Abstract
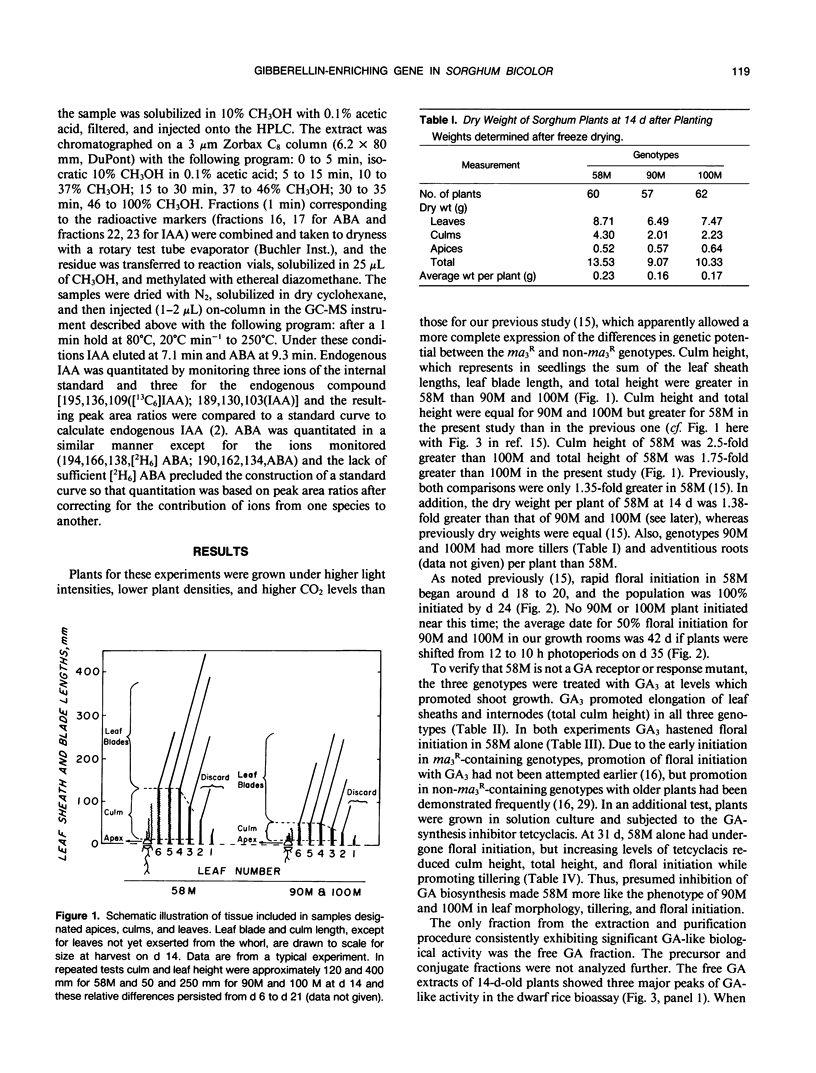
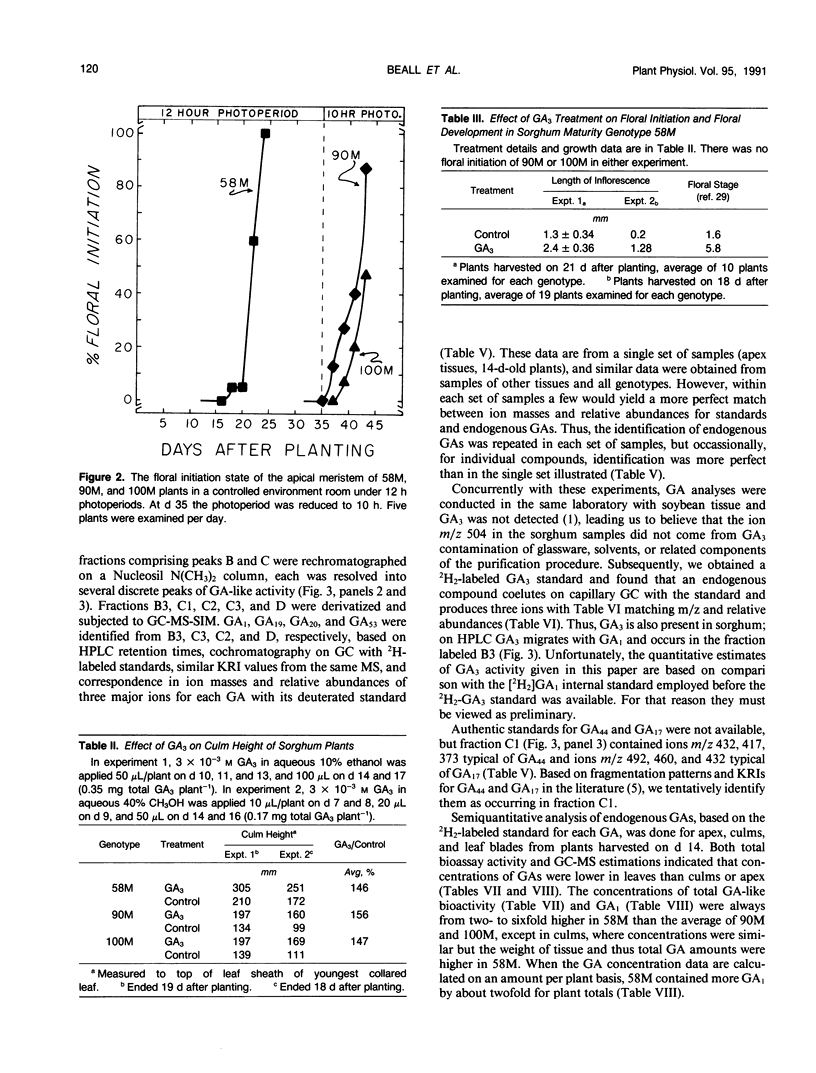
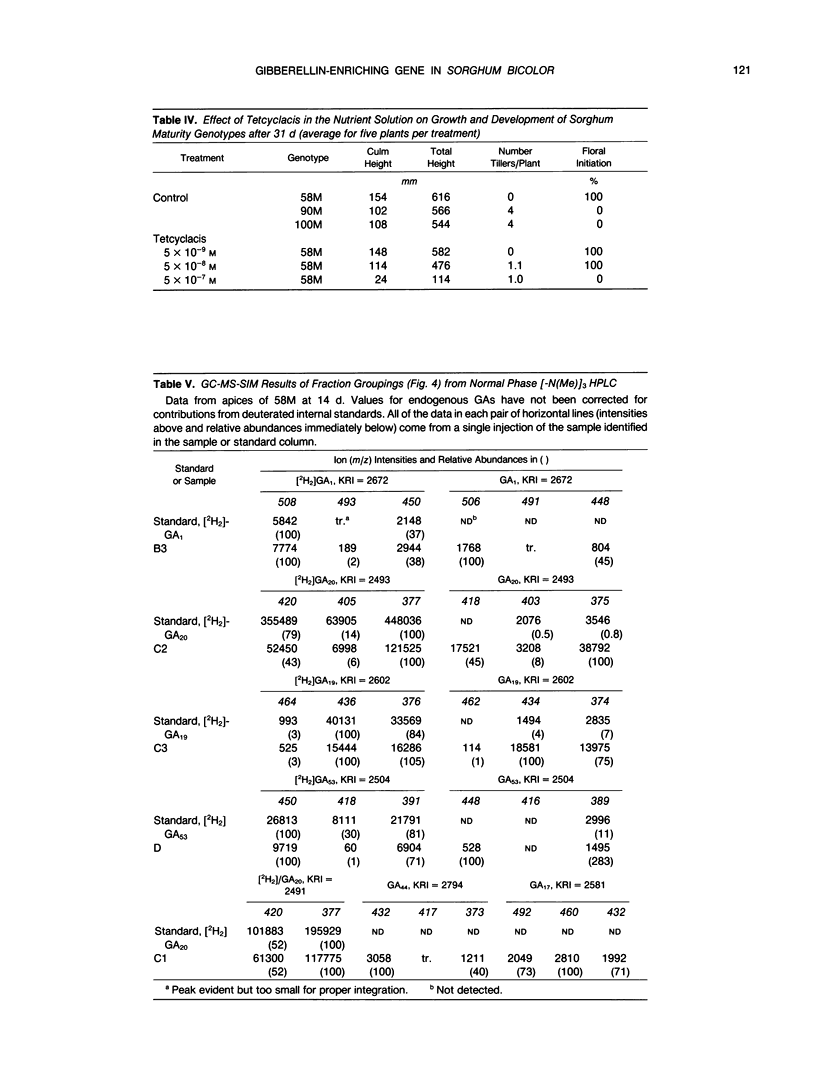
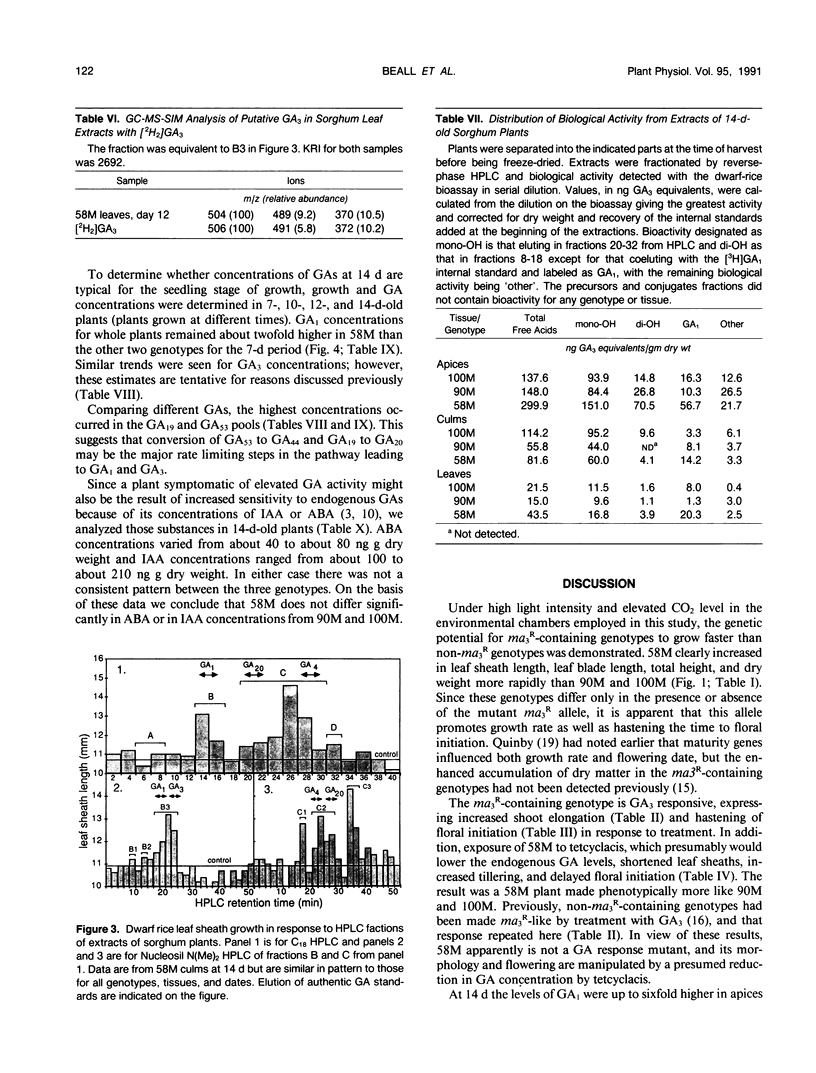
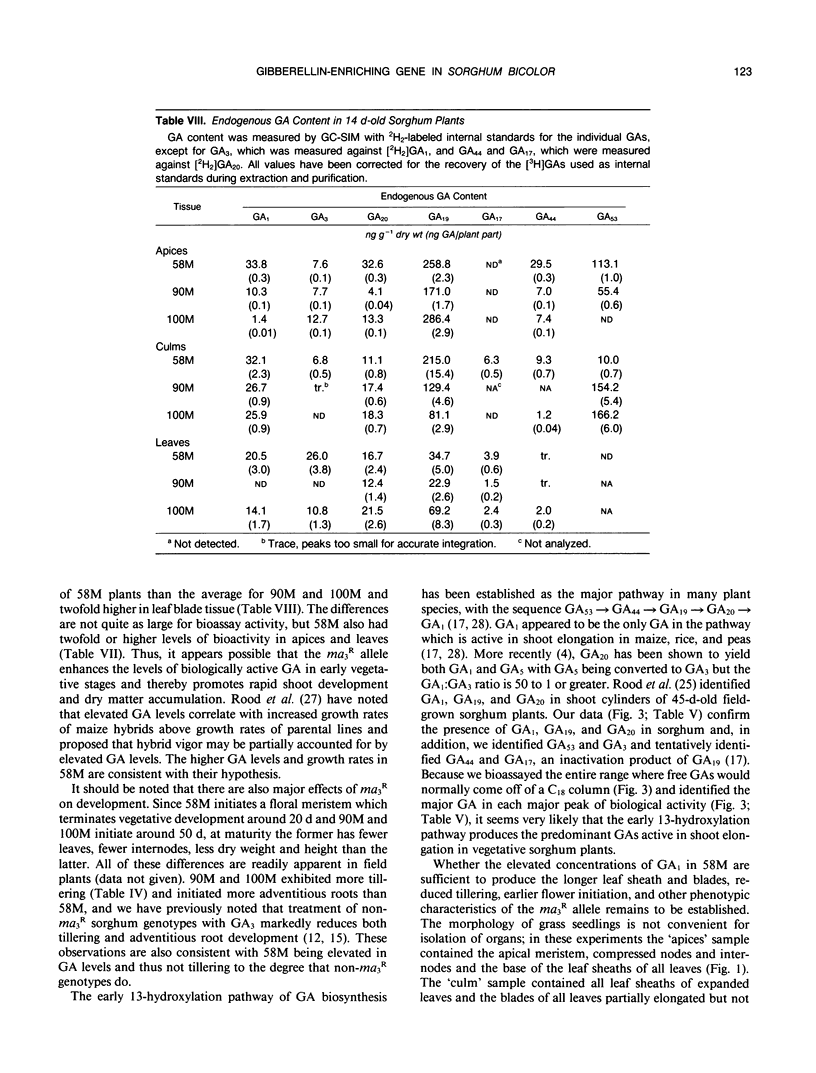
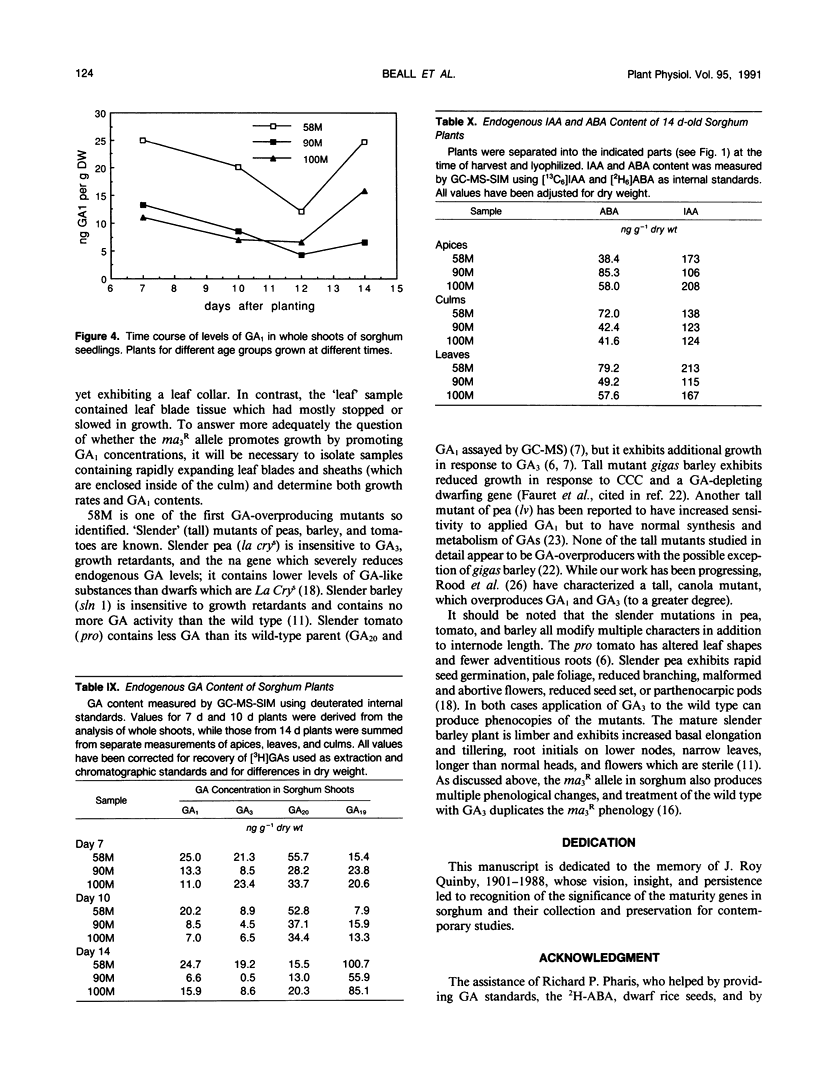
Sorghum bicolor genotypes, near isogenic with different alleles at the third maturity locus, were compared for development, for responsiveness to GA3 and a GA synthesis inhibitor, and occurrence and concentrations of endogenous GAs, IAA, and ABA. At 14 days the genotype 58M (ma3Rma3R) exhibited 2.5-fold greater culm height, 1.75-fold greater total height, and 1.38-fold greater dry weight than 90M (ma3ma3) or 100M (Ma3Ma3). All three genotypes exhibited similar shoot elongation in response to GA3, and 58M showed GA3-mediated hastening of floral initiation when harvested at day 18 or 21. Both 90M and 100M had exhibited hastening of floral initiation by GA3 previously, at later application dates. Tetcyclacis reduced height, promoted tillering, and