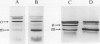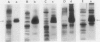Abstract
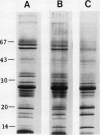
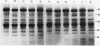
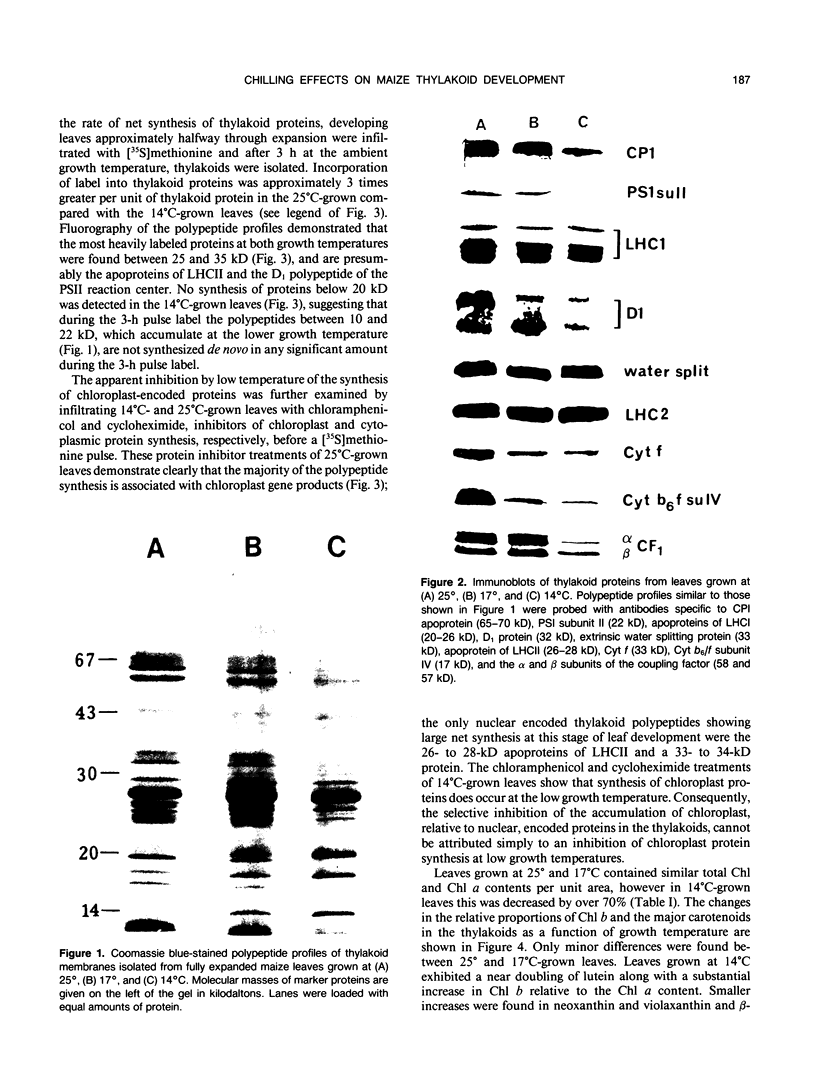
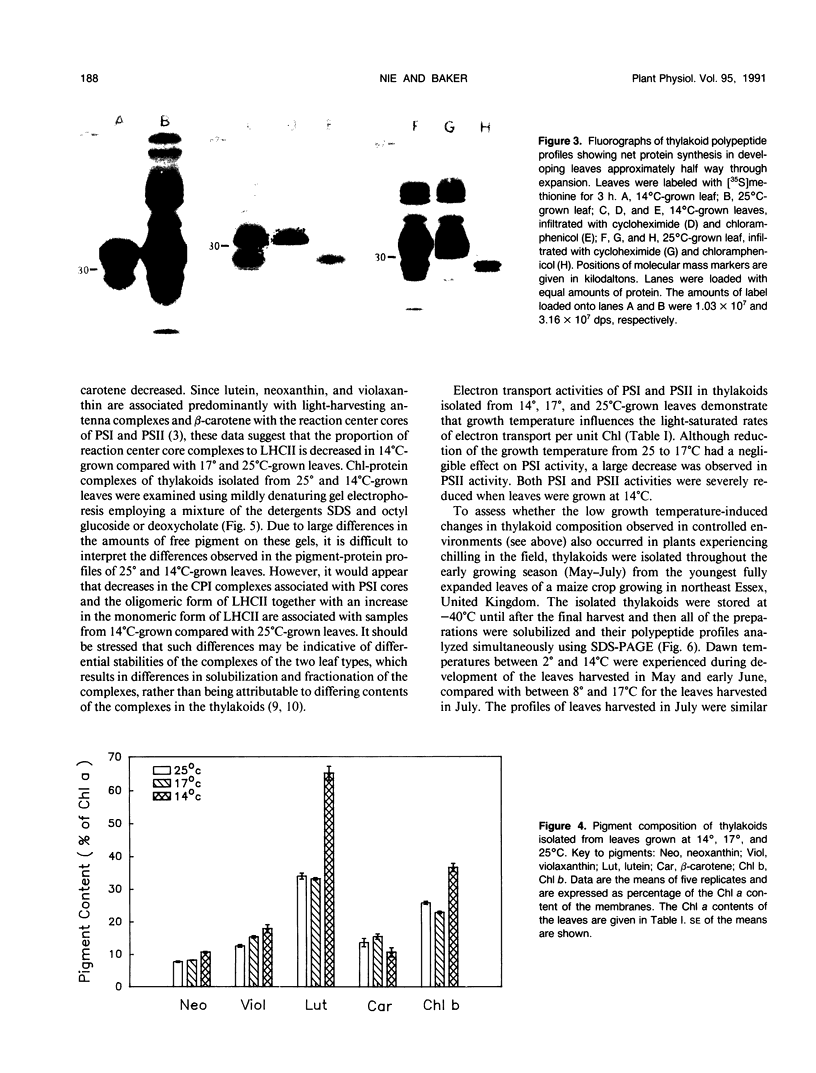
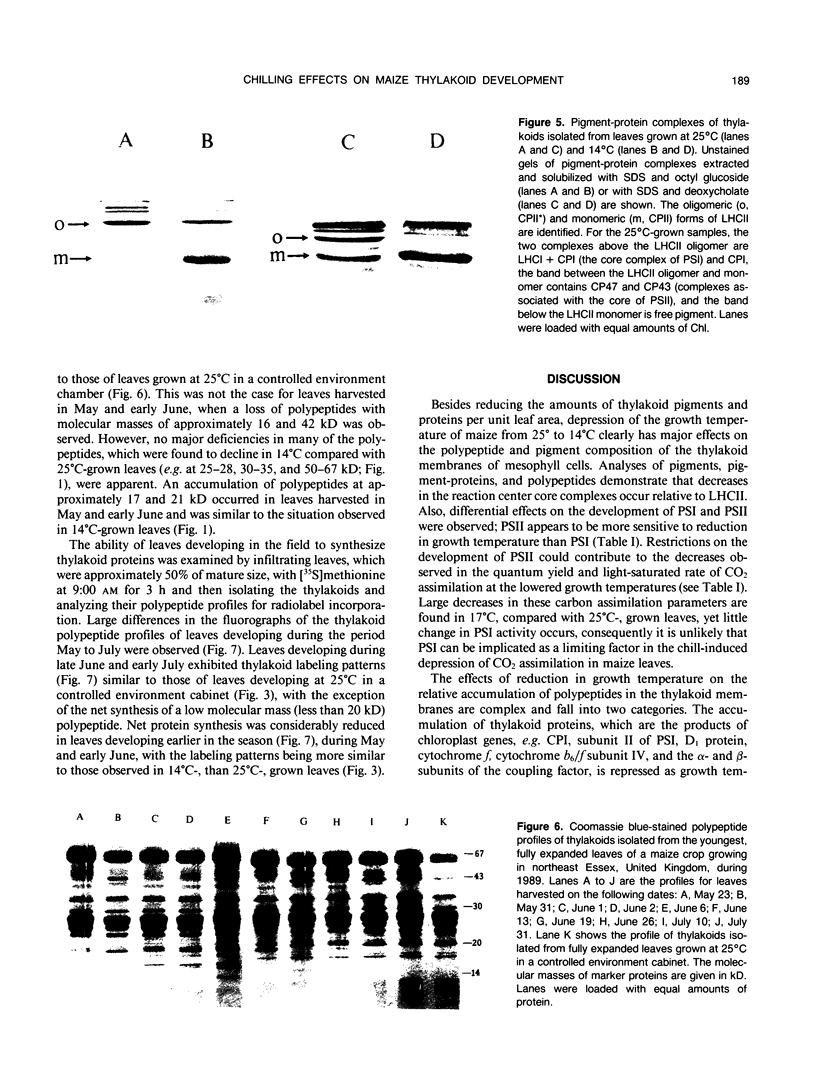
The effects of reductions in growth temperature on the development of thylakoids of maize (Zea mays var LG11) leaves are examined. Thylakoids isolated from mesophyll cells of leaves grown at 17° and 14°C, compared with 25°C, exhibited a decreased accumulation of many polypeptides, which was accompanied by a loss of activity of photosystems (PS) I and II. Probing the polypeptide profiles with a range of antibodies specific for thylakoid proteins demonstrated that a number of polypeptides encoded by the chloroplast genome failed to accumulate at low temperatures. Although thylakoid protein synthesis was reduced severely at 14°C compared with 25°C, major synthesis of both chloroplast and nuclear encoded polypeptides was detected. It is suggested that the lack of accumulation of some thylakoid proteins at low temperatures may be due to an inability to stabilize the proteins in the membranes. A number of thylakoid polypeptides were found to appear as the growth temperature was decreased. Analyses of pigments and polypeptides demonstrated that decreases in the photosystem reaction center core complexes occur relative to the light harvesting complex associated with PS II at reduced growth temperatures. Differential effects on the development of PSI and PSII were also observed, with PSII activity being preferentially reduced. Reductions in PSII content and activity occurred in parallel with decreases in the quantum