Abstract
Background
Previous research has outlined the health benefits of exercise including its therapeutic potential for substance use disorders (SUD). These data have already been utilized and it is now common to find exercise as part of SUD treatment and relapse prevention programs. However, we need to better understand different exercise regimens and determine which would be the most beneficial for SUDs. Recently, high intensity interval training (HIIT) has gained attention in comparison with aerobic and resistance exercise. Little is known regarding the neurobiological mechanisms of HIIT, including its effects on dopamine signaling and receptor levels in the brain. The present study examined the effects of chronic HIIT exercise on dopamine signaling as measured by dopamine type 1-like receptor (D1R)-like, dopamine type 2-like receptor (D2R)-like, and tyrosine hydroxylase (TH) quantification in the brains of male and female rats as measured by [3H] SCH 23390 and [3H] spiperone autoradiography, and TH-immunoreactive optical density values.
Methods
Rats were separated in two groups: sedentary and HIIT exercise. Exercise was on a treadmill for 30 min daily (10 3 min cycles) for six weeks with progressive speed increased up to 0.8 mph (21.5 m/min).
Results
Results showed for D2R-like binding, a significant effect across the ventral caudate putamen (V CPU) between sexes, such that mean D2R-like binding was 14% greater for males than females. In the nucleus accumbens shell (Nac Shell), the HIIT Exercise rats showed 16% greater D2R-like binding as compared to the sedentary rats. No significant effects of HIIT exercise were found across groups for brain D1R-like binding levels or TH expression.
Conclusion
These results suggest that HIIT exercise can modulate dopamine signaling by way of increased D2R. These findings support the premise that HIIT exercise plays an important role in dopamine signaling and, may provide a potential mechanism for how HIIT exercise can impact the brain and behavior.
Keywords: autoradiography, addiction, dopamine, tyrosine, exercise, running, reward deficiency
Introduction
The therapeutic potential of exercise in medicine continues to grow as the application of its benefits is realized (1). Exercise has been shown to reduce the risk of several diseases, and improve overall health (2–4). Exercise intervention has also shown particular efficacy in risk reduction and treatment of substance use disorders (SUDs) (5–11).
In the US there has been a significant increase in substance abuse in the past 5 years, with 40.3 million individuals currently affected, coupled with a $6.8 billion increase in federal spending for drug control from 2015 to 2020 (12). Considering current trends, exercise is a valuable intervention given its cost-effectiveness, accessibility, and ease of use. Clinical trials have proven the efficacy of exercise in treating SUDs while animal models identified its effectiveness in preventing initiation, escalation, and relapse. Exercise is a valuable treatment modality for SUDs as it positively modulates dopamine pathways, specifically the mesolimbic reward pathway, back to a healthy baseline. Given the efficacy of exercise as a therapeutic adjunct for SUDs, research continues with the goal of better understanding the exercise regimen and architecture for maximizing effects and benefits.
As exercise research evolves, more evidence continues to support the potential of high intensity interval training (HIIT) in both preclinical and clinical studies. HIIT is defined by the American College of Sports Medicine as short bouts of high intensity, greater than 65% of maximal capacity, anaerobic exercise “alternating with very short bouts of less intense recovery” (13). In stark contrast, moderate intensity aerobic exercise (MIAE) consists of intensities ranging from 40% to 60% with little fluctuation during exercise (14). HIIT has proven an effective exercise regimen in general given its greater improvements in VO2 max compared to MIAE (15), significant enhancement of working memory capacity and cognitive performance (16), and marked reduction of fasting glucose levels and insulin resistance (17). More important to its application in the treatment of addiction is its outstanding efficacy in the field. HIIT is preferred over MIAE by inactive individuals and is shown to have higher rates of adherence to the regime over time. Moreover, HIIT may possess greater reinforcing properties allowing for better reception and retention among sedentary individuals (18). This increased adherence is especially important given the major difficulties experienced by drug addicts in adhering to certain exercise types (19). HIIT, compared to vigorous-intensity continuous training, was shown to produce higher serum neurotrophin-3 concentrations in humans which plays an important role in the attenuation of craving and reward dependence through a preponderance of dopaminergic regulation (20). When comparing cortisol levels among different endurance exercise protocols, levels were significantly higher with the HIIT protocol (21). Increased cortisol levels following exercise were associated with decreased substance craving. Significantly higher insulin-like growth factor1 (IGF-1) levels were also identified for participants in a HIIT group versus the control resulting in increased hippocampal BDNF expression and neuronal differentiation (22). HIIT provides greater benefits for physical and mental health compared to MIAE (23, 24). Furthermore, patients with SUDs have displayed significant improvements on depression, anxiety, and substance craving scales following a HIIT regimen compared to the control group (22). Other research has confirmed these findings and highlighted additional health benefits conferred by HIIT in those with SUDs such as decreased cardiovascular disease and premature death risk (25).
Exercise’s efficacy in treating addiction is largely linked to its ability to positively modulate the mesolimbic reward pathway. The mesolimbic pathway transports dopamine from the ventral tegmental area (VTA) to the nucleus accumbens and amygdala. The nucleus accumbens is located within the striatum and is of particular importance to addiction as it is believed to modulate reward, desire, and the placebo effect (26). Previous research reported reductions in dopamine D1R-like binding as a neurobiological factor contributing to exercise-induced attenuation of drug-seeking behavior (6–8); however, dopaminergic signaling in response to HIIT exercise has yet to be elucidated. Past studies have shown that chronic treadmill exercise resulted in lower levels of D1R-like binding in the ventral striatum and higher D2R-like binding in several subregions of the dorsal striatum (6–8). Blocking dopamine D1 receptors in the nucleus accumbens core and shell attenuated ethanol-seeking behavior in rats (27). Dopamine D1 receptor knock-out mice were also shown to self-administer significantly less cocaine than wild type (28). This pathway is further supported by previous findings that antagonists of D1-like receptors facilitated drug extinction and attenuated drug-seeking behavior (29). These findings suggest that reductions in D1R-like binding contribute to the attenuation of drug-seeking behavior, thus supporting the efficacy of exercise in treating addiction due to its ability to reduce D1R-like binding.
Conversly, increasing D1R-like binding is linked to exacerbation of addiction symptomology. Previous research has shown that increases in D1R and a decreases in D2R are linked to enhanced compulsive and drug-seeking behavior (30). D1R stimulation has proven important for drug reward and conditioned association (30). This was further supported by postmortem studies of methamphetamine users which revealed significant increases in D1R in the NAc (31). Considering current research, it can be reasonably suggested that increases in D1R exacerbate addiction while reductions in D1R may help alleviate addiction symptomology, specifically drug-seeking behavior.
