Abstract
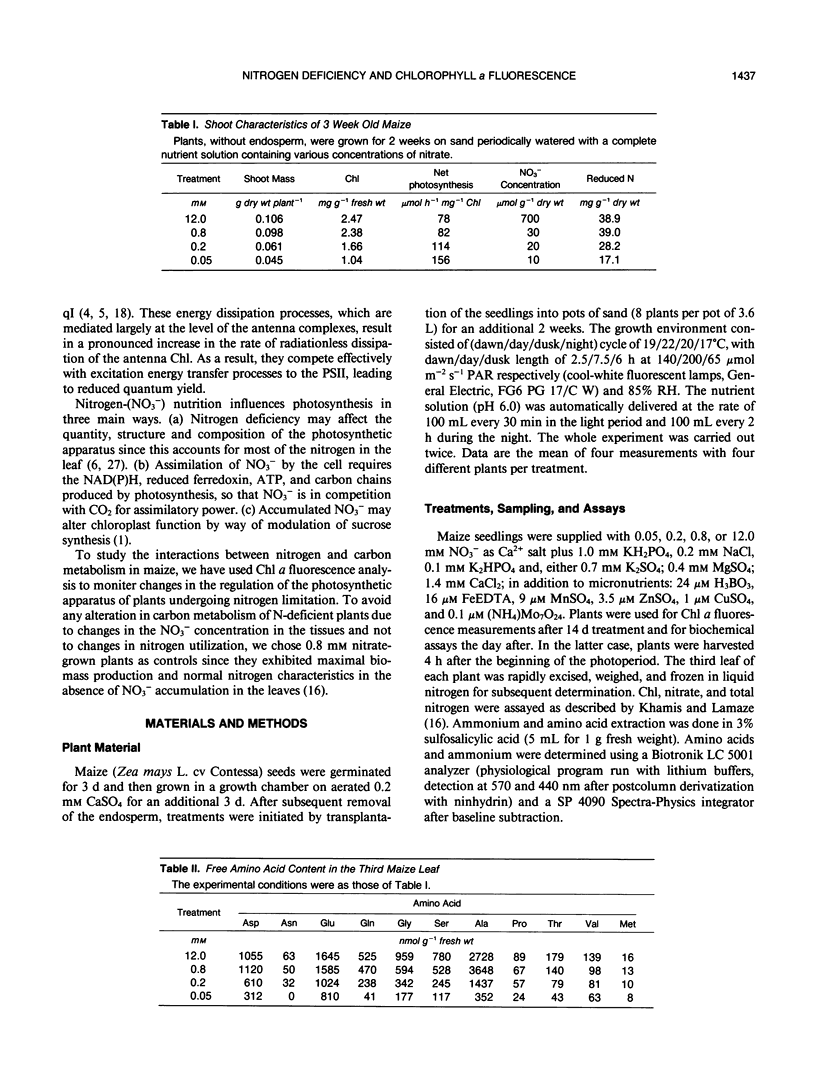
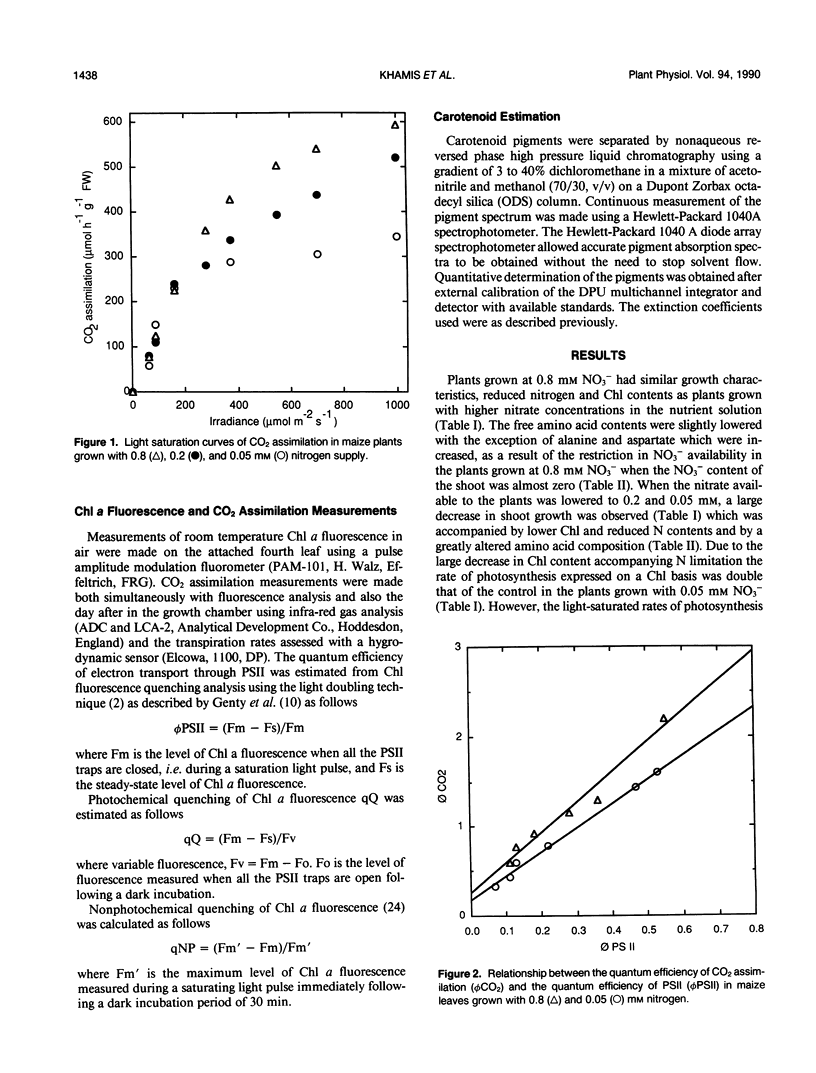
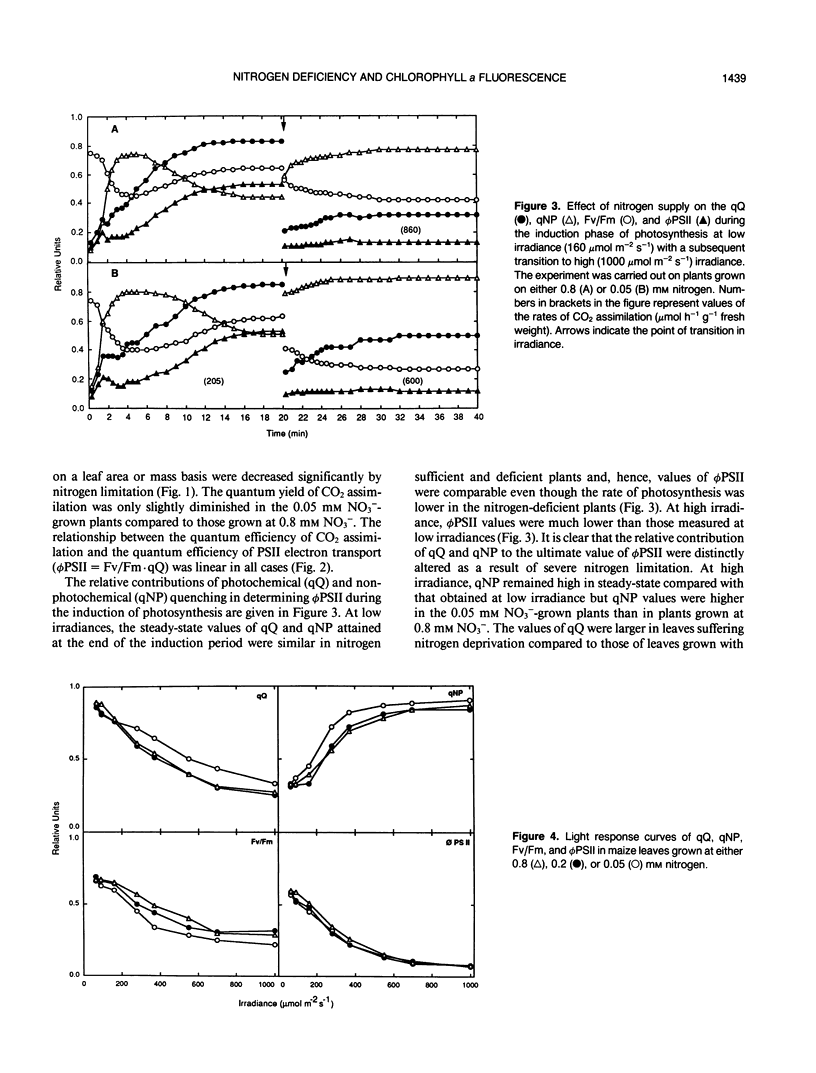
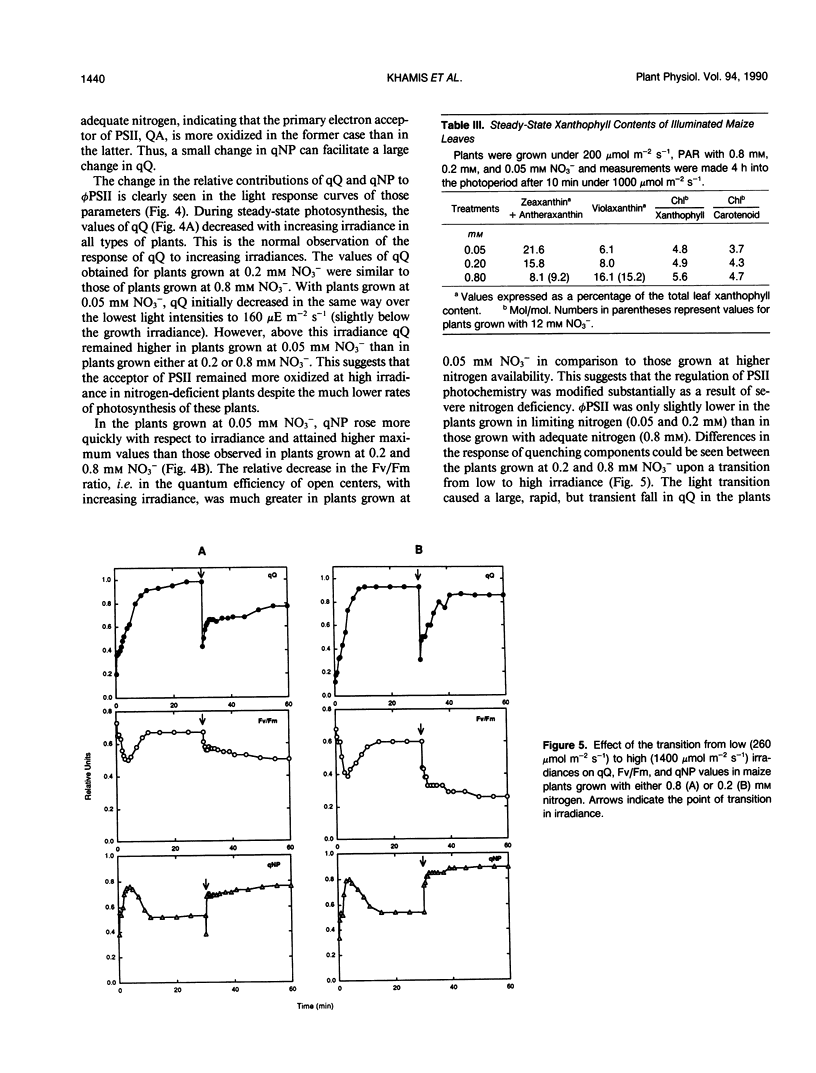
In maize (Zea mays L., cv Contessa), nitrogen (NO3−) limitation resulted in a reduction in shoot growth and photosynthetic capacity and in an increase in the leaf zeaxanthin contents. Nitrogen deficiency had only a small effect on the quantum yield of CO2 assimilation but a large effect on the light-saturated rate of photosynthesis. Linear relationships persisted between the quantum yield of CO2 assimilation and that of photosystem II photochemistry in all circumstances. At high irradiances, large differences in photochemical quenching and nonphotochemical quenching of Chl a fluorescence as well as the ratio of variable to maximal fluorescence (Fv/Fm) were apparent between nitrogen-deficient plants and nitrogen-replete controls, whereas at low irradiances these parameters were comparable in all plants. Light intensity-dependent increases in nonphotochemical quenching were greatest in nitrogen-deficient plants as were the decreases in Fv/Fm ratio. In nitrogen-deficient plants, photochemical