Abstract
The steady state kinetics of glyceraldehyde 3-phosphate:NADP+ oxidoreductase (GNR) (EC 1.2.1.9) have been investigated. The enzyme exhibits hyperbolic behavior over a wide range of substrate concentrations. Double-reciprocal plots are nearly parallel or distantly convergent with limiting Km values of 2 to 5 micromolar for NADP+ and 20 to 40 micromolar for D-glyceraldehyde 3-phosphate (G3P). The velocity response to NADP+ as the varied substrate is however sigmoidal if G3P concentration exceeds 10 micromolar, whereas the response to G3P may show inhibition above this concentration. This `G3P-inhibited state' is alleviated by saturating amounts of NADP+ or NADPH. Product inhibition patterns indicate NADPH as a potent competitive inhibitor to NADP+ (Ki 30 micromolar) and mixed inhibitor towards G3P, and 3-phosphoglycerate (3PGA) as mixed inhibitor to both NADP+ and G3P (Ki 10 millimolar). The data, and those obtained with dead-end inhibitors, are consistent with a nonrapid equilibrium random mechanism with two alternative kinetic pathways. Of these, a rapid kinetic sequence (probably ordered with NADP+ binding first and G3P binding as second substrate) is dominant in the range of hyperbolic responses. A reverse reaction with 3PGA and NADPH as substrates is unlikely, and was not detected. Of a number of compounds tested, erythrose 4-phosphate (Ki 7 micromolar) and Pi (Ki 2.4 millimolar) act as competitive inhibitors to G3P (uncompetitive towards