Previous research has also highlighted exercise’s ability to increase dopamine type 2-like receptor levels and to attenuate drug and alcohol consumption in rats in a sex-dependent (8). These results suggest that an increase in dopamine type 2-like receptor levels may be causally related to attenuation of drug and alcohol consumption (32–37). Robison et al. (8, 11) highlighted how exercise’s benefits on the dopamine (DA) system may be dependent on the intensity or “dose” of the exercise itself; which would impact the reward pathway differently. In mouse models of Parkinson’s disease, high-intensity exercise was shown to increase striatal dopamine D2R expression (38). Additional studies on treadmill running have confirmed the D2R enhancement conferred by exercise (39–41). The increase of D2R-like binding as a result of exercise has also been suggested to attenuate aversion to pain and act as an analgesia (42). In this study, the D2R antagonist was only able to attenuate the pain-blocking response in the presence of the stressor of exercise. Particularly, HIIT would be a high-intensity, short-duration stressor that is suggested to provide a significant analgesic effect. In contrast, chronic pain decreases D2R which may have implications for drug-seeking behavior, suggesting a possible chemical connection between pain medication reliance and addiction (43). Increased D2R-like binding has also been shown to have implications for extending lifespan through the GOA-1-DGK-1-PKC/PKD signaling pathway (44). The dopamine receptor D2 gene (DRD2) is known to play an important role in determining the effectiveness of exercise through the modulation of lipid and carbohydrate metabolism (45). Exercise has also been shown to increase D2R-like binding promoting defensive behavior and conditioned place avoidance (46). Decreased D2 receptor levels, specifically in the striatum, have been associated with increased addictive behavior and increased impulsivity, while increased D2 receptor signaling is associated with increased motivation for recovery from addiction (47). Polymorphisms that elicit reduced D2R densities and binding affinity are significantly associated with nicotine, alcohol, heroin, and cocaine dependence (48–51). Perhaps one of the most replicated findings, however, is the association of cocaine abuse and alcohol dependence with significantly decreased D2R striatal availability (47). Preclinical research has linked increased D2 receptor signaling with enhanced motivation and decreased D2 receptor signaling with attenuated motivation (52). Additionally, successful treatment of depression is correlated with increased striatal D2R-like binding (53). This is important as depressive symptoms including suicide ideation, are known to play a key role in impeding remission efforts, whereby the top candidate gene was the D2R using large GWAS (54, 55).
Tyrosine hydroxylase (TH) levels are another important neurobiological marker of dopamine signaling. TH is the rate-limiting enzyme in the synthesis of catecholamine neurotransmitters, notably dopamine; therefore, TH levels effectively correlate with dopamine bioavailability (56, 57). Past research has indicated that drugs of abuse attenuate tyrosine hydroxylase activity through methylation of the tyrosine hydroxylase gene effectively decreasing TH levels (58, 59). Multiple differing models have highlighted elevations in tyrosine hydroxylase levels conferred by exercise (60–63). Therefore, these studies suggest that exercise modulates TH in such a way that may have therapeutic potential for SUD and, thus, TH levels are important to being examined.
The present study examined the effect of HIIT treadmill exercise on dopamine signaling as measured by D1R, D2R, and TH levels in the brains of rats as compared to sedentary rats. These data will aid our understanding of how varying intensities of prescribed exercise can impact dopamine signaling and this information would be important in exercise neuroscience and medicine.
Methods
Subjects
Male (n = 15) and female (n = 16) Lewis rats (Charles River) at 3 weeks of age were individually housed under standard laboratory conditions at 22.0-C T 2-C with a 12 h reverse light/dark cycle (lights off: 06:00 AM to 06:00 PM). Food and water were available ad libitum for the duration of the study. All subjects were handled daily. The experiment was conducted in accordance with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (1996) and University at Buffalo Institutional Animal Care and Use Committee.
Treadmill
A custom-made motorized treadmill divided into eight lanes by Plexiglas walls and by a sheet of metal at its end to keep the rats enclosed on the treadmill was used, as previosuly described (8). The dimensions of the running lanes were 56 cm long, 9 cm wide, and 31 cm high.
Exercise regimen
The rats received a 7 days acclimation period upon arrival. Groups were randomly assigned. n = 8 male rats and n = 8 female rats were placed in the HIIT group while n = 7 male rats and n = 8 female rats were selected for the sedentary group. On the first day following the acclimation period, animals began their exercise regimen of 6 weeks. The rats within the HIIT cohort began at 10 m/min for 30 min. This regimen consisted of 3 min intervals in which the rats ran for 2 min followed by a 1 min break and was repeated 10 times for a total of 30 min. After 5 days, the speed was increased by increments of 2.87 m/min each day until a speed of 21.46 m/min was reached to allow for steady acclimation to the exercise regimen. This speed was chosen as it essentially doubles previous MIAE speeds of 10 m/min to significantly increase intensity to greater than 65% maximal capacity (8). The exercise continued at the top speed for the remainder of the exercise regimen. The HIIT regimen ran 7 days a week. The animals were monitored for the entire span of their exercise regimen. All exercise sessions were performed during the subjects’ dark cycle (06:00 AM to 06:00 PM). Sedentary rats were exposed to the same environment and hadling but without treadmill being on.
D1 autoradiography
Dopamine type 1-like receptor (D1R) expression was assessed using [3H] SCH 23390 autoradiography. Binding was performed as previously described (8). Briefly, slides were preincubated for 60 min at room temperature in 50 mM Tris HCl buffer (120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH = 7.4). Next, 2.5 nM [3H] SCH 23390 (specific activity = 85 Ci/mmol) and 40 nM ketanserin were added to the pre-incubation buffer followed by an additional 60 min incubation at room temperature. Non-specific binding was determined in the presence of 1 μM flupenthixol. Brain section slides were then washed 2× 5 min at 4°C in pre-incubation buffer followed by brief immersion at 4°C in dH2O.
D2 autoradiography
Dopamine type 2-like receptor (D2R) expression was assessed using [3H] Spiperone autoradiography. Binding was performed as previously described (8). Briefly, slides were preincubated for 60 min at room temperature in 50 mM Tris 7 HCl buffer (120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH = 7.4). Next, 0.5 nM [3H] Spiperone (specific activity = 16.2 Ci/mmol) and 40 nM ketanserin were added to the pre-incubation buffer followed by an additional 60 min incubation at room temperature. Non-specific binding was determined in the presence of 10 μM sulpride. Brain section slides were then washed 2× 5 min at 4°C in pre-incubation buffer followed by brief immersion at 4°C in dH2O.
TH immunohistochemistry
Brain section slides were initially dehydrated in 90% ethanol for 10 min at room temperature (64). TH IHC was performed as previously described but with a few modifications (65). Next, 3× 5 min washes were completed in 1X PBS. Slides were then blocked with a solution containing 0.4% Trition-X, 10% normal goat serum, and 1% H2O2 for 30 min at room temperature followed by 24 h incubation at 4°C with TH primary antibody: Rabbit anti-TH antibody (1:2000, Thermofischer). After five washes in 1X PBS with 0.4% Triton-X (PBS-T), slides were sequentially incubated in biotinylated goat anti-rabbit (1:800, Vector Laboratories, Burlingame, CA) for 1 h and avidin-biotin complex (ABC Kits; Vector Laboratories, Burlingame, CA) for 45 min separated by five washes in PBS-T. Following five washes in PBS-T, immunostaining was visualized using 3,3-diaminobensidine (Vector, Burlingame, CA), mixed with H2O2. Slides were then washed 4× 5 min in PBS-T, dipped into dH2O, and coverslipped with Permount mounting medium (Fisher Scientific). After TH-staining, sections from the striatum were imaged with a digital camera connected to a compound microscope (National DC3-163 Digital Microscope) and with a computer equipped with MOTIC imaging software. Density in the striatum was measured using ImageJ software.
