Abstract
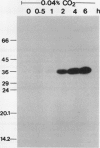
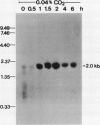
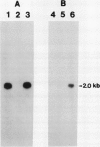
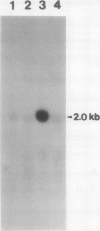
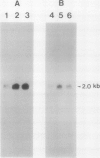
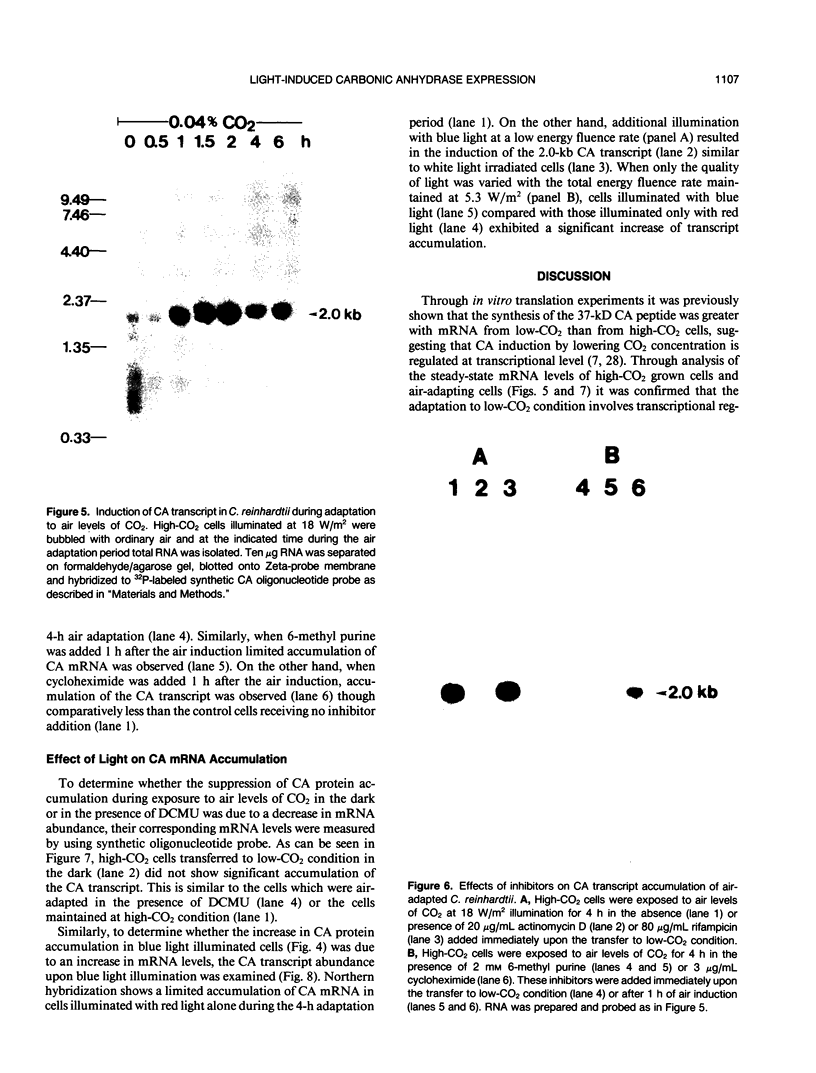
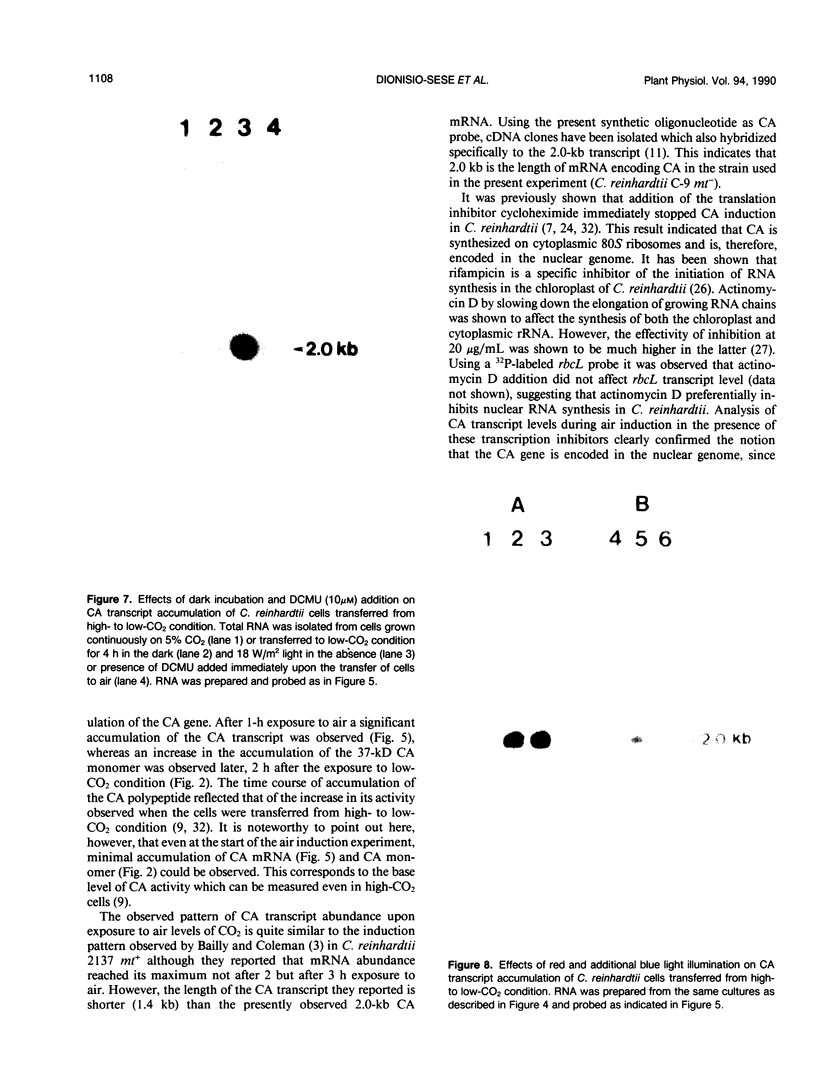
The effect of external inorganic carbon concentration and light on carbonic anhydrase (CA) protein accumulation and steady-state mRNA levels were examined in Chlamydomonas reinhardtii. When photoautotrophically grown high-CO2 cells were transferred to low-CO2 conditions, they exhibited a significant accumulation of the 2.0-kilobase CA transcript after 1 hour with the maximum level reached after 2 hours. An increase in the accumulation of the 37-kilodalton CA monomer was observed after 2-hour exposure to air. Cells allowed to adapt to air levels of CO2 in the dark showed neither an increase in CA mRNA abundance nor in the accumulation of the enzyme. Similarly, addition of 10 micromole 3-(3,4-dichlorophenyl)-1, 1-dimethylurea immediately after transferring high-CO2 cells to low-CO2 condition did not cause an increase in CA transcript abundance and enzyme accumulation, suggesting that photosynthesis is absolutely required in the regulation of CA transcript abundance. In addition to the photosynthesis-dependent process, a blue light stimulated mechanism is also involved in CA transcript regulation. Experiments with transcription inhibitors confirmed the notion that the gene for carbonic anhydrase enzyme is encoded by the nuclear genome and that the induction of the enzyme depends heavily on the transcription of the CA gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S., Mishima H., Miyachi Y. Levels of cyclic-AMP, cyclic-GMP and betamethasone in the aqueous humor following topical administration of betamethasone in rabbit eyes. Hiroshima J Med Sci. 1983 Sep;32(3):301–304. [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly J., Coleman J. R. Effect of CO(2) Concentration on Protein Biosynthesis and Carbonic Anhydrase Expression in Chlamydomonas reinhardtii. Plant Physiol. 