NADP+) and are likely to affect the in vivo activity. Ribose 5-phosphate, phosphoenolpyruvate, ATP, and ADP are also somewhat inhibitory. Full GNR activity in the leaf seems to be allowed only under high photosynthesis conditions, when levels of several inhibitors are low and substrate is high. We suggest that a main function of leaf GNR is to supply NADPH required for photorespiration, the reaction product 3PGA being cycled back to chloroplasts.
Full text
PDF
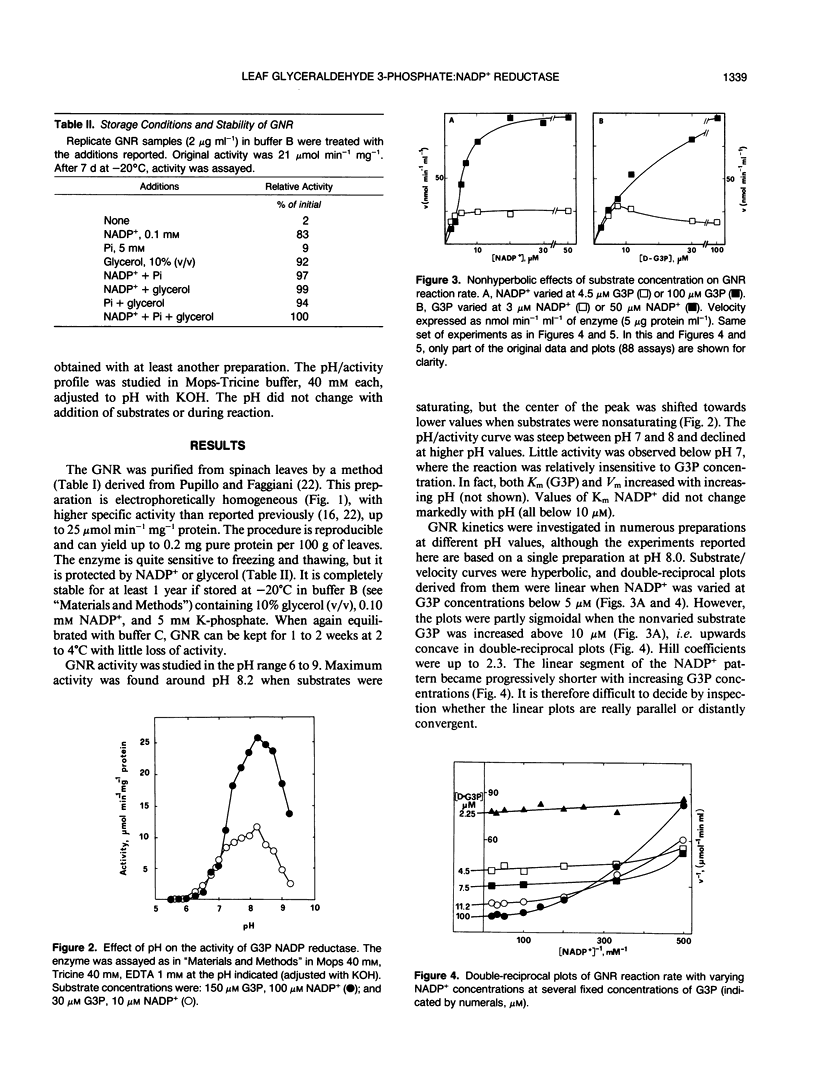
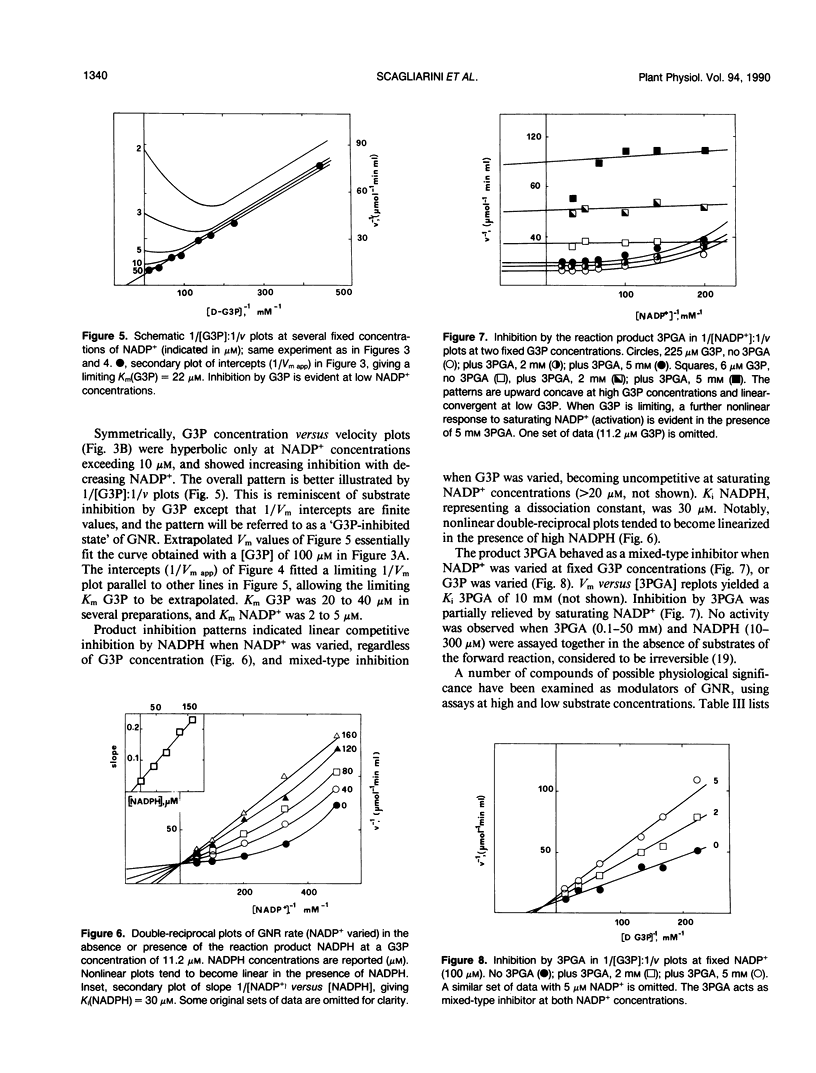
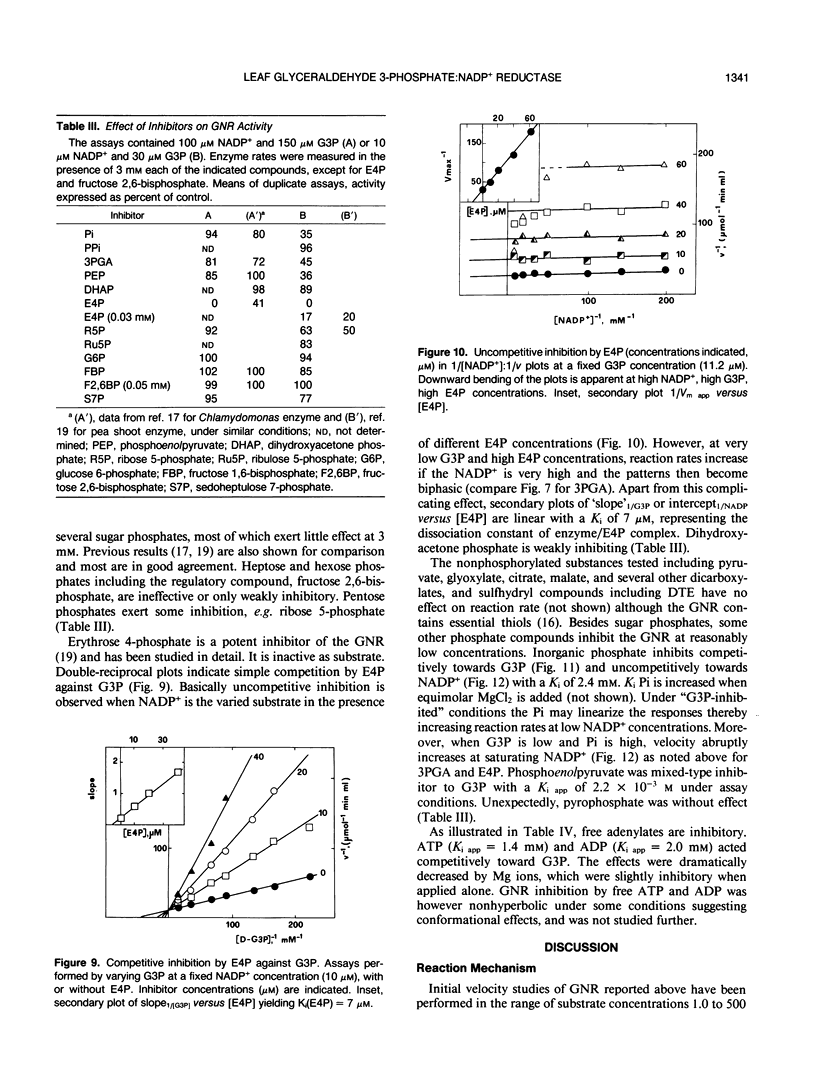
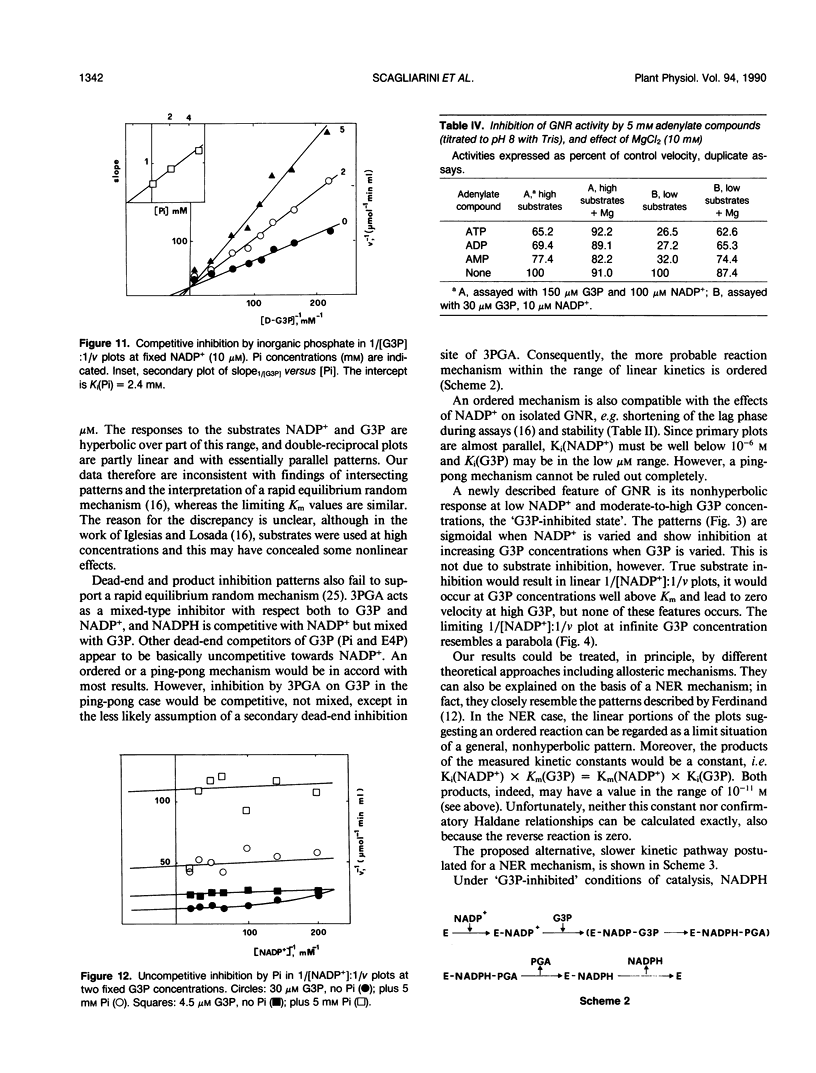


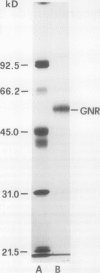
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Ehrlich B. A., Gibbs M. The glyceraldehyde 3-phosphate and glycerate 3-phosphate shuttle and carbon dioxide assimilation in intact spinach chloroplasts. Plant Physiol. 1975 Jun;55(6):1023–1030. doi: 10.1104/pp.55.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides S. M., Deal W. C., Jr Reversible dissociation of tetrameric rabbit muscle glyceraldehyde 3-phosphate dehydrogenase into dimers or monomers by adenosine triphosphate. J Biol Chem. 1969 Oct 25;244(20):5695–5702. [PubMed] [Google Scholar]
- Datta N. S., Shewach D. S., Mitchell B. S., Fox I. H. Kinetic properties and inhibition of human T lymphoblast deoxycytidine kinase. J Biol Chem. 1989 Jun 5;264(16):9359–9364. [PubMed] [Google Scholar]
