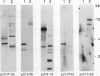
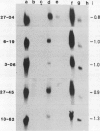
Abstract
Leaves of resurrection plants tolerate desiccation as do embryos of many higher plants. From the resurrection plant Craterostigma plantagineum a number of desiccation-related transcripts have recently been cloned; they are abundantly expressed in dried leaves and abscisic acid-treated dried callus (D Bartels, K Schneider, G Terstappen, D Piatkowski, F Salamini [1990] Planta 18: 27-34). Five distinct cDNA clones representing low copy number genes were selected for further characterization. Their nucleotide sequences were determined and proteins were predicted with a molecular mass between 16 and 34 kilodaltons. Three of these proteins have unusual amino acid compositions and extreme hydrophilic characters. Two of them contain a cluster of contiguous serine residues and lysine-rich repeats. These sequence motifs display homologies to desiccation-related genes expressed in embryos or dehydrated seedlings of several plants. A third cDNA clone contains tracts of sequences which are related to a cotton Lea (late embryogenesis abundant) gene (JC Baker, C Steele, L Dure III [1988] Plant Mol Biol II: 277-291). Secondary structure predictions are discussed and suggest that the deduced proteins could play a role in protecting core cell structures in a dehydrated cell. It is concluded that at least in part the gene products involved in the desiccation-induced pathways are common to leaves of resurrection plants and embryos. Two cDNA clones appear to code for Craterostigma-specific mRNAs. The expression patterns of all five transcripts were studied in comparison to desiccated leaves in dehydrated roots, in wound-stressed leaves and in salt-stressed callus. The data obtained point to the possibility that not only specificity of induction but also the expression level of specific gene products may be of importance for osmoprotection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels D., Thompson R. D. The characterization of cDNA clones coding for wheat storage proteins. Nucleic Acids Res. 1983 May 25;11(10):2961–2977. doi: 10.1093/nar/11.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikhel N. V., Wilkins T. A. Isolation and characterization of a cDNA clone encoding wheat germ agglutinin. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6745–6749. doi: 10.1073/pnas.84.19.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J., Goday A., Freire M. A., Torrent M., Martínez M. C., Torné J. M., Pagès M. Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol. 1990 Mar;14(3):423–432. doi: 10.1007/BF00028778. [DOI] [PubMed] [Google Scholar]
- Vingron M., Argos P. A fast and sensitive multiple sequence alignment algorithm. Comput Appl Biosci. 1989 Apr;5(2):115–121. doi: 10.1093/bioinformatics/5.2.115. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Mundy J., Chua N. H. Four tightly linked rab genes are differentially expressed in rice. Plant Mol Biol. 1990 Jan;14(1):29–39. doi: 10.1007/BF00015652. [DOI] [PubMed] [Google Scholar]