Abstract
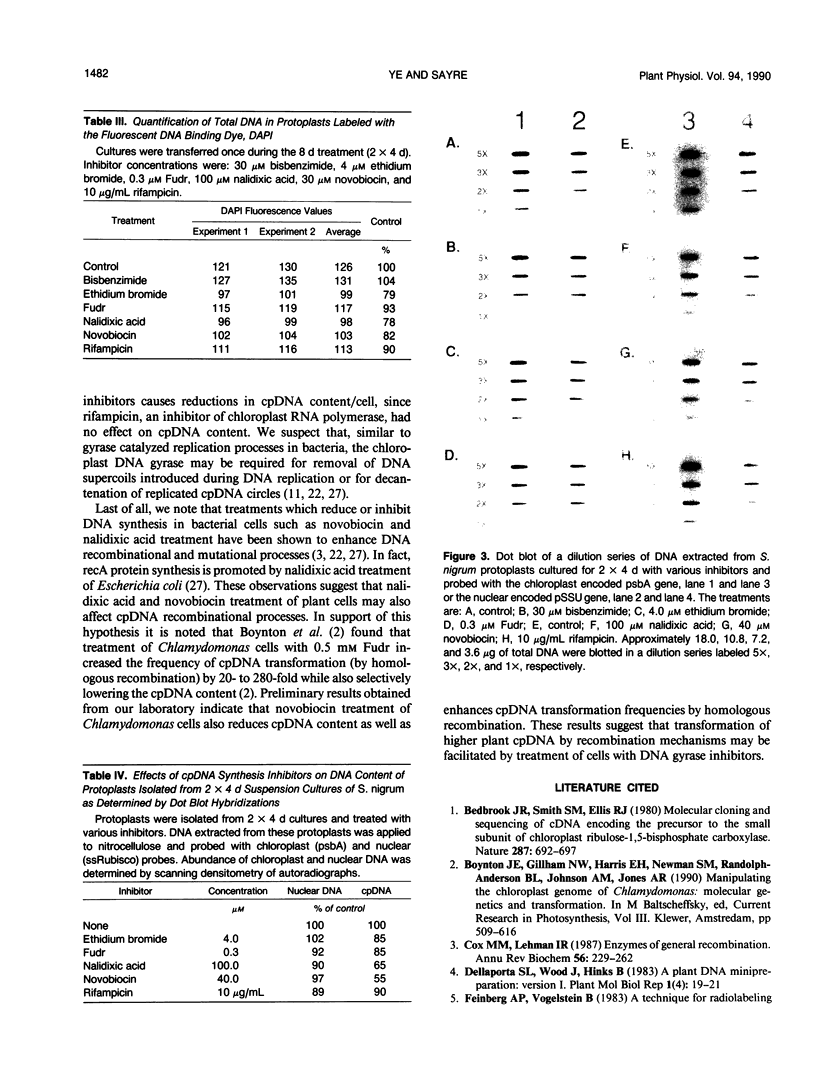
Suspension cell cultures of Solanum nigrum were grown in the presence of six different chloroplast DNA synthesis inhibitors in order to determine whether the pool size of chloroplast DNA (cpDNA) could be selectively reduced relative to the nuclear DNA content. One of the effects of the inhibitors was a reduction in cell growth and viability. Cell growth (fresh weight) was reduced 50% (in 8 day cultures) by: 100 micromolar bisbenzimide, 8 micromolar ethidium bromide, 0.3 micromolar 5-fluordeoxyuridine (Fudr), 200 micromolar nalidixic acid, 30 micromolar novobiocin, or 10 micrograms per milliliter rifampicin. At these concentrations, three of the inhibitors, ethidium bromide, Fudr, and rifampicin, also substantially reduced the viability of the cultures. Analyses of the chloroplast and nuclear DNA content per gram fresh weight by dot blot hybridizations indicated that the reduction of cpDNA content was greatest at inhibitor concentrations which reduced cell growth by more than 50% but this depended on the culture conditions. For example, the two DNA gyrase inhibitors, nalidixic acid and novobiocin, were more effective in lowering cpDNA content in cultures which were transferred (2 × 4 days) once during the eight day incubation. Because several inhibitors were toxic to cell growth, the DNA content of treated cells was also determined on the basis of cell (protoplasts) number. Analyses of nuclear and cpDNA content per cell for each treatment indicated that only the DNA gyrase inhibitors, nalidixic acid, and novobiocin reduced cpDNA content. Neither inhibitor reduced nuclear DNA content. These results suggest that DNA gyrases participate in cpDNA replication. The selective reduction of cpDNA content in regeneratable cultures may facilitate the generation and selection of cpDNA mutants or transformants from higher plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Sager R. Ethidium bromide induced selective and reversible loss of chloroplast DNA. Nat New Biol. 1973 Feb 28;241(113):277–279. doi: 10.1038/newbio241277a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinhorst S., Cannon G., Weissbach A. Chloroplast DNA synthesis during the cell cycle in cultured cells of Nicotiana tabacum: inhibition by nalidixic acid and hydroxyurea. Arch Biochem Biophys. 1985 Jun;239(2):475–479. doi: 10.1016/0003-9861(85)90714-3. [DOI] [PubMed] [Google Scholar]
- Lockshon D., Morris D. R. Positively supercoiled plasmid DNA is produced by treatment of Escherichia coli with DNA gyrase inhibitors. Nucleic Acids Res. 1983 May 25;11(10):2999–3017. doi: 10.1093/nar/11.10.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman H., Jupp A. S., Larrinua I. Action of Nalidixic Acid on Chloroplast Replication in Euglena gracilis. Plant Physiol. 1975 Feb;55(2):390–392. doi: 10.1104/pp.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman H. Specific inhibition of chloroplast replication in Euglena gracilis by nalidixic acid. J Cell Biol. 1967 Dec;35(3):726–730. doi: 10.1083/jcb.35.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P., Walfield A., Hershberger C. L. Effect of nalidixic acid on Euglena gracilis: induced loss of chloroplast deoxyribonucleic acid. Arch Biochem Biophys. 1974 Dec;165(2):548–553. doi: 10.1016/0003-9861(74)90281-1. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Crossland L. D., Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzycki S. J. Genetic functions of the chloroplast of Chlamydomonas reinhardi: effect of rifampin on chloroplast DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1327–1334. doi: 10.1073/pnas.63.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J Bacteriol. 1987 Apr;169(4):1731–1736. doi: 10.1128/jb.169.4.1731-1736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz E. A., Sears B. B., Rabert D. K., Shepherd H. S., Gillham N. W., Boynton J. E. A specific increase in chloroplast gene mutations following growth of Chlamydomonas in 5-fluorodeoxyuridine. Mol Gen Genet. 1979 Mar 5;170(3):235–242. doi: 10.1007/BF00267056. [DOI] [PubMed] [Google Scholar]