Regions of interest
Bound slides and tritium standards on glass slides were opposed to Kodak MR Film. The film was scanned at 1,200 dpi, and images were quantified using ImageJ software. The regions of interest include the nucleus accumbens shell (NAc Shell), nucleus accumbens core (NAc Core), ventromedial caudate putamen (VM CPU), ventrolateral caudate putamen (VL CPU), dorsolateral caudate putamen (DL CPU), dorsomedial caudate putamen (DM CPU), olfactory tubercle (OT), and substantia nigra (SNR) were analyzed for [3H]SCH 23390 and [3H] Spiperone binding and TH Immunohistochemistry.
Statistical analysis
For each region of interest, two-way ANOVA was conducted to determine exercise or sex differences with a dependent variable of D1R, D2R, or TH binding. The significance level was set at α = 0.05 and all statistical analyses were performed with Prism9 Graphpad software version 9.4.1. Post-hoc tests were performed on significant main effects within the region of interest using Sidak’s multiple comparison test (see Figure 1).
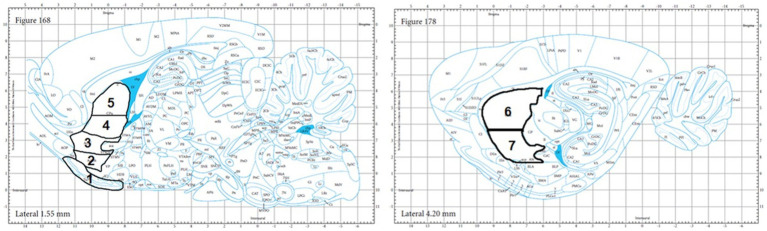
Figure 1.
Brain anatomical atlas images of the regions analyzed: the OT (#1), NAC Shell (#2), NAC Core (#3), VM CPU (#4), DM CPU (#5), DL CPU (#6), and VL CPU (#7) as adopted from the Paxinos & Watson rat brain atlas.
Results
[3H]SCH 23390 autoradiography
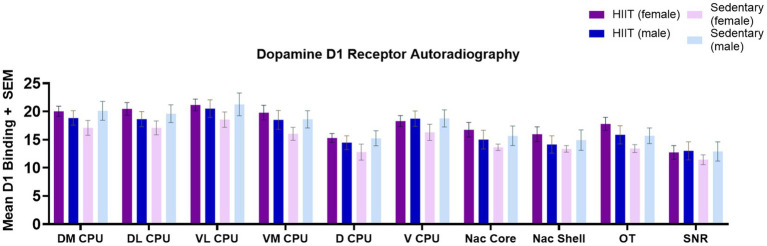
No significant effects of HIIT exercise were found across groups for brain [3H]SCH 23390 binding levels (D1R-like). Specifically, in the DM CPU [F(1,25) = 2.65; p > 0.05], DL CPU [F(1,25) = 2.60; p > 0.05], VL CPU [F(1,25) = 0.48; p > 0.05], VM CPU [F(1,25) = 1.81; p > 0.05], D CPU [F(1,25) = 1.74; p > 0.05], V CPU [F(1,25) = 0.57; p > 0.05], Nac Core [F(1,25) = 1.92; p > 0.05], Nac Shell [F(1,25) = 1.59; p > 0.05], OT [F(1,25) = 2.93; p > 0.05], and SNR [F(1,25) = 0.19; p > 0.05]. Two-way ANOVA found no significant interactions or main effects across groups (p > 0.05; Figure 2). Across D1R-like binding, a general trend towards significance was observed for HIIT females compared to the sedentary female group with the strongest trend towards significance observed in the OT [F(1,25) = 2.93; p < 0.1]. This trend reflected an increase in D1R-like binding in all regions of interest for the HIIT female rats compared with the sedentary female rats.
Figure 2.
Quantitative autoradiography of [3H]SCH 23390 (D1R-like) binding levels within the nucleus accumbens shell (Nac Shell), nucleus accumbens core (Nac Core), ventromedial caudate putamen (VL CPU), ventrolateral caudate putamen (VL CPU), dorsolateral caudate putamen (DL CPU), dorsomedial caudate putamen (DM CPU), dorsal caudate putamen (D CPU), ventral caudate putamen (V CPU), olfactory tubercle (OT), and substantia nigra (SNR) across exercise treatment groups. Measurements of the following regions were carried out at the bregma coordinates taken from the Paxinos & Watson rat brain atlas. Values are expressed as total [3H]SCH 23390 binding means ± S.E.M. for D1R receptors. No significant group differences were observed (p > 0.05). Each bar represents the group mean for dopamine D1 receptor binding.
[3H] Spiperone autoradiography
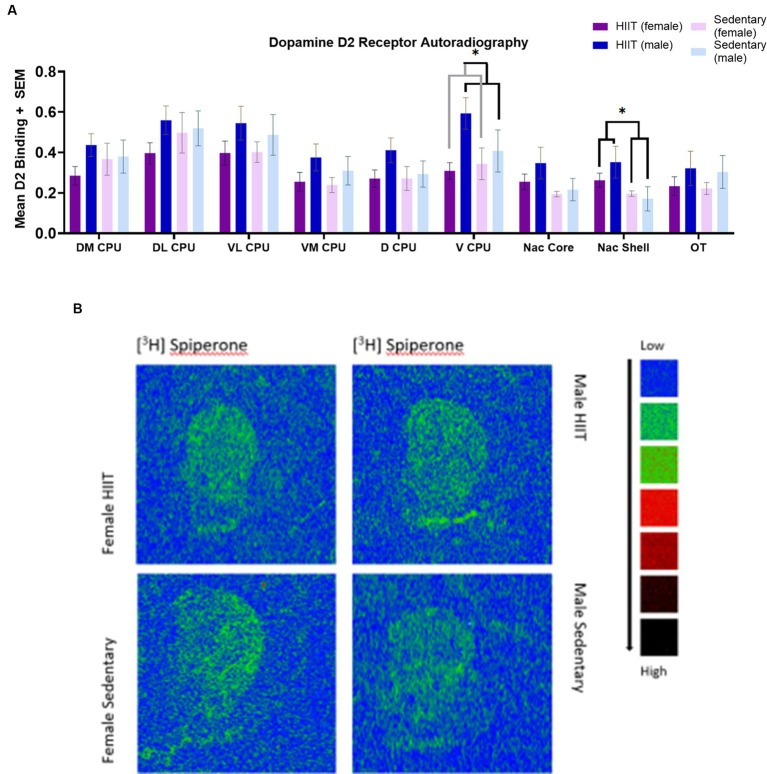
Two-way ANOVA found a main effect across the ventral caudate putamen (V CPU) for sex [F(1,26) = 4.84; p < 0.04; Figures 3A,B], such that mean [3H] spiperone (D2R-like) binding was significantly greater for males than females. The main effect of HIIT Exercise [F(1,26) = 0.91; p > 0.05] and the HIIT-sex interaction [F(1,26) = 1.96; p > 0.05] were not significant in the V CPU.