quenching decreased with increasing irradiance but remained higher than in controls at high irradiances. Thermal dissipative processes were enhanced as a result of nitrogen deficiency (nonphotochemical quenching was elevated and Fv/Fm was lowered) allowing PSII to remain relatively oxidised even when carbon metabolism was limited via nitrogen limitation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979 Oct 10;548(1):128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Light Response of CO(2) Assimilation, Dissipation of Excess Excitation Energy, and Zeaxanthin Content of Sun and Shade Leaves. Plant Physiol. 1989 Jul;90(3):881–886. doi: 10.1104/pp.90.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt E. R., Weber J. A., Gates D. M. Effects of Nitrate Application on Amaranthus powellii Wats. : I. Changes in Photosynthesis, Growth Rates, and Leaf Area. Plant Physiol. 1985 Nov;79(3):609–613. doi: 10.1104/pp.79.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F., Pearcy R. W. The Nitrogen Use Efficiency of C(3) and C(4) Plants: I. Leaf Nitrogen, Growth, and Biomass Partitioning in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987 Jul;84(3):954–958. doi: 10.1104/pp.84.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F., Pearcy R. W. The Nitrogen Use Efficiency of C(3) and C(4) Plants: II. Leaf Nitrogen Effects on the Gas Exchange Characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987 Jul;84(3):959–963. doi: 10.1104/pp.84.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D., Wang J., Osmond C. B. Environmental effects on photosynthesis, nitrogen-use efficiency, and metabolite pools in leaves of sun and shade plants. Plant Physiol. 1987 Jul;84(3):796–802. doi: 10.1104/pp.84.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto B., Miyata K., Nakamoto H., Sasakawa H., Sugiyama T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 1990 Apr;92(4):963–969. doi: 10.1104/pp.92.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. C., Cowan I. R., Farquhar G. D. Leaf Conductance in Relation to Rate of CO(2) Assimilation: I. Influence of Nitrogen Nutrition, Phosphorus Nutrition, Photon Flux Density, and Ambient Partial Pressure of CO(2) during Ontogeny. Plant Physiol. 1985 Aug;78(4):821–825. doi: 10.1104/pp.78.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]


