Abstract
The postnatal mammalian heart undergoes remarkable developmental changes, which are stimulated by the transition from the intrauterine to extrauterine environment. With birth, increased oxygen levels promote metabolic, structural and biophysical maturation of cardiomyocytes, resulting in mature muscle with increased efficiency, contractility and electrical conduction. In this Topical Review article, we highlight key studies that inform our current understanding of human cardiomyocyte maturation. Collectively, these studies suggest that human atrial and ventricular myocytes evolve quickly within the first year but might not reach a fully mature adult phenotype until nearly the first decade of life. However, it is important to note that fetal, neonatal and paediatric cardiac physiology studies are hindered by a number of limitations, including the scarcity of human tissue, small sample size and a heavy reliance on diseased tissue samples, often without age-matched healthy controls. Future developmental studies are warranted to expand our understanding of normal cardiac physiology/pathophysiology and inform age-appropriate treatment strategies for cardiac disease.
Keywords: age dependence, cardiomyocyte, cardiac electrophysiology, development, excitation–contraction coupling, human physiology, maturation
Graphical Abstract
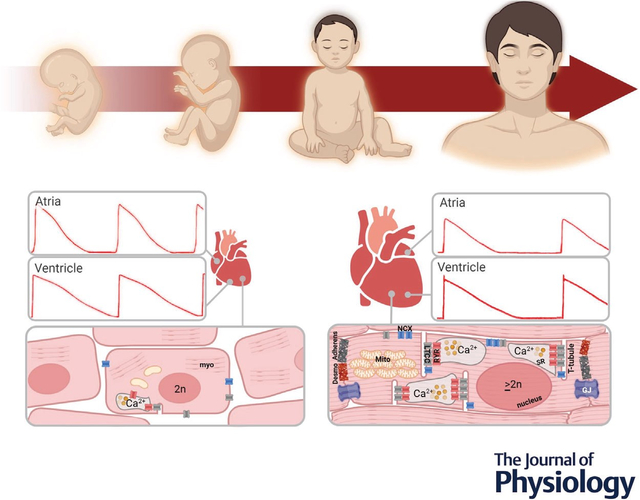
Abstract figure legend Conceptual image of postnatal cardiomyocyte maturation. Human heart development is associated with changes in ionic currents that mediate the cardiac action potential shape (red traces depict atrial and ventricular action potentials) and cardiomyocyte morphological characteristics that collectively influence cell-cell coupling, electrophysiology, metabolism, and contraction. Abbreviations: Adherens, adherens junction; Desmo, desmosome; GJ, gap junction; LTCC, L-type calcium channel; Myo, myofilament; NCX, sodium–calcium exchanger; RYR, ryanodine receptor; SR, sarcoplasmic reticulum. Action potentials are adapted with permission (Cohen & Lederer, 1993). Image created with Biorender.com
Introduction
During postnatal life, the human heart contracts >2 billion times during the average lifespan. Through coordinated activity, the four distinct chambers of the heart work together to deliver oxygenated blood to the body and return deoxygenated blood to the lungs. The cellular composition of the human heart is both heterogeneous and dynamic, and this cellular landscape can change in response to normal development, ageing or disease (Grandi et al., 2023; Litviňuková et al., 2020; Suryawanshi et al., 2020; Tucker et al., 2020). A variety of specialized cell types support heart function, including cardiomyocytes, which compose ~20–40% of the total numberofcells and ~70–85%oftotaltissuevolumeowing to their large size (Bergmann et al., 2015; Litviňuková et al., 2020; Zhou & Pu, 2016). These excitable muscle cells conduct electrical signals and generate contractile force, which pumps blood into the circulatory system in a coordinated rhythmic fashion. Throughout fetal and postnatal development, cardiomyocytes adjust to haemodynamic demands by changing their size, structural organization, metabolism and gene/protein expression profile. These adaptations coincide with a shift in the electrophysiology and contractile properties of the cells.