Figure 3.
(A) Quantitative autoradiography of [3H] Spiperone (D2R-like) binding levels within the nucleus accumbens shell (Nac Shell), nucleus accumbens core (Nac core), ventromedial caudate putamen (VL CPU), ventrolateral caudate putamen (VL CPU), dorsolateral caudate putamen (DL CPU), dorsomedial caudate putamen (DM CPU), dorsal caudate putamen (D CPU), ventral caudate putamen (VCPU), and olfactory tubercle (OT) across exercise treatment groups. Measurements of the following regions were carried out at the bregma coordinates taken from the Paxinos & Watson rat brain atlas. Values are expressed as total [3H] Spiperone binding means ± S.E.M. for D2R receptors. Two-way ANOVA found a main effect across the ventral caudate putamen (V CPU) for sex [F(1,26) = 4.84; p < 0.04], such that D2R-like binding was significantly greater for males than females. Across the nucleus accumbens shell (Nac Shell), a main effect was found for HIIT Exercise [F(1,26) = 5.27; p < 0.03], such that mean D2R-like binding was significantly greater for the HIIT group than the sedentary group. Each bar represents the group mean for dopamine D2 receptor binding. (B) A representative image of D2 autoradiographic binding distribution Specific images were taken from [3H] Spiperone binding in the rat brain. Representative images were taken at approximately bregma level +1.32 mm.
In the nucleus accumbens shell (Nac Shell), a main effect was found for HIIT Exercise [F(1,26) = 5.27; p < 0.03], such that mean D2R-like binding was significantly greater for the HIIT group than the sedentary group. The main effect of sex [F(1,26) = 0.36; p > 0.05] and the HIIT-sex interaction [F(1,26) = 1.14; p > 0.05] were not significant in the Nac Shell. No other main effects were observed in the Nac Shell (p > 0.05; Figures 3A,B).
No significant effects were reported across the DM CPU [F(1,26) = 1.94; p > 0.05], DL CPU [F(1,26) = 0.76; p > 0.05], VL CPU [F(1,26) = 0.17; p > 0.05], VM CPU [F(1,26) = 0.19; p > 0.05], D CPU [F(1,26) = 1.03; p > 0.05], Nac Core [F(1,26) = 0.44; p > 0.05], and OT [F(1,26) = 0.0021; p > 0.05].
TH immunohistochemistry
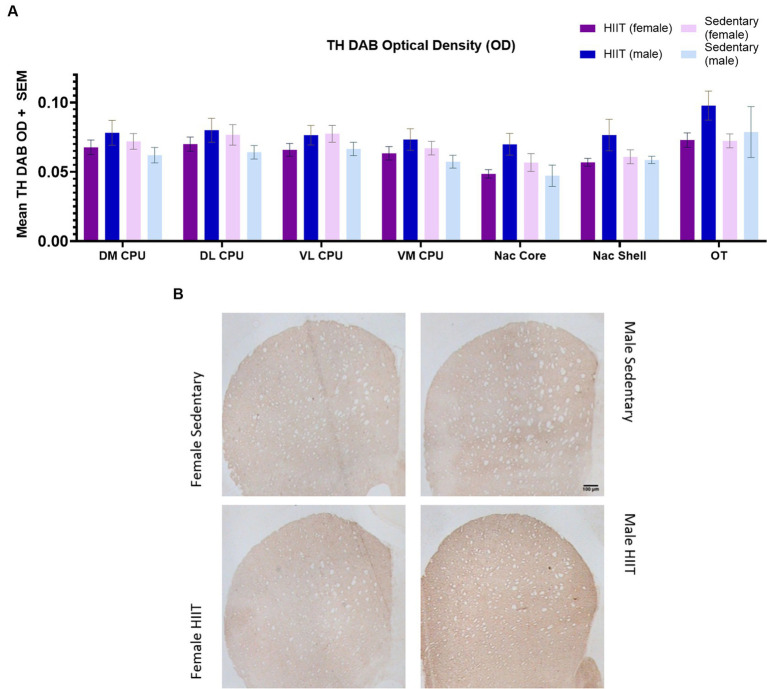
No significant effects of HIIT exercise were found across groups for TH-immunoreactive fiber densities across the DM CPU [F(1,20) = 2.03; p > 0.05], DL CPU [F(1,20) = 2.24; p > 0.05], VL CPU [F(1,20) = 2.93; p > 0.05], VM CPU [F(1,20) = 2.44; p > 0.05], Nac Core [F(1,20) = 4.24; p > 0.05], Nac Shell [F(1,20) = 1.98; p > 0.05], and OT [F(1,20) = 0.99; p > 0.05; Figures 4A,B].
Figure 4.
(A) Two-way ANOVA found no significant interactions or main effects across groups (p > 0.05). Each bar represents the group mean for TH DAB optical density. (B) Effect of treadmill exercise on tyrosine hydroxylase (TH)-immunoreactive fibers in the striatum of the Lewis rats. Photomicrographs of TH-positive fibers in the striatum. The scale bar represents 100 μm.
Discussion
The current study examined the effects of chronic HIIT exercise on dopamine (DA) signaling in rats. Results demonstrated that chronic HIIT exercise produced significant changes in D2R binding levels in the Nac Shell. Specifically, the mean D2R-like binding was 16% greater in the HIIT rats as compared to the sedentary rats. Previous research has reported an increase in D2R after a moderate-intensity treadmill exercise regimen which was shown to attenuate drug-seeking behaviors (8). Sex-dependent differences were mainly observed in the V CPU for D2R-like binding wherein binding was 14% greater for males than females. This finding suggests sex-dependent differences in D2R-binding levels.
For D2R-like binding, we observed an effect of HIIT exercise in the Nac Shell. This brain region is of particular importance due to its critical role in the mesolimbic pathway and association with the modulation of reward and desire. The mesolimbic pathway is highly influenced by dopamine signaling including D1 and D2 receptor levels (66). Past research found that steady moderate treadmill exercise elicited an increase in D2R-like binding in several subregions of the dorsal and ventral striatum, specifically within the DM CPU, VL CPU, VM CPU, and OT (8). The CPU is believed to control compulsive aspects of addiction, such as drug-seeking behavior (67). Previous findings outlined how treadmill exercise enhanced D2R in the basal ganglia, a brain region responsible for SUD habit formation through control of reward (38–40). Additionally, environmental enrichment with wheel running has also been associated with a significant increase in D2R gene expression (68). Clinical research has confirmed this association between D2R-like binding and exercise in which higher-intensity physical activity was associated with greater D2/3 receptor availability (69). Our study’s findings of the increased Nac Shell D2R-like binding agree with these aforementioned studies.
