Abstract
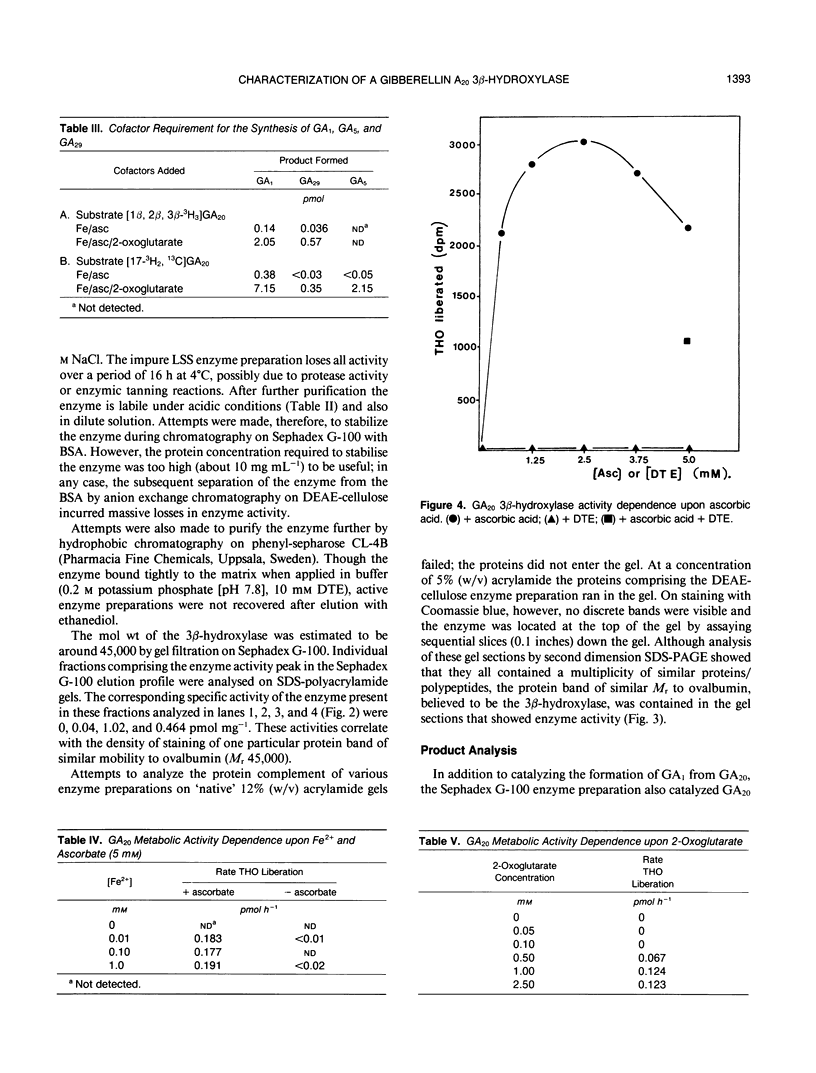
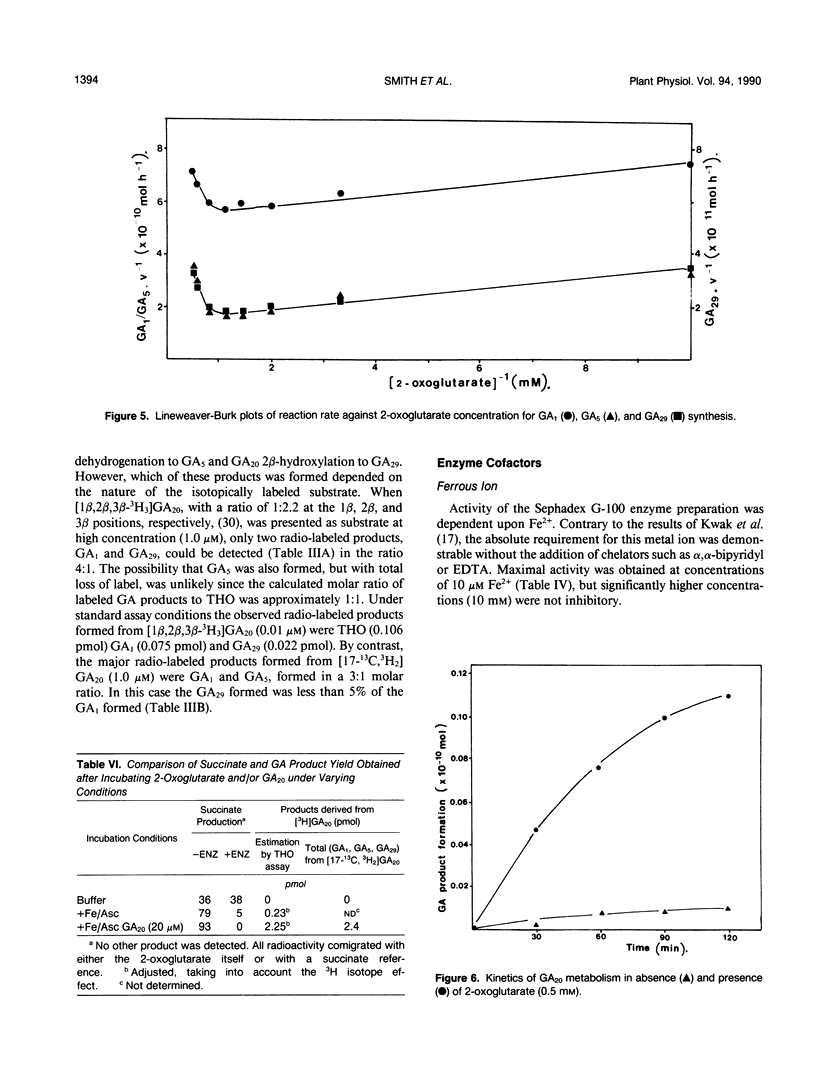
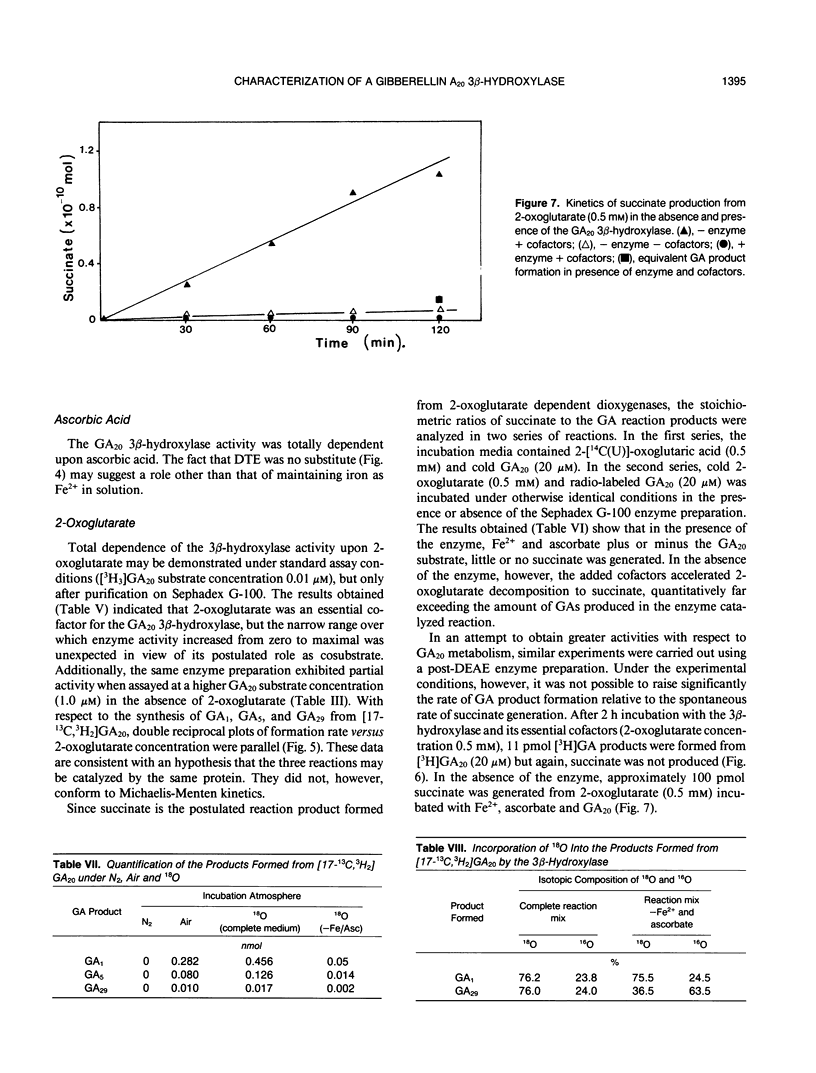
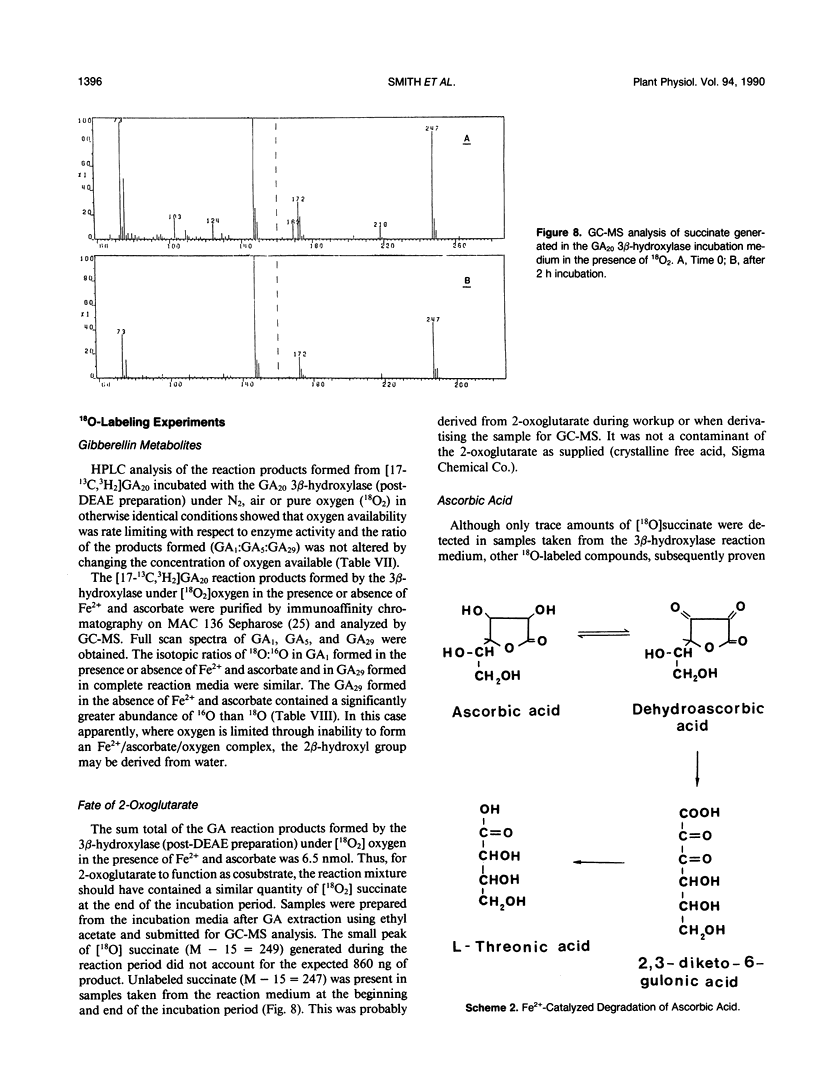
The GA20 3β-hydroxylase present in immature seeds of Phaseolus vulgaris has been partially purified and characterized. The physical characteristics of the enzyme are similar to those of the GA 2β-hydroxylases present in mature and immature seeds of Pisum sativum. It is acid-labile, hydrophobic, and of Mr 45,000. The enzyme catalyzes the synthesis of GA1, GA5, and GA29 from GA20. Activity is dependent upon the presence of Fe2+, ascorbate, 2-oxoglutarate, and oxygen. 2-Oxoglutarate does not function as a cosubstrate; in the presence of the enzyme, succinate is not a reaction product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Abraham E. The biosynthesis of penicillins and cephalosporins. Nat Prod Rep. 1988 Apr;5(2):129–145. doi: 10.1039/np9880500129. [DOI] [PubMed] [Google Scholar]