Despite their critical role in sustaining perinatal life, our current knowledge of human cardiomyocyte physiology and maturation is hindered by the scarcity of healthy heart tissue for biomedical research. Donated biospecimens have provided some insight into cardiomyocyte proliferation, growth and cell structure, but with modest temporal and spatial resolution. Moreover, atrial and ventricular cardiomyocytes are likely to have different maturation trajectories owing to the distinct electrophysiological and mechanical properties of the individual cardiac chambers (Rumyantsev, 1991). Furthermore, fixed tissue specimens provide minimal information on the physiology of living cells. As such, insight into the cardiomyocyte maturation process has largely been extrapolated from either mammalian models (Swift et al., 2020; Velayutham et al., 2020) or human pluripotent stem cell-derived cardiomyocytes (hPSC-CM; Lundy et al., 2013). Although facets of myocyte maturation remain conserved between species, there are significant differences in electrophysiology, calcium handling, contraction and relaxation that can hinder the translational applicability of these findings to humans (Kannan & Kwon, 2020; Milani-Nejad & Janssen, 2014; Noujaim et al., 2004). This is to be expected, because cardiovascular systems have evolved differently across species to adapt to unique physiological demands (West et al., 1997). For example, smaller animals have faster heart rates (e.g. mice ~600 beats min−1 at rest), which require rapid rates of contraction and relaxation to maintain cardiac output, in comparison to larger mammals ((e.g. 75 beats min−1) humans Bratincsák et al., 2020; Jann Hau, 2010; Janssen & Periasamy, 2007; Rudolph, 2009). These physiological differences are supported by both anatomical adaptations and underlying molecular mechanisms (Edwards & Louch, 2017; Milani-Nejad & Janssen, 2014; Odening et al., 2021; Ripplinger et al., 2022).
To bridge this gap, hPSC-CM have gained prominence because these cells have structural features and a gene/protein expression profile that is similar to primary human cardiomyocytes (Casini et al., 2017; Denning et al., 2016; Liang et al., 2013). Moreover, hPSC-CM can be used in the laboratory in large quantity, which is not possible with limited biospecimens that are collected from donated tissues or from patients undergoing corrective surgery. Nevertheless, hPSC-CM lack structural and phenotypical maturity and more closely resemble fetal than adult cardiomyocytes (Bedada et al., 2014; DeLaughter et al., 2016; Kannan & Kwon, 2020; Kannan et al., 2021; Lewandowski et al., 2018; van den Berg et al., 2015). Accordingly, tremendous efforts have been made to increase the maturity of hPSC-CM using biophysical and biochemical cues and to identify regulatory pathways that drive the maturation process (Murphy et al., 2021; Parikh et al., 2017; Ribeiro et al., 2015; Yang et al., 2019; Yoshida et al., 2018). Although animal models and hPSC-CM cannot serve as a replacement for human studies, they are an important resource for expanding our knowledge on postnatal cardiomyocyte maturation. In this article, we aim to highlight key studies that inform our current understanding of human cardiomyocyte maturation, which can help us to use animal or hPSC-CM models better, at appropriate developmental stages and with clear insight into their underlying developmental differences.
Transition from hyperplasia to hypertrophy
At birth, the neonatal heart is roughly 1/10th its adult size, and continued heart growth (e.g. heart weight, ventricular wall thickness and ventricular cavity size) is correlated with advancing age and increasing body size (Scholz et al., 1988; St. John Sutton et al., 1982). At first, fetal cardiac growth is accomplished through hyperplasia (cells proliferate and divide into two daughter cells), then later in development, growth occurs by cardiomyocyte hypertrophy (cell size increases without a change in the total number of cells; Günthel et al., 2018; Tan & Lewandowski, 2020). The fetal myocardium experiences a 6.7-fold increase in the total number of cardiomyocyte nuclei between 16 and 42 weeks of gestation (Mayhew et al., 1997; Table 1). A hallmark of cardiomyocyte maturation is the transition from hyperplasia to hypertrophy. A few studies have found indications that this process begins in utero, supported by the presence of binucleated cardiomyocytes (Kim et al., 1992) and a reduction in proliferative activity (Huttenbach et al., 2001), although it appears that this transition occurs in earnest with the first few months after birth. To date, the exact timing of this event is unknown and differs slightly between experimental studies, probably owing to differences in sample size, species and the experimental technique adopted (Ang et al., 2010; Austin et al., 1995; Botting et al., 2012; Burrell et al., 2003; Jonker et al., 2015; Mandarim-de-Lacerda et al., 1997; Soonpaa & Field, 1998). For example, Mayhew et al. (1997) measured an equivalent number of human cardiomyocyte nuclei at birth (42 gestational weeks) compared with 40 postnatal weeks old. Furthermore, Mollova et al. (2013) reported that the percentage of human cardiomyocytes in mitosis (0.1%) and cytokinesis (0.016%) was highest in infants <1 year old, which then decreased ~11-fold in older heart tissue samples (0.009% mitosis and 0.005% cytokinesis, 10–20 years old). Ye et al. (2016) also found that the window of postnatal proliferation is narrow in humans, because prominent cell cycle activity (0.47%) was observed in atrial myocytes isolated from the youngest congenital heart disease patients, <3 months old, which decreased 27-fold in older infants (0.02% at 7–12 months old).
Table 1.
