Abstract
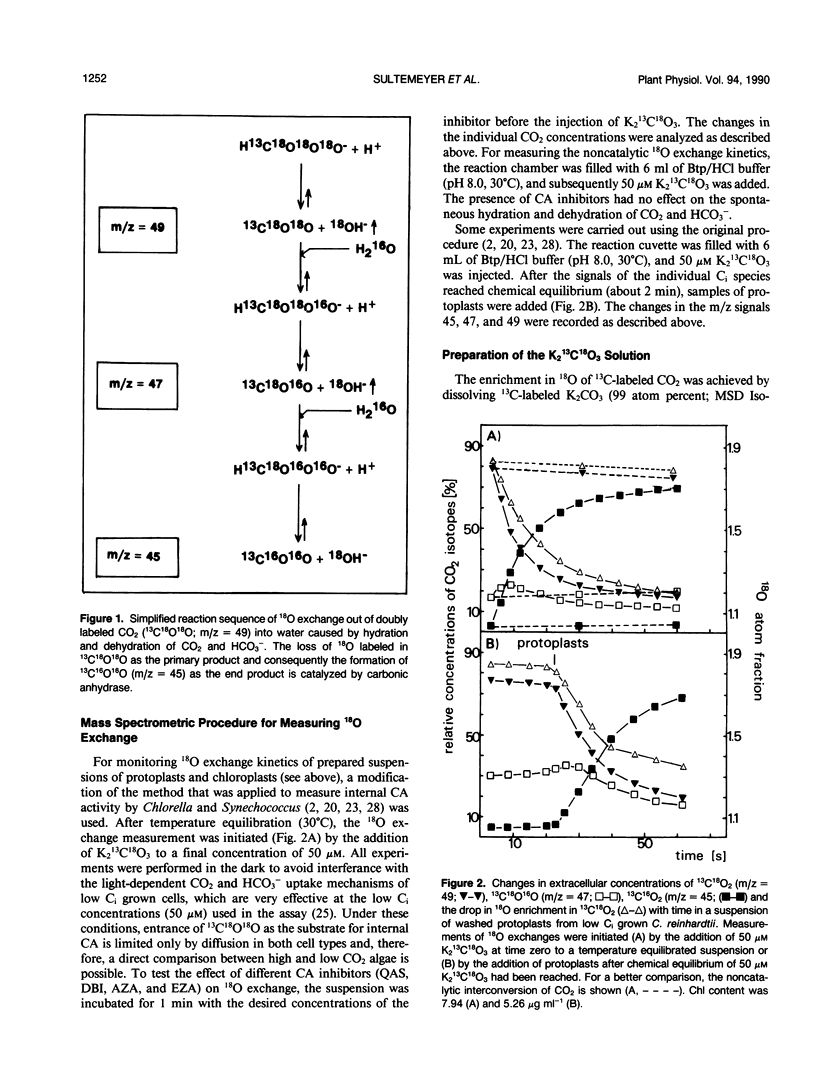
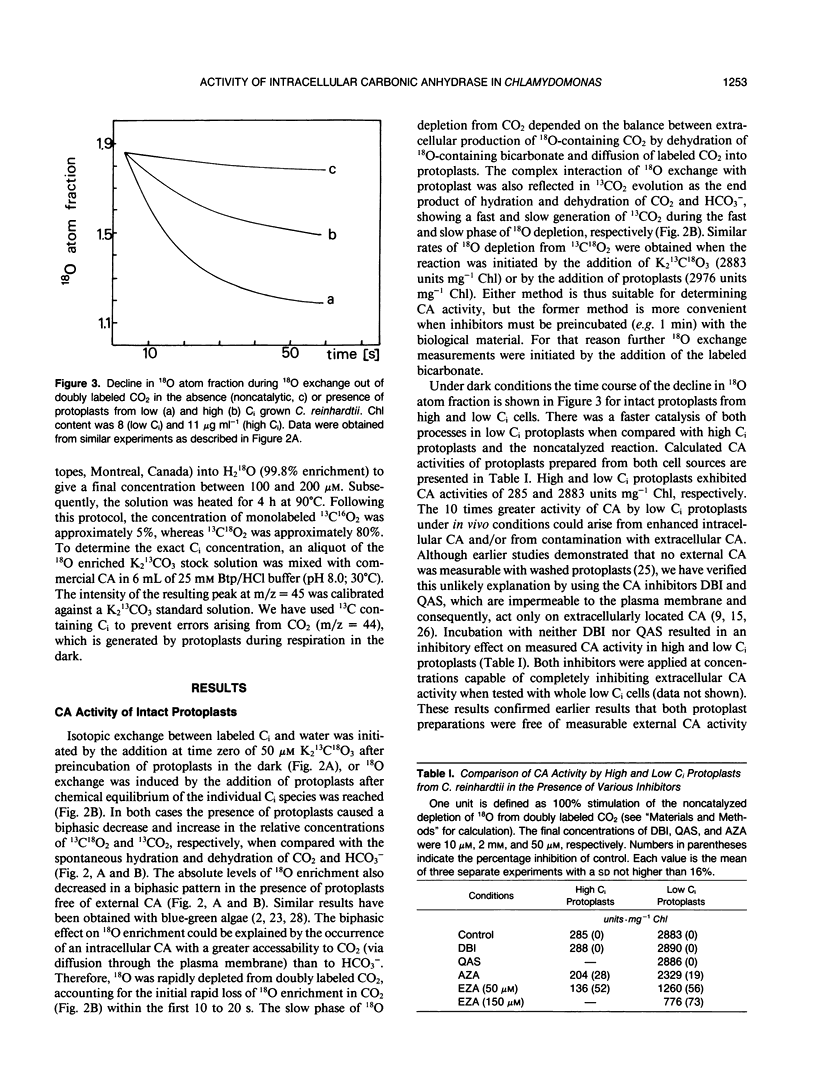
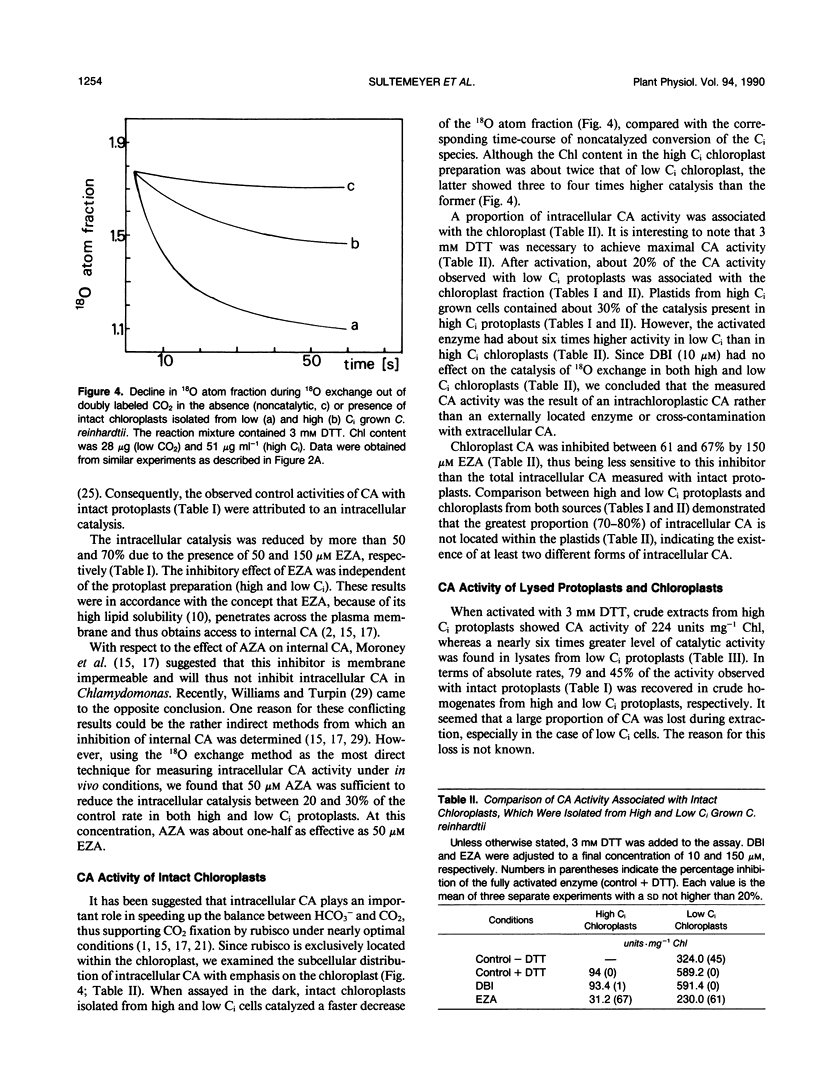
By measuring 18O exchange from doubly labeled CO2 (13C18O18O), intracellular carbonic anhydrase activity was studied with protoplasts and chloroplasts isolated from Chlamydomonas reinhardtii grown either on air (low inorganic carbon [Ci]) or air enriched with 5% CO2 (high Ci). Intact low Ci protoplasts had a 10-fold higher carbonic anhydrase activity than did high Ci protoplasts. Application of dextran-bound inhibitor and quaternary ammonium sulfanilamide, both known as membrane impermeable inhibitors of carbonic anhydrase, had no influence on the catalysis of 18O exchange, indicating that cross-contamination with extracellular carbonic anhydrase was not responsible for the observed activity. This intracellular in vivo activity from protoplasts was inhibited by acetazolamide and ethoxyzolamide. Intracellular carbonic anhydrase activity was partly associated with intact chloroplasts isolated from high and low Ci cells, and the latter had a sixfold greater rate of catalysis. The presence of dextran-bound inhibitor had no effect on chloroplast-associated carbonic anhydrase, whereas 150 micromolar ethoxyzolamide caused a 61 to 67% inhibition of activity. These results indicate that chloroplastic carbonic anhydrase was located within the plastid and that it was relatively insensitive to ethoxyzolamide. Carbonic anhydrase activity in crude homogenates of protoplasts and chloroplasts was about six times higher in the low Ci than in high Ci preparations. Further separation into soluble and insoluble fractions together with inhibitor studies revealed that there are at least two different forms