Abstract
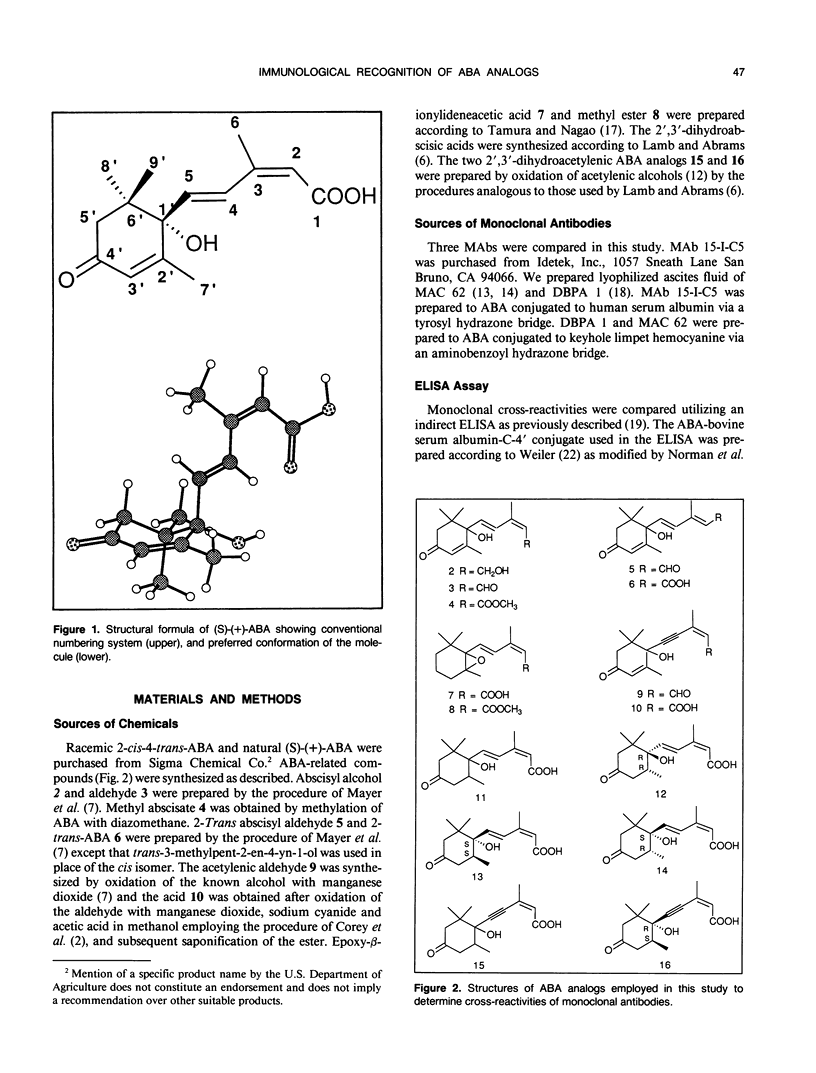
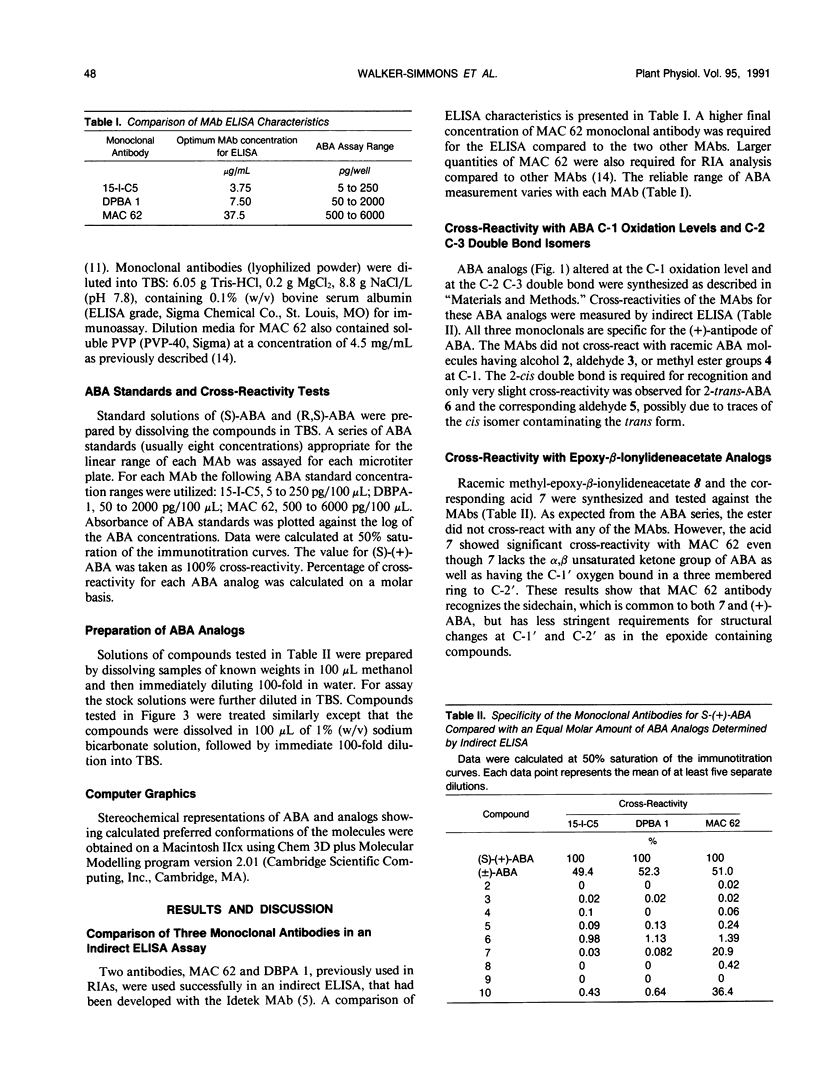
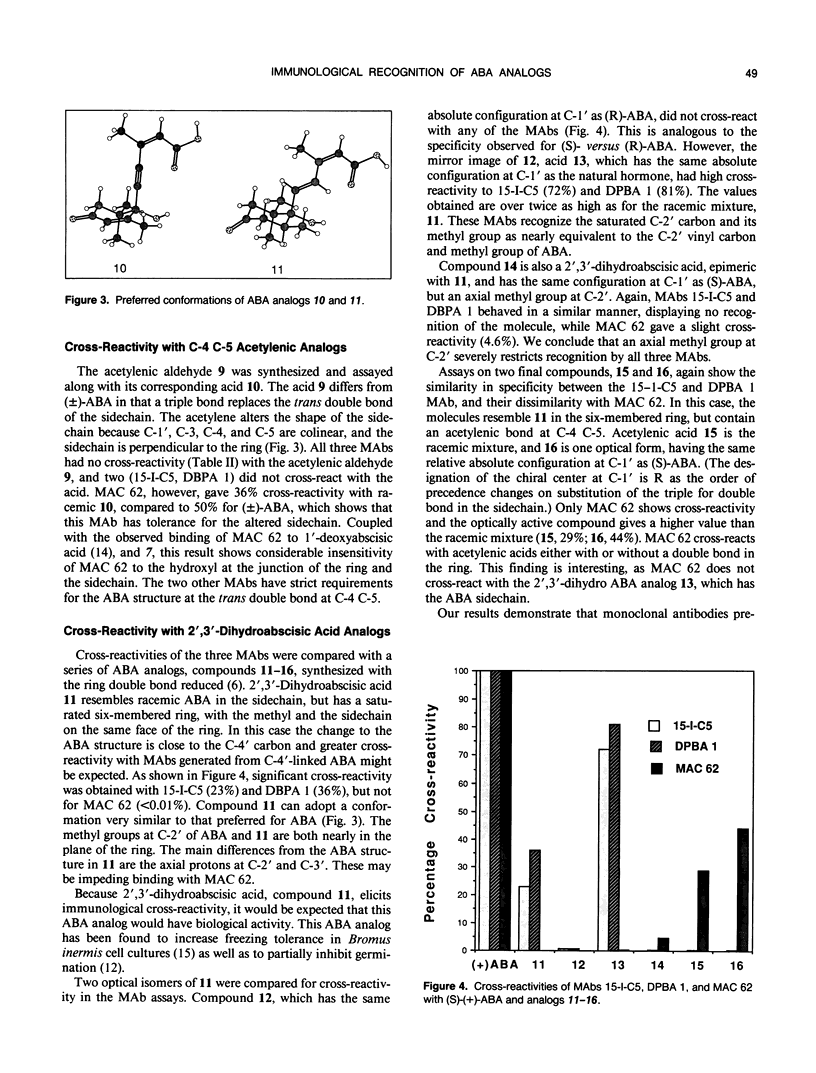
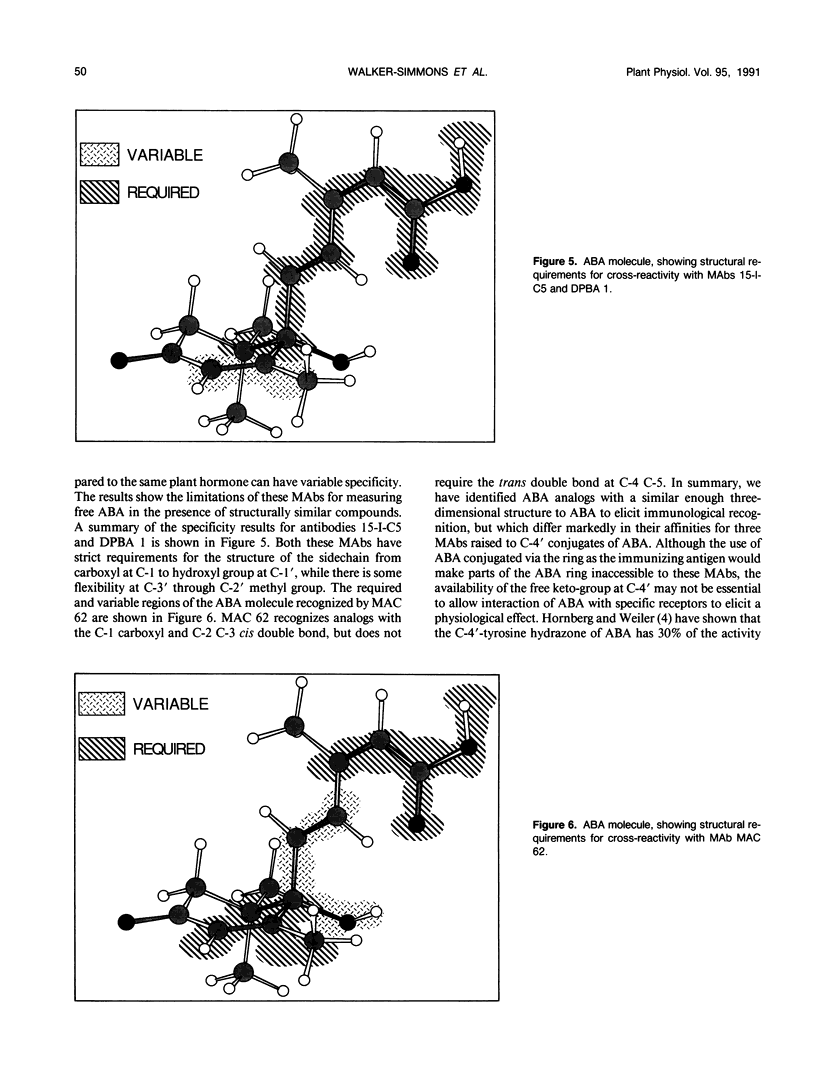
Specificities of three monoclonal antibodies (15-I-C5, DBPA 1, and MAC 62) raised against the plant hormone (S)-(+)-abscisic acid (ABA) have been compared. Immunological cross-reactivities against fifteen biologically active analogs of ABA were measured. The ABA analogs were altered at one or more of four positions: the double bonds in the ring, at C-2 C-3 and at C-4 C-5, and in the oxidation level at C-1. Several analogs were optically active with chiral centers at C-1′ and C-2′. For cross-reactivity, all three monoclonal antibodies required the carboxylic acid group, and the cis configuration of the double bond at C-2 C-3 of the ABA molecule. Monoclonals 15-I-C5 and DBPA 