delayed flowering of 58M resulting in plants which were near phenocopies of 90M and 100M. Based on bioassay activity, HPLC retention times, cochromatography with 2H2-labeled standards on capillary column GC and matching mass spectrometer fragmentation patterns (ions [m/z] and relative abundances), GA1, GA19, GA20, GA53, and GA3 were identified in extracts of all three genotypes. In addition, based on published Kovats retention index values and correspondence in ion masses and relative abundances, GA44 and GA17 were detected. Quantitation was based on recovery of coinjected, 2H2-labeled standards. In 14 day-old-plants, total GA-like bioactivity and GA1 concentrations (nanograms GA/gram dry weight) were two- to six-fold higher in 58M than 90M and 100M in leaf blades, apex samples, and whole plants while concentrations in culms were similar. Similar trends occurred if data were expressed on a per plant basis. GA1 concentrations for whole plants were about two-fold higher in 58M than 90M and 100M from day 7 to day 14. Concentrations of ABA and IAA did not vary between the genotypes. The results indicate the mutant allele ma3R causes a two- to six-fold increase in GA1 concentrations, does not result in a GA-receptor or transduction mutation and is associated with phenotypic characteristics that can be enhanced by GA3 and reduced by GA synthesis inhibitor. These observations support the hypothesis that the allele ma3R causes an overproduction of GAs which results in altered leaf morphology, reduced tillering, earlier flowering, and other phenotypic differences between 58M and 90M or 100M.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensen R. J., Beall F. D., Mullet J. E., Morgan P. W. Detection of endogenous gibberellins and their relationship to hypocotyl elongation in soybean seedlings. Plant Physiol. 1990 Sep;94(1):77–84. doi: 10.1104/pp.94.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Baldi B. G., Slovin J. P. C(6)-[benzene ring]-indole-3-acetic Acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic Acid in plants. Plant Physiol. 