Thus, we may speculate that HIIT also attenuates drug-seeking behavior through its modulation of D2R binding. The present study, along with many others, have confirmed the increase in D2R conferred by exercise, including HIIT exercise (8, 11, 38–41). Rodent models have specifically outlined a causal link between exercise-induced D2R enhancement and attenuation of drug consumption (6–8). This exercise-induced enhancement of D2R is also linked to conditoned place avoidance, enhanced motivation, and decreased depressive symptoms (46, 52, 53). These are important factors known to play key roles in remission efforts. Furthermore, enhancement of D2R signaling is associated with increased motiviation for recovery from addiction, while reduction in D2R levels in the striatum is associated with increased impulsivity and addictive behavior (47). Since previous research suggests that positive modulation of dopamine is likely dependent on exercise intensity, we may speculate that exercise remains a beneficial treatment modality for addiction due to its enhancement of D2R and that HIIT may provide exceptional efficacy due to its greater intensity.
In contrast to D2R, HIIT exercise did not to have a significant effect on D1R-like binding, across all regions of interest in both male and female rats. Previous research using a steady and moderate-intensity treadmill exercise regimen—showed a reduction of D1R (8). Compared to moderate-intensity aerobic continuous exercise (MI-ACE), our findings point to neurochemical differences in D1R levels between a HIIT exercise regimen and a MI-ACE regimen.
TH levels are known to correlate with dopamine bioavailability and effectively predict dopamine levels (56, 57). As indicated by previous research, TH activity decreases with the loss of dopamine neurons as TH is the rate-limiting enzyme in the synthesis of dopamine (70). The present study showed that chronic HIIT exercise did not produce significant changes in TH levels, thus suggesting insignificant changes in DA levels.
Insignificant modulation of TH and D1R may be explained by a suggested upper limit to neurotrophic factors. Previous studies have indicated that exercise-induced modulation of neurotrophic factors is occasionally absent in healthy controls, despite observing significant effects of exercise in animal models representing a diseased condition subject to the same protocol (63). This phenomenon may be explained by a sort of natural ceiling or upper limit to neurotrophic modulation. The healthy rats are already at homeostasis and thus much closer to an upper limit than rats of a diseased model such as Parkinson’s disease or SUDs where baseline dopamine signaling is already significantly imparied. Therefore, the healthy rats may not show a significant effect when the diseased model does because the diseased model has a much larger increase in neurotrophic factor from deficit to upper limit rather than from homeostasis to upper limit. Likewise, the rodents utlized in our study were healthy and thus theoretically had a less significant range baseline to upper limit than would a diseased or addicted model. Hence, it would be unreasonable to assume that an addicted model would not exhibit positive modulation of TH and D1R with HIIT solely based on the insignificant effects observed in our healthy model. This topic warrants further exploration and comparison between addicted and healthy models of exercise-induced dopamine signaling.
The disparate findings in D1R binding demonstrated by HIIT exercise compared to moderate-intensity exercise could be explained by exercise intensity. Chronic high-level voluntary wheel running has been shown to produce lower levels of D2R mRNA and higher levels of ΔFosB/FosB immunoreactivity and TH mRNA in the NAc Core, NAc, and the ventral tegmental area (VTA) respectively, exhibiting a resemblance to chronic drug exposure (71, 72). Stimulation of D1-expressing medium spiny neurons has been shown to enhance sensitivity to drugs of abuse (26, 73–75). Additionally, chronic methamphetamine users are known to exhibit significantly higher D1R levels (31). Previous research has outlined D1R stimulation as the primary mechanism for drug reward and conditioned association (30). The results of the present study suggest that HIIT exercise regimen resulted in increased D2R levels in the brain. Therefore, it would be beneficial for future studies to directly examine various intensities of exercise and their potential effects on substance abuse behavior (76).
A plausible contributor to varied responses of exercise on D1R, D2R and TH expression can be overtraining; however, this notion was refuted for our study. A recent study on HIIT analyzed the threshold for overtraining in humans (77). Current HIIT training recommendations advise that HIIT be followed by low-to-moderate intensity training for at least the first 48 h to ensure proper recovery, especially in untrained individuals (13, 77). Furthermore, HIIT guidelines also recommend 1–2 recovery days per week and no more than a weekly increase of 10% in training time or intensity (78). Overtraining has been shown on numerous accounts to induce neurotransmitter and enzyme disturbances including disturbances of dopamine and modulation of TH expression (60–63, 79, 80). Furthermore, overtraining can dampen and even reverse some of the positive effects of exercise; therefore, it is imperative to screen for signs and symptoms of overtraining syndrome to validate related findings.
Our present study utilized a HIIT exercise regimen consisting of daily 30 min training sessions amounting to a total of 210 min of HIIT per week for 6 weeks. Although a HIIT overtraining threshold has yet to be identified for rodents, corticosterone ELISA analysis found no significant difference in corticosterone levels between HIIT treated rats relative to sedentary rats (72). Additionally, a significant decrease in animal body weight, which is an accepted sign of overtraining, was not observed throughout this HIIT regimen (81, 82). These findings indicate that our HIIT regimen did not induce overtraining syndrome in the exercise group thus validating our findings.
The varied effects of our HIIT results compared to other exercise routines point to the importance of exercise specificity in clinical application. It is evident that different exercise regimens are better at producing certain results and treating specific conditions. Resistance training is a prime example with its unmatched efficacy in treating osteoporosis and sarcopenia (83). Other research suggests that MIAE may be the best regimen for reducing all-cause mortality and that high-dose resistance training may be the worst followed by vigorous exercise possibly due to increased vessel calcification (84, 85). These differences are likely transferable to treating addiction; wherein, there is a most effective and least effective or possibly even counterproductive exercise regimen in treating addiction. The present study findings support further research to elucidate exercise regimen or exercise perscription differences and their efficacy in therapeutic tools combating many psychiatric disorders, including substance use disorders.
