, patient 02 experienced aGvHD on day 111 after SCT and was treated by steroids (1 mg/kg, days 111–119, then 0.5 mg/kg, days 120–125), anti-CD25 monoclonal antibody (days 111, 113, 118, 125 and 133) and ruxolitinib (5 mg, days 112–127). When patient 02 started to receive CMV-TCR-T cell infusion on day 120 and the CMV-TCR-T cell expansion was first detected on day 123 post-SCT, the anti-GvHD reagents were still being used (tables 1 and 2). The CMV-TCR-T cells expanded and persisted in this patient despite the use of these immunosuppressive medications, indicating that CMV-TCR-T cells have high proliferation potential. The administration of anti-GvHD reagents has been completed before CMV-TCR-T cell infusion in the other three patients who experienced aGvHD after SCT.
CMV-specific TCRs can be identified from patients by VDJ sequencing
To monitor the clone frequencies of infused CMV-TCR-T cells at different time points, TCRβ chain sequencing was performed for patients 01, 03 and 05. In patient 01 who received the lowest dose of TCR-T cells, the transgenic CMV-specific TCR sequence was not detected in the peripheral blood on days 216, 310 and 387 post-SCT (online supplemental figure 2 and online supplemental table 1), which was consistent with the qPCR data. In patient 03, the clone frequency of the CMV-specific TCR remained at 6–8% during days 103–139 post-SCT and became undetectable on day 313 (online supplemental figure 2), indicating that detectable CMV-TCR-T cells persisted for at least 101 days (days 39–139 post-SCT) according to both the qPCR and TCRβ sequencing data (figure 4 and online supplemental figure 2). In patient 05, the clone frequency of the CMV-specific TCR remained at 11–13% during days 110–188 post-SCT (online supplemental figure 2), indicating the persistence of CMV-TCR-T cells lasted for at least 125 days (days 64–188 post-SCT), by combining the data of qPCR and TCRβ sequencing.
jitc-2023-007735supp003.xlsx (25.5KB, xlsx)
Toxicities
No severe adverse effects (SAE) were observed. No immune effector cell-associated neurotoxicity syndrome or TCR-T cell-related GvHD occurred in any patient. Only patient 04 who received 1×105 CMV-TCR-T cells/kg per dose for three doses experienced grade 1 cytokine release syndrome (CRS) with a fever of 39.3℃ which lasted for 1 day. Patient 03 had fevers of 38.0 and 38.4℃ which lasted for less than 2 days after receiving the second and third doses of TCR-T cell infusion, respectively. A fever of 38.4℃ lasting for only 4 hours was recorded for patient 05 after the second infusion. No medication was used to treat fever in these patients. No other adverse effects were observed (table 2).
Discussions
To date, there has been no report of clinical trials of CMV-TCR-T cell as a first-line therapy for patients with CMV reactivation after haploidentical SCT. We conducted the single-arm, open-label, phase I clinical trial evaluating the safety and efficacy of CMV-TCR-T cell therapy as the first-line pre-emptive therapy for patients with CMV reactivation post-haploidentical PBSCT. The donors-derived TCR-T cells were adoptively transferred by one to three doses of CMV-TCR-T cells in six patients. The therapy was very safe. Except for the grade 1 CRS observed in one patient and mild fever in two patients, no other adverse events were observed. Four patients (66.7%) with CMV viremia and/or colitis showed response without the administration of any antiviral agents. Our study first showed CMV-TCR-T cell as a highly feasible, safe and effective first-line pre-emptive treatment for CMV reactivation after haploidentical SCT.
This trial was a dose-escalation study with 1×103, 1×105 and 5×105 CMV-TCR-T cells/kg body weight per dose for one to three doses. Non-DLT or SAEs were observed at all dose levels, indicating all three doses are safe and tolerable and the maximum tolerated dose was at least 5×105 CMV-TCR-T cells/kg. Because the infusion of 1×103 CMV-TCR-T cells/kg per dose for two doses alone did not successfully clear the CMV viremia and the expansion of TCR-T was not detected in the peripheral blood of patient 01, it is possible that this low dose of TCR-T cells was not effective in treating CMV reactivation. In comparison, both 1×105 and 5×105 CMV-TCR-T cells/kg showed sufficient potency to rapidly clear CMV in patients with viremia and colitis and displayed robust expansion and persistence, which will provide long-term protection against CMV reactivation in patients. Therefore, the minimal effective dose level can be as low as 1×105 CMV-TCR-T cells/kg body weight per dose for one to three doses. This result reflected the extraordinary advantage of this new type of therapy that it only needs a very low dose to work, expand and persist. In comparison, the effective dose of HPV-specific TCR-T cells was 1–170×109, which was in the range of 1×107–1×109 human papillomavirus (HPV)-TCR-T cells/kg body for treating HPV-associated cancers.23 24 The low effective dose of CMV-TCR-T cells also reduces the difficulty in timely manufacture. In addition, because all three dose levels of CMV-TCR-T cells in our study were very safe and tolerable, it is worth exploring for higher doses in the future clinical trials for treating more refractory CMV diseases.
