Abstract
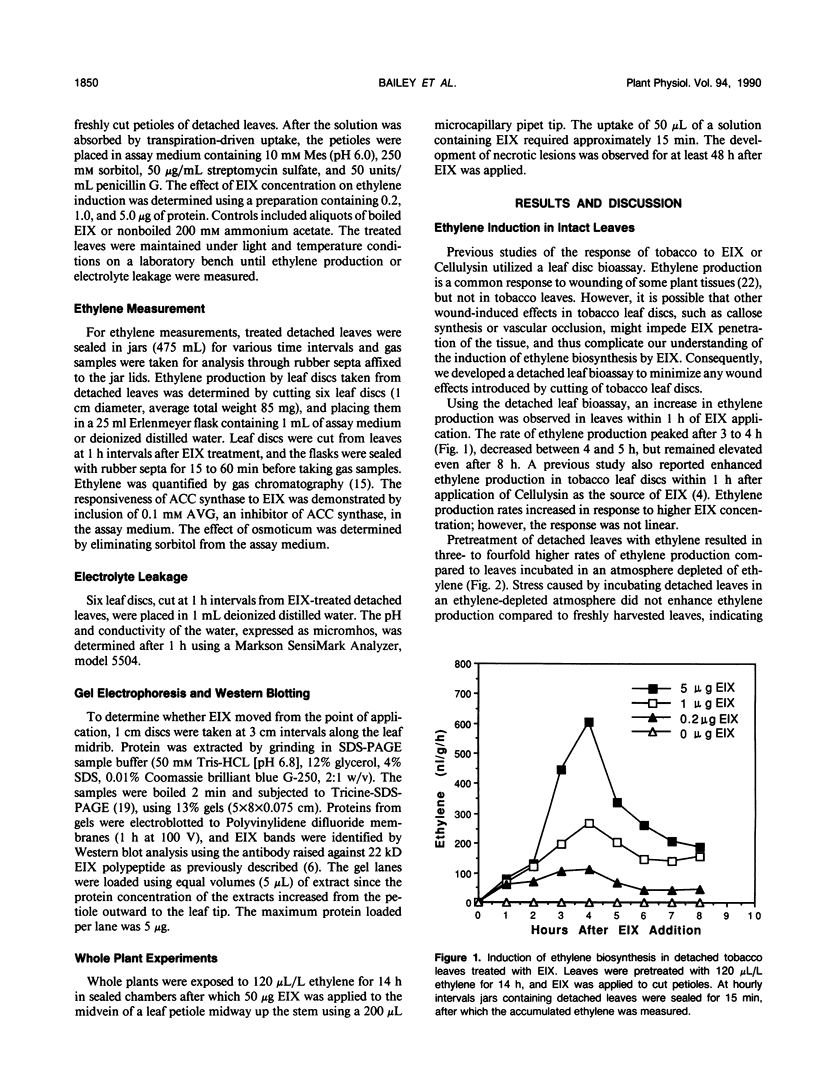
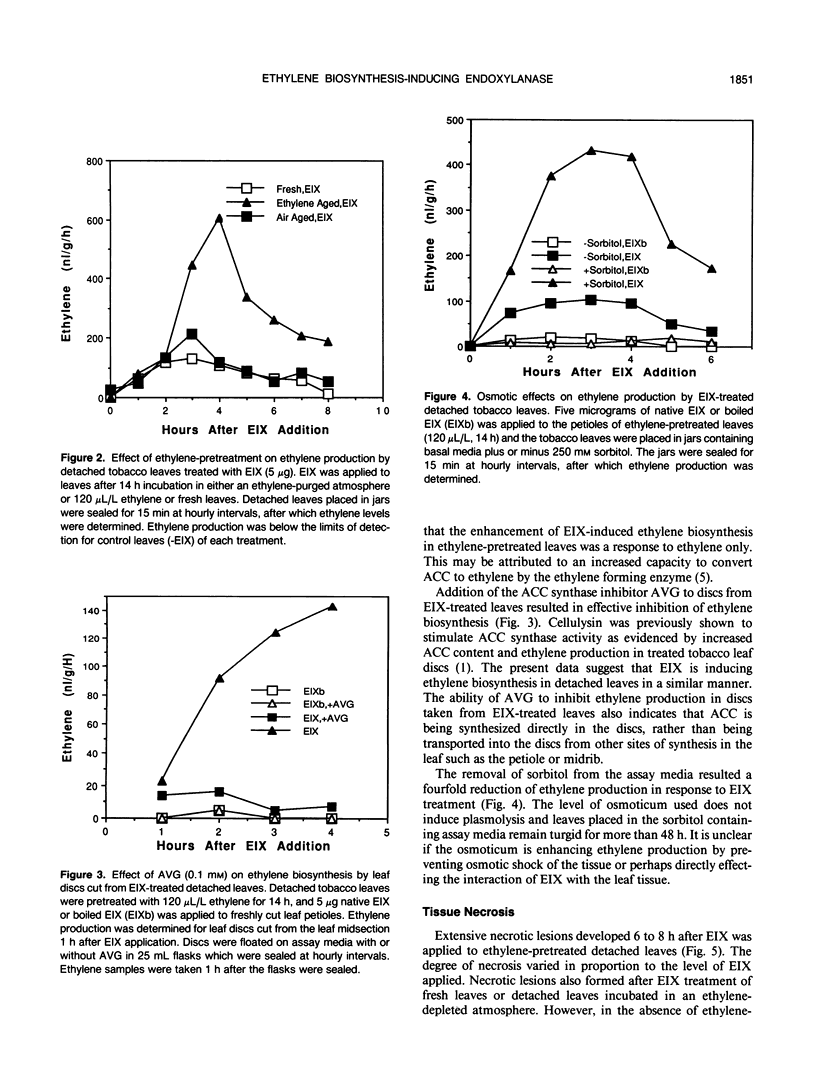
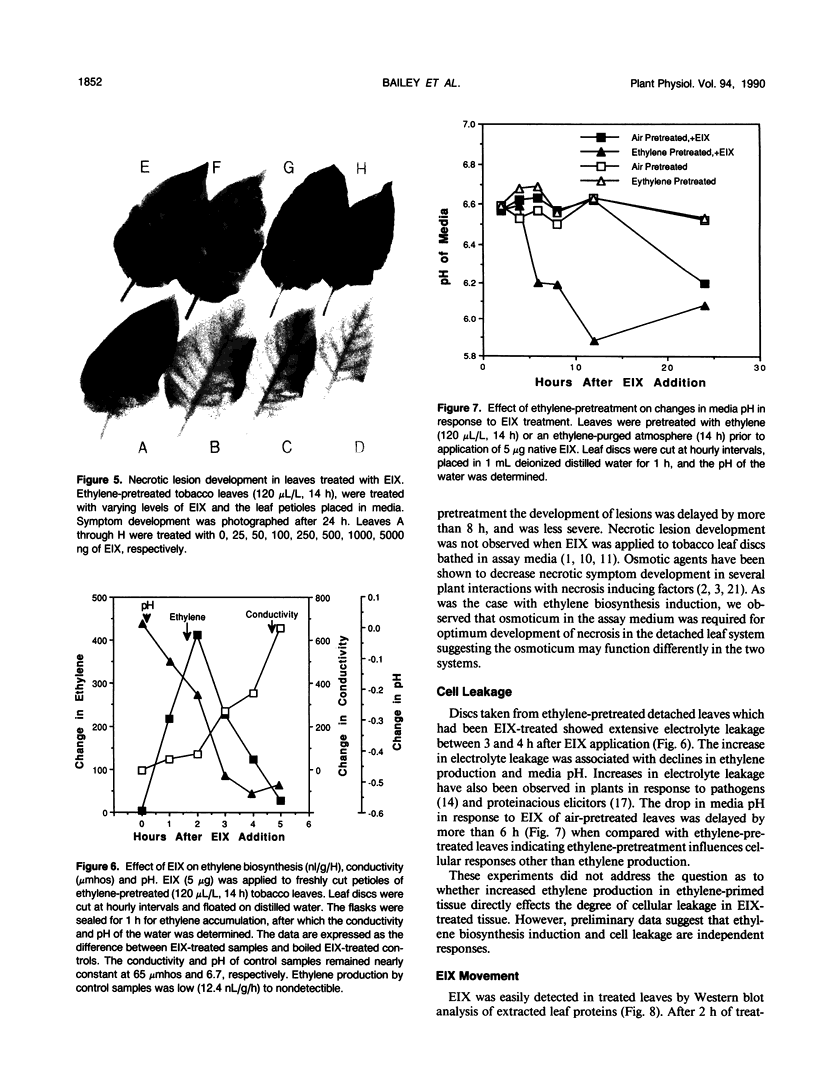
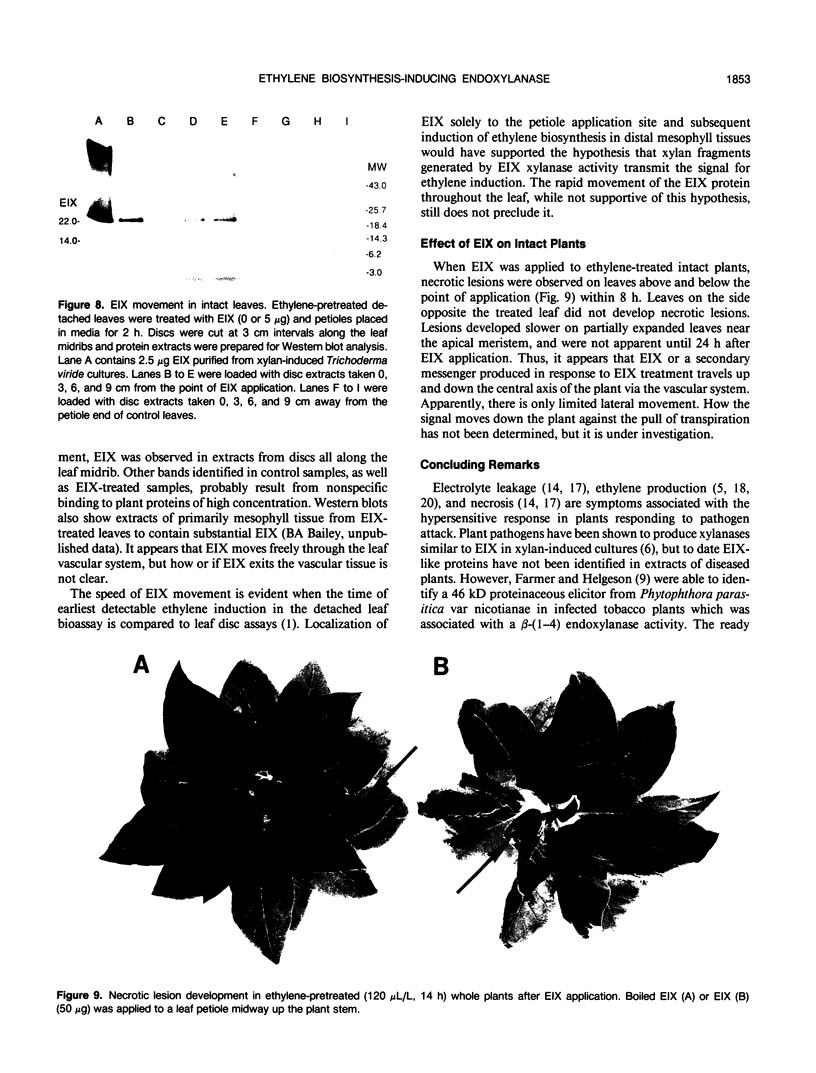
We have previously demonstrated that a protein purified from xylan-induced culture filtrates of Trichoderma viride contains β-1,4-endoxylanase activity and induces ethylene biosynthesis in tobacco (Nicotiana tabacum cv Xanthi) leaf discs. When the ethylene biosynthesis-inducing xylanase (EIX) was applied to cut petioles of detached tobacco leaves, it induced ethylene biosynthesis within 1 hour and extensive electrolyte leakage and necrosis were observed in tobacco leaf tissue within 5 hours. Ethylene-pretreatment (120 microliters per liter ethylene for 14 hours) of tobacco leaves enhanced ethylene biosynthesis in response to EIX by more than threefold and accelerated development of cellular leakage and necrosis. In intact plants, similar symptoms could be induced in leaves that were distant from the point of the enzyme application. The evidence suggests that EIX is translocated via the vascular system and elicits plant responses similar