yield and light-saturated rate of CO2 assimilation. Fractionation of thylakoid pigment-protein complexes showed that the ratio of monomeric:oligomeric form of the light harvesting complex associated with PSII increased at low growth temperature, which is consistent with a chill-induced modification of thylakoid organization. Many, but not all, of the characteristic changes in thylakoid protein metabolism, which were observed when leaves were grown at low temperatures in controlled environments, were identified in leaves of a field maize crop during the early growing season when low temperatures were experienced by the crop. Chill-induced perturbations of thylakoid development can occur in the field in temperate regions and may have implications for the photosynthetic productivity of the crop.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R., Long S. P., Ort D. R. Photosynthesis and temperature, with particular reference to effects on quantum yield. Symp Soc Exp Biol. 1988;42:347–375. [PubMed] [Google Scholar]
- Cooper P., Ort D. R. Changes in protein synthesis induced in tomato by chilling. Plant Physiol. 1988 Oct;88(2):454–461. doi: 10.1104/pp.88.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunahay T. G., Staehelin L. A. Isolation of photosystem I complexes from octyl glucoside/sodium dodecyl sulfate solubilized spinach thylakoids : characterization and reconstitution into liposomes. Plant Physiol. 1985 Jul;78(3):606–613. doi: 10.1104/pp.78.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M., Huner N. P., Hayden D. B. Low temperature development of winter rye leaves alters the detergent solubilization of thylakoid membranes. Plant Physiol. 1986 Jun;81(2):471–477. doi: 10.1104/pp.81.