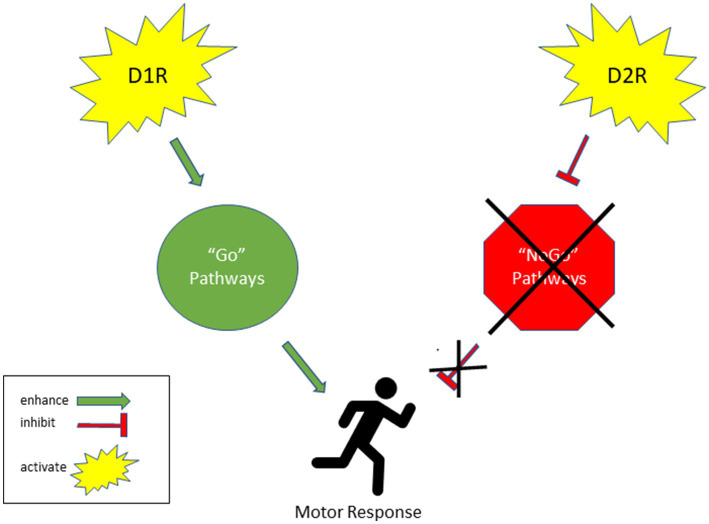
The results of this study alluded to sex-dependent differences in dopamine signaling. As discussed, past studies have shown that female rats are more susceptible to addiction (86–88). These sex-dependent effects were observed in our D2R data. The male rats showed 14% greater D2R binding levels in the V CPU as compared to females. The greater levels of D2R indicate sex-dependent differences in D2R binding. Striatal D1 receptors enhance the synaptic activity of “Go” pathways, promoting the likelihood of triggering a specific response. Conversely, striatal D2 receptor activation inhibits “NoGo” pathways, which in turn increases the probability of initiating a particular response (89). Moreover, D1-expressing neurons directly activate motor responses, while D2-expressing neurons indirectly trigger motor responses by removing inhibitory signals (Figure 5). This data alludes to the notion that there are sex differences in how exercise may mediate neurochemistry and thus a sex difference in their utility and therapeutic potential. Careful consideration is needed in terms of exercise prescription. Furthermore, exercise prescription may be further influenced by sex and so further research is warranted on this. Since our study focused on the neurological effects of exercise, future studies should explore how HIIT exercise affects drug-seeking and even non-drug seeking behaviors like overeating and gambling. This may provide insight into the best exercise-based treatment for drug-use attenuation.
Figure 5.
Effect of D1R and D2R activation on motor responses.
Conclusion
SUD programs have had considerable interest in dopamine as relative dopamine deficiences have been considered central to the pathophysiology of human addiction, drugs of abuse-related dysphoria, and craving (90, 91). The present findings showed that daily HIIT treadmill exercise had a significant effect on D2R-like binding in the Nac Shell, as the exercise group displayed 16% higher D2R-like binding compared to the sedentary group. Sex differences were also observed in the V CPU wherein males showed 14% greater D2R-like binding than females. HIIT exercise did not impact D1R-like binding or TH expression as compared to a sedentary group.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JT: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MP: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. BR: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. NR: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. NH: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. JH: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. KB: Writing – review & editing. MG: Funding acquisition, Writing – review & editing. DB: Writing – review & editing. PT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by NY Research Foundation (RIAQ1094) and partially supported by the University of Buffalo ELN fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Swenson S, Blum K, McLaughlin T, Gold MS, Thanos PK. The therapeutic potential of exercise for neuropsychiatric diseases: a review. J Neurol Sci. (2020) 412:116763. doi: 10.1016/j.jns.2020.116763, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. (2017) 32:541–56. doi: 10.1097/HCO.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 3.Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. (2018) 11:e005263. doi: 10.1161/CIRCOUTCOMES.118.005263 [DOI] [PubMed] [Google Scholar]
- 4.Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. (2018) 9:509. doi: 10.3389/fpsyg.2018.00509, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman N, Mihalkovic A, Geary O, Haffey R, Hamilton J, Thanos PK. Chronic aerobic exercise: autoradiographic assessment of Gaba (a) and mu-opioid receptor binding in adult rats. Pharmacol Biochem Behav. (2020) 196:172980. doi: 10.1016/j.pbb.2020.172980, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, et al. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res. (2010) 215:77–82. doi: 10.1016/j.bbr.2010.06.033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanos PK, Stamos J, Robison LS, Heyman G, Tucci A, Wang GJ, et al. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav Brain Res. (2013) 239:8–14. doi: 10.1016/j.bbr.2012.10.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LS, Swenson S, Hamilton J, Thanos PK. Exercise reduces dopamine D1r and increases D2r in rats: implications for addiction. Med Sci Sports Exerc. (2018) 50:1596–602. doi: 10.1249/MSS.0000000000001627, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Smith M, Lynch W. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry. (2012) 2:82. doi: 10.3389/fpsyt.2011.00082, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Wang Y, Wang Y, Li R, Zhou C. Impact of physical exercise on substance use disorders: a meta-analysis. PLoS One. (2014) 9:e110728. doi: 10.1371/journal.pone.0110728, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robison LS, Alessi L, Thanos PK. Chronic forced exercise inhibits stress-induced reinstatement of cocaine conditioned place preference. Behav Brain Res. (2018) 353:176–84. doi: 10.1016/j.bbr.2018.07.009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knopf A. NSDUH 2020: 40 million adults with SUD. Alcohol Drug Abuse Wkly. (2021) 33:1–3. doi: 10.1002/adaw.33251 [DOI] [Google Scholar]
- 13.Liguori G, American College of Sport Medicine . ACSM’s guidelines for exercise testing and prescription Lippincott Williams & Wilkins; (2020). [Google Scholar]
- 14.De Feo P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Nutr Metab Cardiovasc Dis. (2013) 23:1037–42. doi: 10.1016/j.numecd.2013.06.002, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Milanovic Z, Sporis G, Weston M. Effectiveness of high-intensity interval training (hit) and continuous endurance training for Vo2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. (2015) 45:1469–81. doi: 10.1007/s40279-015-0365-0, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Moreau D, Kirk IJ, Waldie KE. High-intensity training enhances executive function in children in a randomized, placebo-controlled trial. eLife. (2017) 6:6. doi: 10.7554/eLife.25062, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. (2015) 16:942–61. doi: 10.1111/obr.12317, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Epstein LH, O’Donnell S, Biondolillo MJ, Hostler D, Roemmich JN. Comparing the reinforcing value of high intensity interval training versus moderate intensity aerobic exercise in sedentary adults. Physiol Behav. (2021) 238:113468. doi: 10.1016/j.physbeh.2021.113468, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrantes AM, Blevins CE. Exercise in the context of substance use treatment: key issues and future directions. Curr Opin Psychol. (2019) 30:103–8. doi: 10.1016/j.copsyc.2019.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Li X, Han T, Zou X, Zhang H, Feng W, Wang H, et al. Long-term high-intensity interval training increases serum neurotrophic factors in elderly overweight and obese Chinese adults. Eur J Appl Physiol. (2021) 121:2773–85. doi: 10.1007/s00421-021-04746-w, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Wahl P, Mathes S, Kohler K, Achtzehn S, Bloch W, Mester J. Acute metabolic, hormonal, and psychological responses to different endurance training protocols. Horm Metab Res. (2013) 45:827–33. doi: 10.1055/s-0033-1347242 [DOI] [PubMed] [Google Scholar]