of intracellular carbonic anhydrase. One enzyme, which was rather insoluble and relatively insensitive to ethoxyzolamide, is likely an intrachloroplastic carbonic anhydrase. The second carbonic anhydrase, which was soluble and sensitive to ethoxyzolamide, is most probably located in an extrachloroplastic compartment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Price G. D. Carbonic Anhydrase Activity Associated with the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989 Jan;89(1):51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Grossman A. R. Biosynthesis of carbonic anhydrase in Chlamydomonas reinhardtii during adaptation to low CO(2). Proc Natl Acad Sci U S A. 1984 Oct;81(19):6049–6053. doi: 10.1073/pnas.81.19.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder L. B., Hayes S. L. Diffusion of sulfonamides in aqueous buffers and into red cells. Mol Pharmacol. 1965 Nov;1(3):266–279. [PubMed] [Google Scholar]
- Husic H. D., Kitayama M., Togasaki R. K., Moroney J. V., Morris K. L., Tolbert N. E. Identification of Intracellular Carbonic Anhydrase in Chlamydomonas reinhardtii which Is Distinct from the Periplasmic Form of the Enzyme. Plant Physiol. 1989 Mar;89(3):904–909. doi: 10.1104/pp.89.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Active Transport of CO(2) by the Cyanobacterium Synechococcus UTEX 625 : Measurement by Mass Spectrometry. Plant Physiol. 1988 Mar;86(3):677–683. doi: 10.1104/pp.86.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E. Effect of Carbonic Anhydrase Inhibitors on Inorganic Carbon Accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985 Sep;79(1):177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Kitayama M., Togasaki R. K., Tolbert N. E. Evidence for