- Duff S. M., Moorhead G. B., Lefebvre D. D., Plaxton W. C. Phosphate Starvation Inducible ;Bypasses' of Adenylate and Phosphate Dependent Glycolytic Enzymes in Brassica nigra Suspension Cells. Plant Physiol. 1989 Aug;90(4):1275–1278. doi: 10.1104/pp.90.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Nicotinamide adenine dinucleotide-specific glyceraldehyde 3-phosphate dehydrogenase from Pisum sativum. Assay and steady state kinetics. J Biol Chem. 1974 Jan 10;249(1):167–174. [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. A possible mechanism for phosphofructokinase. Biochem J. 1966 Jan;98(1):278–283. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardeström P., Wigge B. Influence of Photorespiration on ATP/ADP Ratios in the Chloroplasts, Mitochondria, and Cytosol, Studied by Rapid Fractionation of Barley (Hordeum vulgare) Protoplasts. Plant Physiol. 1988 Sep;88(1):69–76. doi: 10.1104/pp.88.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W., Beevers H. Subcellular Distribution of Enzymes of Glycolate Metabolism in the Alga Cyanidium caldarium. Plant Physiol. 1989 Jul;90(3):799–805. doi: 10.1104/pp.90.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A. A., Losada M. Purification and kinetic and structural properties of spinach leaf NADP-dependent nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys. 1988 Feb 1;260(2):830–840. doi: 10.1016/0003-9861(88)90514-0. [DOI] [PubMed] [Google Scholar]