Of the four responders, patients 05 and 06 had CMV viremia. In patient 05, two doses of 5×105 CMV-TCR-T cells/kg were infused 14 and 18 days after CMV reactivation was detected, respectively. It took 28 days to clear the blood viral load after first dose of TCR-T in this patient. However, in patient 06, one dose of 5×105 CMV-TCR-T cells/kg was infused on the same day when CMV reactivation was detected and CMV viremia was cleared within only 4 days after TCR-T cell infusion. By comparing the information of these two patients, it is likely that earlier infusion of CMV-TCR-T cells when CMV viremia is first detected may result in more rapid CMV clearance and better clinical outcome.
CMV-TCR-T cells showed a robust expansion and persistence after infusion, which can provide a relatively long-term protection to prevent CMV infection recurrence. It seems that the persistence time and highest value of DNA copies were not exactly associated with the doses of CMV-TCR-T cells as patient 04 from the middle-dose group displayed the most robust expansion and persistence pattern, including the highest copy number of transgenic TCR and the longest persistence time to date. It is worth noticing that the CMV-TCR-T cells can expand in patients even when immunosuppressive drugs for treating GvHD were administered. For example, in patient 02, the CMV-TCR-T cells started to expand despite the anti-GvHD reagents were still being used and their persistence lasted for 12 days. This result further suggested that CMV-TCR-T cells have high proliferation potential and can overcome the immunosuppressive effects resulted from anti-GvHD reagents. In terms of the methodologies, TCRβ sequencing was used in addition to qPCR to monitor the expansion and persistence of the transferred CMV-TCR-T cells. However, this approach could not discriminate the transgenic TCR from any other endogenous TCR using the same β chain.
There were a few hypotheses why CMV-TCR-T cells alone did not control CMV infection in patient 04, despite their robust expansion and persistence. First, the CMV reactivation progressed very rapidly, with highest peak viral load among all the patients (>5×105 copies/mL). The first dose of 1×105 CMV-TCR-T cells/kg was not sufficient to immediately control this severe and rapidly evolved CMV disease. It was possible that higher doses of CMV-TCR-T cells would be more effective. Second, the salvage administration of ganciclovir and foscarnet 4 days after the first dose might masked the efficiency of the CMV-TCR-T cells. Furthermore, a portion of CMV-infected cells might carry mutations in the epitopes recognized by CMV-TCR-T cells.
In this study, three CMV epitopes and three HLA haplotypes were covered: NLVPMVATV (HLA-A*02:01-restricted), ATVQGQNLK (HLA-A*11:01-restricted), QYDPVAALF (HLA-A*24:02-restricted). These three epitopes are all from pp65 protein. The limited coverage of epitopes and HLA haplotypes was the biggest disadvantage of this study. In the future, more epitopes from pp65 and other CMV proteins need to be tested to ensure the eradication of infected cells presenting various CMV epitopes. Though HLA-A*11:01, HLA-A*24:02 and HLA-A*02:01 are the most frequent HLA-A haplotypes in Chinese populations, other HLA-restricted TCRs should be isolated and functionally validated to benefit more patients.
This study also showed that though CMV-TCR-T cell therapy as a pre-emptive treatment for CMV reactivation after SCT was less toxic and more tolerable than currently available antiviral reagents, it has several limitations. This new treatment modality was more challenging to administer and not universally available, and likely more costly at the current time.
In conclusion, our study showed that adoptive T cell therapy using SCT donors-derived CMV-TCR-T cells is a feasible and safe approach with potential therapeutic efficacy in treating patients with CMV reactivation after haploidentical SCT. However, the small sample size of this study might limit definitive conclusions about safety and efficacy of this new treatment modality. In the future, more clinical trials with larger sample size were still needed to confirm whether this approach can serve as an alternative treatment strategy for CMV reactivation after haploidentical SCT.
jitc-2023-007735supp004.pdf (120.6KB, pdf)
Acknowledgments
The authors would like to acknowledge the patients and donors who participated in this study.
Footnotes
Contributors: Study design—D-HL, LD and CM. Acquisition and analysis of data—CM, PC, JD, LW and NL. CMV-TCR identification and TCR-T cell manufacture—JS, XQ and YW. Drafting of the manuscript—CM, D-HL and LD. Study supervision—D-HL and LD. Guarantor—D-HL. Final approval of the manuscript—all authors.
Funding: This work was partially supported by grants from the National Natural Science Foundation of China (nos. 82070178, 82270162, 82270224, and 82200169), the National Key R&D Program of China (2023YFC2507800 and 2021YFA1100904), the Beijing Natural Science Foundation of China (no. 7222175), military medical support innovation and generate special program (21WQ034), Special Research Found for Health Protection (21BJZ30), and the Logistics Independent Research Program (2023hqzz09).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by the Ethics Committee of the Chinese PLA General Hospital (ethics number: S2021-344-01). Written informed consent was obtained from all patients and donors after SCT.