1 required the entire ABA sidechain from the C-1 to C-1′, but these monoclonals did cross-react with analogs with the ring double bond reduced and the C-2′ methyl cis to the sidechain. Only MAC 62 recognized analogs containing an acetylene at C-4 C-5. MAC 62 had more strict requirements for the ring double bond, but gave some cross-reactivity with acetylenic analogs having a saturated ring. All three monoclonals had higher specificity for analogs having the same absolute configuration at C-1′ as (S)-(+)-ABA. This work provides new information about the spatial regions of the ABA molecule that elicit immunological recognition, and serves as a basis for future investigations of the ABA receptor using ABA analogs and anti-idiotypic antibodies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gaulton G. N., Greene M. I. Idiotypic mimicry of biological receptors. Annu Rev Immunol. 1986;4:253–280. doi: 10.1146/annurev.iy.04.040186.001345. [DOI] [PubMed] [Google Scholar]
- Milborrow B. V. The conformation of abscisic acid by n.m.r. and a revision of the proposed mechanism for cyclization during its biosynthesis. Biochem J. 1984 May 15;220(1):325–332. doi: 10.1042/bj2200325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarrie S. A., Galfre G. Use of different hapten-protein conjugates immobilized on nitrocellulose to screen monoclonal antibodies to abscisic acid. Anal Biochem. 1985 Dec;151(2):389–399. doi: 10.1016/0003-2697(85)90193-9. [DOI] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M. ABA Levels and Sensitivity in Developing Wheat Embryos of Sprouting Resistant and Susceptible Cultivars. Plant Physiol. 1987 May;84(1):61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