to those observed in a hypersensitive response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Mattoo A. K., Lieberman M. Induction of ethylene biosynthesis in tobacco leaf discs by cell wall disesting enzymes. Biochem Biophys Res Commun. 1982 Jul 30;107(2):588–596. doi: 10.1016/0006-291x(82)91532-7. [DOI] [PubMed] [Google Scholar]
- Chalutz E., Mattoo A. K., Solomos T., Anderson J. D. Enhancement by ethylene of cellulysin-induced ethylene production by tobacco leaf discs. Plant Physiol. 1984 Jan;74(1):99–103. doi: 10.1104/pp.74.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Helgeson J. P. An Extracellular Protein from Phytophthora parasitica var nicotianae Is Associated with Stress Metabolite Accumulation in Tobacco Callus. Plant Physiol. 1987 Nov;85(3):733–740. doi: 10.1104/pp.85.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Anderson J. D. Purification and characterization of ethylene inducing proteins from cellulysin. Plant Physiol. 1987 Jul;84(3):732–736. doi: 10.1104/pp.84.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Saxena A., Gamble H. R., Anderson J. D. Ethylene biosynthesis-inducing protein from cellulysin is an endoxylanase. Plant Physiol. 1989 Jan;89(1):138–143. doi: 10.1104/pp.89.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S. Factors Influencing Protoplast Viability of Suspension-Cultured Rice Cells during Isolation Process. Plant Physiol. 1988 Sep;88(1):26–29. doi: 10.1104/pp.88.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T., Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990 Jun;93(2):811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever T. L., Higgins V. J. Electrolyte Leakage, Lipoxygenase, and Lipid Peroxidation Induced in Tomato Leaf Tissue by Specific and Nonspecific Elicitors from Cladosporium fulvum. Plant Physiol. 1989 Jul;90(3):867–875. doi: 10.1104/pp.90.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki N., Fry S. C., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXIV. Fragments Isolated from Suspension-Cultured Sycamore Cell Walls Inhibit the Ability of the Cells to Incorporate [C]Leucine into Proteins. Plant Physiol. 1983 Jul;72(3):864–869. doi: 10.1104/pp.72.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]