Cardiomyocyte transition from hyperplasia to hypertrophy
| Age range | Cardiomyocyte type | Key points | Citation |
|---|---|---|---|
|
| |||
| <0 weeks | Ventricular |
Hyperplasia contributes to increased heart size before birth Total cell volume per cardiomyocyte nucleus remains consistent before birth |
Mayhew et al. (1998) |
| ≤16 weeks | Ventricular |
Hyperplasia ceases soon after birth Number of cardiomyocyte nuclei increases throughout gestation |
Mayhew et al. (1997) |
| ≤1 year | Atrial |
Narrow window of postnatal proliferation Postnatal cardiomyocyte proliferative potential is highest in patients <3 months old |
Ye et al. (2016) |
| 0–59 years | Left ventricular |
Hypertrophy begins after birth Infants have highest rates of cardiomyocyte mitosis and cytokinesis. Cardiomyocyte volume is eight times larger in adults |
Mollova et al. (2013) |
| 0–73 years | Left and right ventricular |
Transition to hypertrophy occurs almost immediately Total number of cardiomyocytes is established postnatally within 1 month. Volume of myocyte nuclei decreases, whereas hypertrophy increases from birth to adulthood |
Bergmann et al. (2015) |
| 0–75 years | Left ventricular |
One nucleus per cardiomyocyte Cardiomyocytes remain mononucleated; nuclear content increases with age |
Takamatsu et al. (1983) |
| 0–90 years | Left ventricular |
DNA synthesis occurs with hypertrophy Myocytes are diploid at birth, but increased polyploidy is observed with age |
Eisenstein & Wied (1970) |
However, it is important to consider that cell cycle activity is not only indicative of cell proliferation; cells can replicate DNA with or without karyokinesis, resulting in the formation of multinucleated cardiomyocytes or multiple chromosomes within a single nucleus. In the aforementioned studies, the time line of cell cycle withdrawal is in agreement with work by Bergmann et al. (2015), who used a specific marker for cardiomyocyte nuclei to calculate that the final number of cardiomyocytes in the heart (~3.2 × 109) was established within 1 month of birth (the youngest age assessed). Although the total number of myocytes remained relatively constant between 1 month and 73 years old, the number of endothelial cells and mesenchymal cells continued to increase, by 6.5- and 8.2-fold, respectively.
The mechanisms driving cardiomyocyte cell-cycle arrest are still poorly understood in humans but remain an active area of investigation (Ball & Levine, 2005; Lock et al., 2018), because identifying strategies to ‘restart’ myocyte proliferation could lead to new therapeutic interventions to regenerate and repair heart tissue after injury, as recently demonstrated in several animal models (Abouleisa et al., 2022; Porrello et al., 2011, 2013; Soonpaa & Field, 1997; Zgheib et al., 2014). Further, a newborn child with severe myocardial infarction rapidly recovered left ventricular function within weeks after injury (Haubner et al., 2016). Additional studies are needed to understand the timing and mechanistic regulators of cell-cycle arrest in humans, in order to evaluate potential heterogeneities within different regions of the heart and to assess the functional outcomes of this process.
As cardiomyocytes develop and acquire structural maturity [e.g. sarcomere assembly and expansion, intercellular coupling via intercalated discs and invaginations of the sarcolemma to form transverse tubules (T-tubules)], it can become disadvantageous to undergo cell proliferation as a means of continued growth, because proliferation requires dedifferentiation, a process by which sarcomeres disassemble and neighbouring cardiomyocytes become uncoupled (Jopling et al., 2010; O’Meara et al., 2015; Zhu et al., 2021). Accordingly, in the perinatal period, human cardiomyocytes undergo hypertrophy to increase their overall size and expand the number of connected sarcomeres (the contractile unit of the cardiomyocyte). Mayhew et al. (1998) reported that the total cell volume per cardiomyocyte nucleus remained constant during fetal development (16–35 weeks gestation). Thereafter, Mollova et al. (2013) demonstrated that cardiomyocyte size begins to increase after birth, with an average cardiomyocyte volume increasing 8.6-fold from infants (0–1 year) to older individuals (10–20 years). Cardiomyocyte hypertrophy was also demonstrated by a 6.6-fold decrease in the density of nuclei within myocardial tissue, from 2.4 × 108 nuclei cm−3 in infants to 0.36 × 108 nuclei cm−3 in older individuals. This is in agreement with the study by Bergmann et al. (2015), who reported an even greater (15-fold) decrease in myocyte nuclei density per volume of ventricular tissue, from birth to adulthood. Cardiomyocyte hypertrophy occurs in tandem with increased myofibrillar density and alignment, allowing the heart to adapt and respond to the increasing workload that accompanies rapid postnatal growth.
