Abstract
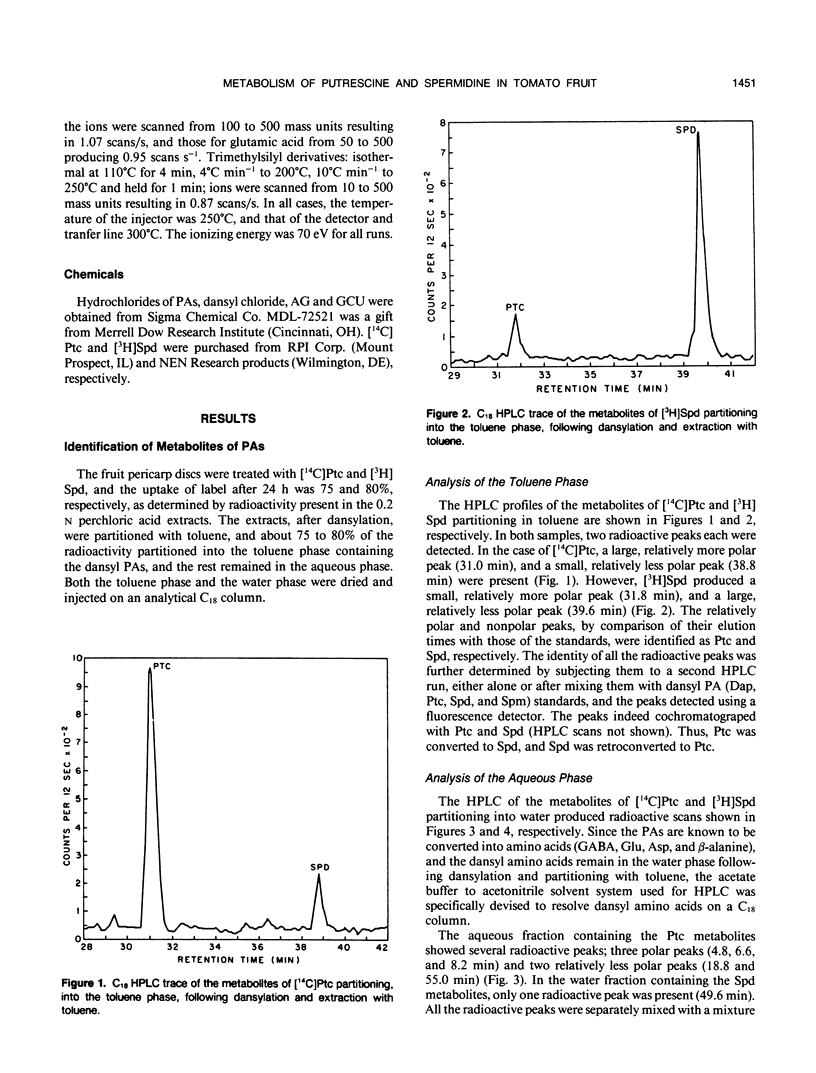
The metabolism of [1,4-14C]putrescine and [terminal methylene-3H]spermidine was studied in the fruit pericarp (breaker stage) discs of tomato (Lycopersicon esculentum Mill.) cv Rutgers, and the metabolites identified by high performance liquid chromatography and gas chromatography-mass spectrometry. The metabolism of both putrescine and spermidine was relatively slow; in 24 hours about 25% of each amine was metabolized. The 14C label from putrescine was incorporated into spermidine, γ-aminobutyric acid (GABA), glutamic acid, and a polar fraction eluting with sugars and organic acids. In the presence of gabaculine, a specific inhibitor of GABA:pyruvate transaminase, the label going into glutamic acid, sugars and organic acids decreased by 80% while that in GABA increased about twofold, indicating that the transamination reaction is probably a major fate of GABA produced from putrescine in vivo. [3H]Spermidine was catabolized into putrescine and β-alanine. The conversion of putrescine into GABA, and that of spermidine into putrescine, suggests the presence of polyamine oxidizing enzymes in tomato pericarp tissues. The possible pathways of putrescine and spermidine metabolism are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Canellakis Z. N., Applewhite P. B., Kaur-Sawhney R., Galston A. W. Binding of spermidine to a unique protein in thin-layer tobacco tissue culture. Plant Physiol. 1988;88:996–998. doi: 10.1104/pp.88.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Bitonti A. J., McCann P. P. Assaying ornithine and arginine decarboxylases in some plant species. Plant Physiol. 1985 Oct;79(2):509–514. doi: 10.1104/pp.79.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble A. R., Davies P. J., Mutschler M. A. Polyamine content of long-keeping alcobaca tomato fruit. Plant Physiol. 1988 Feb;86(2):338–340. doi: 10.1104/pp.86.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Miyazawa S., Endo A. Isolation and inhibitory activity of gabaculine, a new potent inhibitor of gamma-aminobutyrate aminotransferase produced by a Streptomyces. FEBS Lett. 1977 Apr 15;76(2):207–210. doi: 10.1016/0014-5793(77)80153-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Horikoshi K. Purification and characterization of extracellular polyamine oxidase produced by Penicillium sp. no. PO-1. Biochim Biophys Acta. 1982 Jul 26;705(2):133–138. doi: 10.1016/0167-4838(82)90171-6. [DOI] [PubMed] [Google Scholar]
- Michaels R., Kim K. H. Comparative studies of putrescine degradation by microorganisms. Biochim Biophys Acta. 1966 Jan 25;115(1):59–64. doi: 10.1016/0304-4165(66)90048-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Al-Therib M. J. Putrescine catabolism in mammalian brain. Biochem J. 1974 Oct;144(1):29–35. doi: 10.1042/bj1440029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J. 1985 Jan 1;225(1):219–226. doi: 10.1042/bj2250219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., al-Therib M. J., Kataoka K. Formation of GABA from putrescine in the brain of fish (Salmo irideus Gibb.). J Neurochem. 1973 Mar;20(3):699–708. doi: 10.1111/j.1471-4159.1973.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Smith T. A. Polyamine oxidase in higher plants. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1452–1456. doi: 10.1016/0006-291x(70)90549-8. [DOI] [PubMed] [Google Scholar]
- Streeter J. G., Thompson J. F. In Vivo and In Vitro Studies on gamma-Aminobutyric Acid Metabolism with the Radish Plant (Raphanus sativus, L.). Plant Physiol. 1972 Apr;49(4):579–584. doi: 10.1104/pp.49.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tago K., Kurioka S., Matsuda M. 4-Aminobutyraldehyde dehydrogenase activity in rat brain. J Neurochem. 1982 Sep;39(3):803–809. doi: 10.1111/j.1471-4159.1982.tb07963.x. [DOI] [PubMed] [Google Scholar]


