Abstract
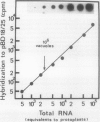
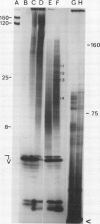
We have shown that highly purified vacuoles of suspension-cultured tomato (Lycopersicon esculentum) cells contain RNA-oligonucleotides, using two different approaches to label and detect RNA: (a) in vivo labeling of cellular RNA with [5-3H]uridine, followed by preparation of vacuoles from protoplasts and by quantification of radioactively labeled material; and (b) in vitro labeling and analysis on sequencing gels of nucleic acids prepared from tomato vacuoles and their identification as RNA. The intravacuolar location of the RNA found in vacuolar preparations was concluded from analyzing for RNA intact organelles after repeated flotation steps as well as ribonuclease A treatment. About 3% of the RNA in protoplasts was localized within vacuoles, exceeding by severalfold the contribution made by contamination with unlysed protoplasts and subcellular organelles. Investigation of the size distribution of vacuolar RNA revealed an oligonucleotide pattern strikingly different from that which would arise from contaminating protoplasts; vacuolar RNA fragments are considerably shorter than 80 nucleotides. Characterization of these oligoribonucleotides (3′-phosphorylated termini; relatively rich in pyrimidines) as possible products of tomato vacuolar ribonuclease I action, and, in addition, enzymatic hydrolysis of vacuolar RNA by inherent enzyme activities in lysed vacuole preparations support the hypothesis that plant vacuoles are involved in cellular nucleolytic processes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H., Alibert G., Boudet A. M. Hydrolysis of Intracellular Proteins in Vacuoles Isolated from Acer pseudoplatanus L. Cells. Plant Physiol. 1985 Dec;79(4):1090–1093. doi: 10.1104/pp.79.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H., Alibert G., Carrasco A., Boudet A. M. Rapid Degradation of Abnormal Proteins in Vacuoles from Acer pseudoplatanus L. Cells. Plant Physiol. 1986 Jun;81(2):460–463. doi: 10.1104/pp.81.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobianchi F., Wilson S. H. Enzymes for modifying and labeling DNA and RNA. Methods Enzymol. 1987;152:94–110. doi: 10.1016/0076-6879(87)52013-4. [DOI] [PubMed] [Google Scholar]
- Kiss T., Tóth M., Solymosy F. Plant small nuclear RNAs. Nucleolar U3 snRNA is present in plants: partial characterization. Eur J Biochem. 1985 Oct 15;152(2):259–266. doi: 10.1111/j.1432-1033.1985.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Walker-Simmons M. Plant vacuoles. Methods Enzymol. 1983;96:580–589. doi: 10.1016/s0076-6879(83)96050-0. [DOI] [PubMed] [Google Scholar]
- Schoelz J. E., Zaitlin M. Tobacco mosaic virus RNA enters chloroplasts in vivo. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4496–4500. doi: 10.1073/pnas.86.12.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Schatz G. DNA-protein conjugates can enter mitochondria via the protein import pathway. Nature. 1989 Mar 9;338(6211):170–172. doi: 10.1038/338170a0. [DOI] [PubMed] [Google Scholar]