- Campell B. R., Bonner B. A. Evidence for Phytochrome Regulation of Gibberellin A(20) 3beta-Hydroxylation in Shoots of Dwarf (lele) Pisum sativum L. Plant Physiol. 1986 Dec;82(4):909–915. doi: 10.1104/pp.82.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale G. J., Rhoads R. E., Udenfriend S. Simultaneous incorporation of 18 O into succinate and hydroxyproline catalyzed by collagen proline hydroxylase. Biochem Biophys Res Commun. 1971 May 7;43(3):537–543. doi: 10.1016/0006-291x(71)90647-4. [DOI] [PubMed] [Google Scholar]
- Fujioka S., Yamane H., Spray C. R., Gaskin P., Macmillan J., Phinney B. O., Takahashi N. Qualitative and Quantitative Analyses of Gibberellins in Vegetative Shoots of Normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 Seedlings of Zea mays L. Plant Physiol. 1988 Dec;88(4):1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S., Yamane H., Spray C. R., Phinney B. O., Gaskin P., Macmillan J., Takahashi N. Gibberellin A(3) Is Biosynthesized from Gibberellin A(20) via Gibberellin A(5) in Shoots of Zea mays L. Plant Physiol. 1990 Sep;94(1):127–131. doi: 10.1104/pp.94.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall B. G., Hartl D. L. Regulation of newly evolved enzymes. II. The ebg repressor. Genetics. 1975 Nov;81(3):427–435. doi: 10.1093/genetics/81.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme E., Lindstedt G., Lindstedt S., Tofft M. 18-O studies of the 2-ketoglutarate-dependent sequential oxygenation of thymine to 5-carboxyuracil. J Biol Chem. 1971 May 25;246(10):3314–3319. [PubMed] [Google Scholar]
- Ikeda K., Weir E. C., Sakaguchi K., Burtis W. J., Zimering M., Mangin M., Dreyer B. E., Brandi M. L., Aurbach G. D., Broadus A. E. Clonal rat parathyroid cell line expresses a parathyroid hormone-related peptide but not parathyroid hormone itself. Biochem Biophys Res Commun. 1989 Jul 14;162(1):108–115. doi: 10.1016/0006-291x(89)91969-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Nietfeld J. J., Kemp A. Properties of prolyl 4-hydroxylase containing firmly-bound iron. Biochim Biophys Acta. 1980 Jun 13;613(2):349–358. doi: 10.1016/0005-2744(80)90089-3. [DOI] [PubMed] [Google Scholar]
- Smith V. A., Macmillan J. An immunological approach to gibberellin purification and quantification. Plant Physiol. 1989 Jul;90(3):1148–1155. doi: 10.1104/pp.90.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]