1988 Aug;87(4):833–840. doi: 10.1104/pp.87.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Fukuzawa H., Fujiwara S., Yamamoto Y., Dionisio-Sese M. L., Miyachi S. cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: regulation by environmental CO2 concentration. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4383–4387. doi: 10.1073/pnas.87.11.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J. 125I-Labeled protein A: reactivity with IgG and use as a tracer in radioimmunoassay. Methods Enzymol. 1980;70(A):356–375. doi: 10.1016/s0076-6879(80)70064-2. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Kitayama M., Togasaki R. K., Tolbert N. E. Evidence for Inorganic Carbon Transport by Intact Chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzycki S. J. Genetic functions of the chloroplast of Chlamydomonas reinhardi: effect of rifampin on chloroplast DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1327–1334. doi: 10.1073/pnas.63.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzycki S. J., Rochaix J. D. Transcriptional mapping of ribosomal RNA genes of the chloroplast and nucleus of Chlamydomonas reinhardi. J Mol Biol. 1971 Nov 28;62(1):89–109. doi: 10.1016/0022-2836(71)90133-1. [DOI] [PubMed] [Google Scholar]
- Sültemeyer D. F., Miller A. G., Espie G. S., Fock H. P., Canvin D. T. Active CO(2) Transport by the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 1989 Apr;89(4):1213–1219. doi: 10.1104/pp.89.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toguri T., Muto S., Miyachi S. Biosynthesis and intracellular processing of carbonic anhydrase in Chlamydomonas reinhardtii. Eur J Biochem. 1986 Aug 1;158(3):443–450. doi: 10.1111/j.1432-1033.1986.tb09773.x. [DOI] [PubMed] [Google Scholar]