- Jacob J. L., D'Auzac J. La glyćeraldehyde-3-phosphate déshydrogénalse du latex d'Hevea brasiliensis. Comparaison avec son homologue phosphorylante. Eur J Biochem. 1972 Dec 4;31(2):255–265. doi: 10.1111/j.1432-1033.1972.tb02528.x. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Givan C. V., Hodgson J. M., Randall D. D. Subcellular Location of NADPH-Dependent Hydroxypyruvate Reductase Activity in Leaf Protoplasts of Pisum sativum L. and Its Role in Photorespiratory Metabolism. Plant Physiol. 1988 Dec;88(4):1182–1185. doi: 10.1104/pp.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo P., Faggiani R. Subunit structure of three glyceraldehyde 3-phosphate dehydrogenases of some flowering plants. Arch Biochem Biophys. 1979 May;194(2):581–592. doi: 10.1016/0003-9861(79)90653-2. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. L., ARNON D. I. The preparation and properties of a new glyceraldehyde-3-phosphate dehydrogenase from photosynthetic tissues. J Biol Chem. 1955 Nov;217(1):361–371. [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E., Loescher W. H. A pathway for photosynthetic carbon flow to mannitol in celery leaves : activity and localization of key enzymes. Plant Physiol. 1983 Dec;73(4):869–873. doi: 10.1104/pp.73.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Geiger D. R., Tucci M. A., Fondy B. R. Leaf Carbon Metabolism and Metabolite Levels during a Period of Sinusoidal Light. Plant Physiol. 1989 Feb;89(2):403–408. doi: 10.1104/pp.89.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]