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Walbot V. Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol. 1989 Nov;91(3):930–938. doi: 10.1104/pp.91.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Krol M., Williams J. P., Maissan E., Low P. S., Roberts D., Thompson J. E. Low Temperature Development Induces a Specific Decrease in trans-Delta-Hexadecenoic Acid Content which Influences LHCII Organization. Plant Physiol. 1987 May;84(1):12–18. doi: 10.1104/pp.84.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa Z., Huner N. P., Williams J. P., Maissan E., James D. R. Development at Cold-Hardening Temperatures : The Structure and Composition of Purified Rye Light Harvesting Complex II. Plant Physiol. 1987 May;84(1):19–24. doi: 10.1104/pp.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Basso L., Alberdi M., Raynal M., Ferrero-Cadinanos M. L., Delseny M. Changes in Protein Synthesis in Rapeseed (Brassica napus) Seedlings during a Low Temperature Treatment. Plant Physiol. 1986 Nov;82(3):733–738. doi: 10.1104/pp.82.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano X., Lane D. P. Monoclonal antibody to simian virus 40 small t. J Virol. 1984 Sep;51(3):760–767. doi: 10.1128/jvi.51.3.760-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Waldron J. C., Anderson J. M. Chlorophyll-protein complexes from thylakoids of a mutant barley lacking chlorophyll b. Eur J Biochem. 1979 Dec 17;102(2):357–362. doi: 10.1111/j.1432-1033.1979.tb04250.x. [DOI] [PubMed] [Google Scholar]
- Zilber A. L., Malkin R. Ferredoxin Cross-Links to a 22 kD Subunit of Photosystem I. Plant Physiol. 1988 Nov;88(3):810–814. doi: 10.1104/pp.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]