References
- 1.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 2019;29:e2034. 10.1002/rmv.2034 [DOI] [PubMed] [Google Scholar]
- 2.Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest 2011;121:1673–80. 10.1172/JCI45449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaly RF, El Haddad L, Winston DJ, et al. Cytomegalovirus (CMV) cell-mediated immunity and CMV infection after allogeneic hematopoietic cell transplantation: the REACT study. Clin Infect Dis 2020;71:2365–74. 10.1093/cid/ciz1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho SY, Lee DG, Kim HJ. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci 2019;20:11. 10.3390/ijms20112666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol 2021;19:759–73. 10.1038/s41579-021-00582-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeckh M, Murphy WJ, Peggs KS. Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant 2015;21:24–9. 10.1016/j.bbmt.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Ljungman P. Prevention and treatment of viral infections in stem cell transplant recipients. Br J Haematol 2002;118:44–57. 10.1046/j.1365-2141.2002.03515.x [DOI] [PubMed] [Google Scholar]
- 8.Sutrave G, Blyth E, Gottlieb DJ. Cellular therapy for multiple pathogen infections after hematopoietic stem cell transplant. Cytotherapy 2017;19:1284–301. 10.1016/j.jcyt.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen Q, Champlin R, Giralt S, et al. Late cytomegalovirus pneumonia in adult allogeneic blood and marrow transplant recipients. Clin Infect Dis 1999;28:618–23. 10.1086/515146 [DOI] [PubMed] [Google Scholar]
- 10.Ko J-H, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis 2015;60:e20–6. 10.1093/cid/ciu969 [DOI] [PubMed] [Google Scholar]
- 11.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016;3:e119–27. 10.1016/S2352-3026(15)00289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanada M, Yamamoto K, Emi N, et al. Cytomegalovirus antigenemia and outcome of patients treated with pre-emptive Ganciclovir: retrospective analysis of 241 consecutive patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2003;32:801–7. 10.1038/sj.bmt.1704232 [DOI] [PubMed] [Google Scholar]
- 13.Montesinos P, Sanz J, Cantero S, et al. Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2009;15:730–40. 10.1016/j.bbmt.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Salzberger B, Bowden RA, Hackman RC, et al. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood 1997;90:2502–8. [PubMed] [Google Scholar]
- 15.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 2003;362:1375–7. 10.1016/S0140-6736(03)14634-X [DOI] [PubMed] [Google Scholar]
- 16.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013;121:3745–58. 10.1182/blood-2012-08-448977 [DOI] [PubMed] [Google Scholar]
- 17.Zhao X-Y, Pei X-Y, Chang Y-J, et al. First-line therapy with donor-derived human cytomegalovirus (HCMV)-Specific T cells reduces persistent HCMV infection by promoting antiviral immunity after allogenic stem cell transplantation. Clin Infect Dis 2020;70:1429–37. 10.1093/cid/ciz368 [DOI] [PubMed] [Google Scholar]
- 18.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of Pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010;116:4360–7. 10.1182/blood-2010-01-262089 [DOI] [PubMed] [Google Scholar]
- 19.van der Heiden P, Marijt E, Falkenburg F, et al. Control of cytomegalovirus viremia after allogeneic stem cell transplantation: a review on CMV-specific T cell reconstitution. Biol Blood Marrow Transplant 2018;24:1776–82. 10.1016/j.bbmt.2018.03.028 [DOI] [PubMed] [Google Scholar]
- 20.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-Specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002;99:3916–22. 10.1182/blood.v99.11.3916 [DOI] [PubMed] [Google Scholar]
- 21.Schub A, Schuster IG, Hammerschmidt W, et al. CMV-specific TCR-transgenic T cells for immunotherapy. J Immunol 2009;183:6819–30. 10.4049/jimmunol.0902233 [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Chen H, Cao X, et al. Efficacy of pp65-specific TCR-T cell therapy in treating cytomegalovirus infection after hematopoietic stem cell transplantation. Am J Hematol 2022;97:1453–63. 10.1002/ajh.26708 [DOI] [PubMed] [Google Scholar]
- 23.Doran SL, Stevanović S, Adhikary S, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol 2019;37:2759–68. 10.1200/JCO.18.02424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagarsheth NB, Norberg SM, Sinkoe AL, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med 2021;27:419–25. 10.1038/s41591-020-01225-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007735supp001.pdf (111.3KB, pdf)
jitc-2023-007735supp002.pdf (1.9MB, pdf)
jitc-2023-007735supp003.xlsx (25.5KB, xlsx)
jitc-2023-007735supp004.pdf (120.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information.