1986 Jan;80(1):14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S., Yamane H., Spray C. R., Gaskin P., Macmillan J., Phinney B. O., Takahashi N. Qualitative and Quantitative Analyses of Gibberellins in Vegetative Shoots of Normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 Seedlings of Zea mays L. Plant Physiol. 1988 Dec;88(4):1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshioka M., Takeno K., Beall F. D., Pharis R. P. Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol. 1983 Oct;73(2):398–406. doi: 10.1104/pp.73.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi S., Muir R. M. Increase in Diffusible Auxin after Treatment with Gibberellin. Science. 1962 Sep 7;137(3532):760–761. doi: 10.1126/science.137.3532.760. [DOI] [PubMed] [Google Scholar]
- Pao C. I., Morgan P. W. Genetic Regulation of Development in Sorghum bicolar: I. Role of the Maturity Genes. Plant Physiol. 1986 Oct;82(2):575–580. doi: 10.1104/pp.82.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. I., Morgan P. W. Genetic Regulation of Development in Sorghum bicolar: I. Role of the Maturity Genes. Plant Physiol. 1986 Oct;82(2):575–580. doi: 10.1104/pp.82.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. I., Morgan P. W. Genetic Regulation of Development in Sorghum bicolor: II. Effect of the ma(3) Allele Mimicked by GA(3). Plant Physiol. 1986 Oct;82(2):581–584. doi: 10.1104/pp.82.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Blake T. J., Pharis R. P. Gibberellins and Heterosis in Maize : II. Response to Gibberellic Acid and Metabolism of [H]Gibberellin A(20). Plant Physiol. 1983 Mar;71(3):645–651. doi: 10.1104/pp.71.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood S. B., Williams P. H., Pearce D., Murofushi N., Mander L. N., Pharis R. P. A mutant gene that increases gibberellin production in brassica. Plant Physiol. 1990 Jul;93(3):1168–1174. doi: 10.1104/pp.93.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]