Inorganic Carbon Transport by Intact Chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987 Mar;83(3):460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Togasaki R. K., Husic H. D., Tolbert N. E. Evidence That an Internal Carbonic Anhydrase Is Present in 5% CO(2)-Grown and Air-Grown Chlamydomonas. Plant Physiol. 1987 Jul;84(3):757–761. doi: 10.1104/pp.84.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N. Carbonic anhydrase: oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller H., Wynns G. C., Tu C. Role of Photosynthetic Reactions in the Activity of Carbonic Anhydrase in Synechococcus sp. (UTEX 2380) in the Light : Inhibitor Studies Using the O-Exchange in C/O-Labeled Bicarbonate. Plant Physiol. 1988 Apr;86(4):1185–1192. doi: 10.1104/pp.86.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sültemeyer D. F., Miller A. G., Espie G. S., Fock H. P., Canvin D. T. Active CO(2) Transport by the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 1989 Apr;89(4):1213–1219. doi: 10.1104/pp.89.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker J. P., Coulson R., Weiner I. M. Dextran-bound inhibitors of carbonic anhydrase. J Pharmacol Exp Ther. 1981 Sep;218(3):600–607. [PubMed] [Google Scholar]
- Toguri T., Muto S., Miyachi S. Biosynthesis and intracellular processing of carbonic anhydrase in Chlamydomonas reinhardtii. Eur J Biochem. 1986 Aug 1;158(3):443–450. doi: 10.1111/j.1432-1033.1986.tb09773.x. [DOI] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. G., Turpin D. H. The Role of External Carbonic Anhydrase in Inorganic Carbon Acquisition by Chlamydomonas reinhardii at Alkaline pH. Plant Physiol. 1987 Jan;83(1):92–96. doi: 10.1104/pp.83.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]