- 22.Tas Durmus P, Vardar ME, Kaya O, Tayfur P, Sut N, Vardar SA. Evaluation of the effects of high intensity interval training on cytokine levels and clinical course in treatment of opioid use disorder. Turk Psikiyatri Derg. (2020) 31:151–8. doi: 10.5080/u25070, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Bahdur K, Gilchrist R, Park G, Nina L, Pruna R. Effect of Hiit on cognitive and physical performance. Apunts Medicina de l’Esport. (2019) 54:113–7. doi: 10.1016/j.apunts.2019.07.001 [DOI] [Google Scholar]
- 24.Loprinzi PD, Frith E, Edwards MK, Sng E, Ashpole N. The effects of exercise on memory function among young to middle-aged adults: systematic review and recommendations for future research. Am J Health Promot. (2018) 32:691–704. doi: 10.1177/0890117117737409, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Flemmen G, Unhjem R, Wang E. High-intensity interval training in patients with substance use disorder. Biomed Res Int. (2014) 2014:616935: 1–8. doi: 10.1155/2014/616935, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaess S, Stott SRW, Ang SL. Chapter 17-the generation of midbrain dopaminergic neurons In: Rubenstein J, Rakic P, Chen B, Kwan KY, editors. Patterning and cell type specification in the developing CNS and PNS. 2nd ed: Academic Press; (2020). 369–98. [Google Scholar]
- 27.Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. (2009) 207:303–14. doi: 10.1007/s00213-009-1657-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. (2007) 27:13140–50. doi: 10.1523/JNEUROSCI.2284-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahveisi K, Abdoli N, Farnia V, Khazaie H, Hosseini M, Ghazvini H, et al. Rem sleep deprivation before extinction or reinstatement alters methamphetamine reward memory via D1-like dopamine receptors. Pharmacol Biochem Behav. (2022) 213:173319. doi: 10.1016/j.pbb.2021.173319, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. (2019) 99:2115–40. doi: 10.1152/physrev.00014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worsley J, Moszczynska A, Falardeau P, Kalasinsky K, Schmunk G, Guttman M, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. (2000) 5:664–72. doi: 10.1038/sj.mp.4000760, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. (2001) 78:1094–103. doi: 10.1046/j.1471-4159.2001.00492.x, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Hitzemann R, Hitzemann B, Rivera S, Gatley J, Thanos P, Shou LL, et al. Dopamine D2 receptor binding, Drd 2 expression and the number of dopamine neurons in the BXD recombinant inbred series: genetic relationships to alcohol and other drug associated phenotypes. Alcohol Clin Exp Res. (2003) 27:1–11. doi: 10.1097/01.Alc.0000047862.40562.27, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. (2004) 28:720–8. doi: 10.1097/01.alc.0000125270.30501.08, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, et al. Dopamine D2r DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. (2005) 77:130–9. doi: 10.1016/j.lfs.2004.10.061, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Delis F, Benveniste H, Xenos M, Grandy D, Wang GJ, Volkow ND, et al. Loss of dopamine D2 receptors induces atrophy in the temporal and parietal cortices and the caudal thalamus of ethanol-consuming mice. Alcohol Clin Exp Res. (2012) 36:815–25. doi: 10.1111/j.1530-0277.2011.01667.x, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2r DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. (2008) 62:481–6. doi: 10.1002/syn.20523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vučcković MG, Li Q, Fisher B, Nacca A, Leahy RM, Walsh JP, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: in vivo imaging with [18F]Fallypride. Mov Disord. (2010) 25:2777–84. doi: 10.1002/mds.23407, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. (2004) 77:378–90. doi: 10.1002/jnr.20162, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. NeuroMolecular Med. (2008) 10:67–80. doi: 10.1007/s12017-008-8032-3, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Cho HS, Baek DJ, Baek SS. Effect of exercise on hyperactivity, impulsivity and dopamine D2 receptor expression in the substantia Nigra and striatum of spontaneous hypertensive rats. J Exerc Nutr Biochem. (2014) 18:379–84. doi: 10.5717/jenb.2014.18.4.379, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noursadeghi E, Rashvand M, Haghparast A. Nucleus accumbens dopamine receptors mediate the stress-induced analgesia in an animal model of acute pain. Brain Res. (1784) 1784:147887. doi: 10.1016/j.brainres.2022.147887 [DOI] [PubMed] [Google Scholar]
- 43.Gee TA, Weintraub NC, Lu D, Phelps CE, Navratilova E, Heien ML, et al. A pain-induced tonic hypodopaminergic state augments phasic dopamine release in the nucleus Accumbens. Pain. (2020) 161:2376–84. doi: 10.1097/j.pain.0000000000001925, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Gaur U, Cao Z, Hou S-T, Zheng W. Dopamine D1-and D2-like receptors oppositely regulate lifespan via a dietary restriction mechanism in Caenorhabditis elegans. BMC Biol. (2022) 20:71. doi: 10.1186/s12915-022-01272-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Świtała K, Bojarczuk A, Hajto J, Piechota M, Buryta M, Leońska-Duniec A. Impact of the DRD2 polymorphisms on the effectiveness of the training program. Int J Environ Res Public Health. (2022) 19:4942. doi: 10.3390/ijerph19094942, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood BN. The role of dopamine in overcoming aversion with exercise. Brain Res. (2019) 1713:102–8. doi: 10.1016/j.brainres.2018.08.030, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. (2014) 76 Pt B:498–509. doi: 10.1016/j.neuropharm.2013.06.031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lachowicz M, Chmielowiec J, Chmielowiec K, Suchanecka A, Masiak J, Michałowska-Sawczyn M, et al. Significant association of DRD2 and ANKK1 genes with rural heroin dependence and relapse in men. Ann Agric Environ Med. (2020) 27:269–73. doi: 10.26444/aaem/119940, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Blum K, Chen TJ, Downs BW, Bowirrat A, Waite RL, Braverman ER, et al. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing “deprivation-amplification relapse therapy” (DART). Postgrad Med. (2009) 121:176–96. doi: 10.3810/pgm.2009.11.2087, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahlgren A, Wargelius HL, Berglund KJ, Fahlke C, Blennow K, Zetterberg H, et al. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol Alcohol. (2011) 46:509–13. doi: 10.1093/alcalc/agr045, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Blum K, Han D, Bowirrat A, Downs BW, Bagchi D, Thanos PK, et al. Genetic addiction risk and psychological profiling analyses for “Preaddiction” severity index. J Pers Med. (2022) 12:1772. doi: 10.3390/jpm12111772, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. (2007) 191:461–82. doi: 10.1007/s00213-006-0668-9, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Klimke A, Larisch R, Janz A, Vosberg H, Müller-Gärtner H-W, Gaebel W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I] IBZM SPECT. Psychiatry Res Neuroimaging. (1999) 90:91–101. doi: 10.1016/S0925-4927(99)00009-8, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Levey DF, Stein MB, Wendt FR, Pathak GA, Zhou H, Aslan M, et al. Bi-ancestral depression Gwas in the million veteran program and Meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci. (2021) 24:954–63. doi: 10.1038/s41593-021-00860-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimbrel NA, Ashley-Koch AE, Qin XJ, Lindquist JH, Garrett ME, Dennis MF, et al. A genome-wide association study of suicide attempts in the million veterans program identifies evidence of Pan-ancestry and ancestry-specific risk loci. Mol Psychiatry. (2022) 27:2264–72. doi: 10.1038/s41380-022-01472-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS One. (2012) 7:e29867. doi: 10.1371/journal.pone.0029867, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. (2011) 508:1–12. doi: 10.1016/j.abb.2010.12.017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaillancourt K, Chen GG, Fiori L, Maussion G, Yerko V, Théroux J-F, et al. Methylation of the tyrosine hydroxylase gene is dysregulated by cocaine dependence in the human striatum. iScience. (2021) 24:103169. doi: 10.1016/j.isci.2021.103169, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine-and cocaine-regulated phosphoproteins. Brain Res. (1991) 561:147–50. doi: 10.1016/0006-8993(91)90759-O, PMID: [DOI] [PubMed] [Google Scholar]
- 60.da Silva WAB, Ferreira Oliveira K, Caroline Vitorino L, Ferreira Romão L, Allodi S, Lourenço CC. Physical exercise increases the production of tyrosine hydroxylase and CDNF in the spinal cord of a Parkinson’s disease mouse model. Neurosci Lett. (2021) 760:136089. doi: 10.1016/j.neulet.2021.136089 [DOI] [PubMed] [Google Scholar]
- 61.Baek DJ, Lee CB, Baek SS. Effect of treadmill exercise on social interaction and tyrosine hydroxylase expression in the attention-deficit/hyperactivity disorder rats. J Exerc Rehabil. (2014) 10:252–7. doi: 10.12965/jer.140162, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold JC, Salvatore MF. Exercise-mediated increase in Nigral tyrosine hydroxylase is accompanied by increased Nigral Gfr-Α1 and Eaac 1 expression in aging rats. ACS Chem Neurosci. (2016) 7:227–39. doi: 10.1021/acschemneuro.5b00282, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fallah Mohammadi Z, Falah Mohammadi H, Patel DI. Comparing the effects of progressive and mild intensity treadmill running protocols on neuroprotection of parkinsonian rats. Life Sci. (2019) 229:219–24. doi: 10.1016/j.lfs.2019.05.036, PMID: [DOI] [PubMed] [Google Scholar]
- 64.Shi SR, Liu C, Pootrakul L, Tang L, Young A, Chen R, et al. Evaluation of the value of frozen tissue section used as “gold standard” for immunohistochemistry. Am J Clin Pathol. (2008) 129:358–66. doi: 10.1309/7cxuyxt23e5al8kq, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM. Expression of human A53t alpha-synuclein in the rat substantia Nigra using a novel Aav1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol Neurodegener. (2010) 5:43. doi: 10.1186/1750-1326-5-43, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pariyadath V, Gowin JL, Stein EA. Chapter 8-resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks In: Ekhtiari H, Paulus MP, editors. Progress in brain research: Elsevier; (2016). 155–73. [DOI] [PubMed] [Google Scholar]
- 67.Yoo JH, Chun JW, Choi MR, Cho H, Kim JY, Choi J, et al. Caudate nucleus volume mediates the link between glutamatergic neurotransmission and problematic smartphone use in youth. J Behav Addict. (2021) 10:338–46. doi: 10.1556/2006.2021.00024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thanos PK, Hamilton J, O’Rourke JR, Napoli A, Febo M, Volkow ND, et al. Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget. (2016) 7:19111–23. doi: 10.18632/oncotarget.8088, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Köhncke Y, Papenberg G, Jonasson L, Karalija N, Wåhlin A, Salami A, et al. Self-rated intensity of habitual physical activities is positively associated with dopamine D (2/3) receptor availability and cognition. NeuroImage. (2018) 181:605–16. doi: 10.1016/j.neuroimage.2018.07.036 [DOI] [PubMed] [Google Scholar]
- 70.Haavik J, Toska K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol. (1998) 16:285–309. doi: 10.1007/bf02741387 [DOI] [PubMed] [Google Scholar]
- 71.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. (2011) 217:354–62. doi: 10.1016/j.bbr.2010.11.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammond N, Rahman N, Zhan S, Yim YY, Nestler EJ, Thanos PK. Chronic high intensity interval training (HIIT) exercise in adolescent rats results in cocaine place aversion and ΔFosB induction. (2023).
- 73.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. (2010) 66:896–907. doi: 10.1016/j.neuron.2010.05.011, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. (2011) 5:41. doi: 10.3389/fnana.2011.00041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreitzer AC, Berke JD. Investigating striatal function through cell-type-specific manipulations. Neuroscience. (2011) 198:19–26. doi: 10.1016/j.neuroscience.2011.08.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hatoum AS, Colbert SMC, Johnson EC, Huggett SB, Deak JD, Pathak GA, et al. Multivariate genome-wide association Meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat Ment Health. (2023) 1:210–23. doi: 10.1038/s44220-023-00034-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanley J, Peake JM, Buchheit M. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med. (2013) 43:1259–77. doi: 10.1007/s40279-013-0083-4, PMID: [DOI] [PubMed] [Google Scholar]
- 78.Brenner JS. Overuse injuries, overtraining, and burnout in child and adolescent athletes. Pediatrics. (2007) 119:1242–5. doi: 10.1542/peds.2007-0887, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Anish EJ. Exercise and its effects on the central nervous system. Curr Sports Med Rep. (2005) 4:18–23. doi: 10.1097/01.CSMR.0000306066.14026.77 [DOI] [PubMed] [Google Scholar]
- 80.Meeusen R, Piacentini M. Exercise and neurotransmission: a window to the future? Eur J Sport Sci. (2001) 1:1–12. doi: 10.1080/17461390100071103 [DOI] [Google Scholar]
- 81.Kreher JB, Schwartz JB. Overtraining syndrome: a practical guide. Sports Health. (2012) 4:128–38. doi: 10.1177/1941738111434406, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kadaja L, Eimre M, Paju K, Roosimaa M, Põdramägi T, Kaasik P, et al. Impaired oxidative phosphorylation in overtrained rat myocardium. Exp Clin Cardiol. (2010) 15:e116–27. PMID: [PMC free article] [PubMed] [Google Scholar]
- 83.Tyler J, Thanos PK. Raising the bar for public health: resistance training and health benefits. Int J Strength Cond. (2023) 3. doi: 10.47206/ijsc.v3i1.195 [DOI] [Google Scholar]
- 84.Lee DH, Rezende LFM, Joh HK, Keum N, Ferrari G, Rey-Lopez JP, et al. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of us adults. Circulation. (2022) 146:523–34. doi: 10.1161/circulationaha.121.058162, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Momma H, Kawakami R, Honda T, Sawada SS. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: a systematic review and meta-analysis of cohort studies. Br J Sports Med. (2022) 56:755–63. doi: 10.1136/bjsports-2021-105061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS one. (2013) 8:e79465. doi: 10.1371/journal.pone.0079465, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. (2012) 37:2605–14. doi: 10.1038/npp.2012.99, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kerstetter KA, Kippin TE. Impact of sex and gonadal hormones on cocaine and food reinforcement paradigms. J Addict Res Ther. (2011) 1:2963. doi: 10.4172/2155-6105.s4-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. (2009) 199:141–56. doi: 10.1016/j.bbr.2008.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. (1985) 9:469–77. doi: 10.1016/0149-7634(85)90022-3 [DOI] [PubMed] [Google Scholar]
- 91.Blum K, Liu Y, Shriner R, Gold MS. Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des. (2011) 17:1158–67. doi: 10.2174/138161211795656819, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.