With increased age, a substantial number of human cardiomyocytes also undergo polyploidization (Adler, 1975; Adler et al., 1996; Eisenstein & Wied, 1970), a process in which DNA content increases without subsequent karyokinesis and cytokinesis. Given the large size of adult cardiomyocytes, increasing the DNA content might help to support transcriptional needs for protein biosynthesis. Takamatsu et al. (1983) estimated that 94.3% of infant cardiomyocytes are diploid (2n), with an increased incidence of hyperploidy (>2n) at later stages of development (13.6% at 1–9 years, 26.7% at 9–22 years and 31.4% at 22–75 years). A comparable trend was reported by Mollova et al. (2013), whereby the percentage of hyperploid cardiomyocytes increased steadily during development, from 16.3% in the first year of life to 54.2% in individuals >40 years old. Likewise, Bergmann et al. (2015) reported that almost all cardiomyocyte nuclei were diploid within the first few years of life, with polyploidization occurring much later in development, during the second and third decade of life, although other studies suggest that polyploidization occurs even earlier in life, particularly in cases of congenital or acquired heart diseases (Adler, 1975; Adler & Costabel, 1975; Brodsky et al., 1994). Overall, the majority of human cardiomyocytes remain mononucleated (~63–80%) throughout development, with a smaller proportion (20–37%) becoming multinucleated (karyokinesis without cytokinesis), although there is no discernible difference between age groups (Bergmann et al., 2015; Takamatsu et al., 1983). Similar to human cardiomyocytes, hPSC-CM are primarily mononucleated, with a smaller percentage of cells becoming multinucleated (Kong et al., 2017; Laflamme & Murry, 2011). This is in stark contrast to many experimental mammalian models, thus highlighting the risk of inaccuracy when extrapolating animal data to humans. For example, ~50% of sheep cardiomyocytes become binucleated inutero (Jonker et al., 2015; Jonker, Zhang et al., 2007), ~90% of rodent cardiomyocytes become binucleated within the first 2 weeks after birth (Bensley et al., 2016; Clubb & Bishop, 1984; Li et al., 1996, 1997; Soonpaa et al., 1996), and swine cardiomyocytes become multinucleated within 6 months and often present with 8–16 nuclei per cell (Velayutham et al., 2020). The mechanisms triggering these adaptations are understudied, but are likely to be influenced by changes in haemodynamics, hormones and oxygen levels (Jonker & Louey, 2016; Jonker, Faber et al., 2007; Jonker, Louey et al., 2018; Naqvi et al., 2014; Puente et al., 2014). Of interest, differences in cell nucleation have been shown to alter cardiomyocyte physiology, with mononucleated rabbit cardiomyocytes exhibiting a faster beating rate, larger calcium transients and increased ryanodine receptor (RYR) density, compared with binucleated cells (Huang et al., 2012). The functional outcomes of multinucleation and polyploidization are still incompletely understood, as is the impact of congenital heart defects, preterm delivery and intrauterine growth restriction on these maturation patterns (Adler & Costabel, 1980; Botting et al., 2014; Brodsky et al., 1994; Jonker, Kamna et al., 2018). Furthermore, the mechanisms underlying species-specific differences remain understudied, which can limit the human applicability of experimental animal models in developmental research (Kannan & Kwon, 2020).
Structural maturation of the human cardiomyocyte
Given the rapid rate of body growth, postnatal development is accompanied by an increase in metabolic demand, cardiac workload and cardiac output, culminating in a 6- to 12-fold increase in stroke volume from birth until adulthood (De Simone et al., 1998). To adapt, cardiomyocytes undergo developmental hypertrophy (discussed above), resulting in a significant increase in left ventricular mass. Concurrent with this increase in cell size, myocytes acquire structural adaptations to improve the efficiency and capacity of muscle contraction. Key components of the cardiomyocyte architecture have been reviewed in detail previously (Ehler, 2016; Gautel & Djinovic-Carugo, 2016;´ Henderson et al., 2017). Briefly, human adult cardiomyocytes are large in size, rod shaped and contain densely packed myofibrils, the contractile machinery of cardiac muscle (Gerdes et al., 1992). Myofibrils are composed of actin and myosin filaments that are intricately arranged to facilitate cross-bridge formation, sarcomere shortening (contraction) and subsequent relaxation (Ehler, 2016) and are composed of multiple sarcomeres in series, defined at the ends by Z-lines or lateral boundaries. In adult cardiomyocytes, the sarcolemma is invaginated to form T-tubules near these Z-lines (Richards et al., 2011). The T-tubules are a key structural component of excitation–contraction coupling, localizing L-type calcium channels in close proximity to ryanodine receptors (RYR) on the junctional sarcoplasmic reticulum (SR), which supports synchronized activation of the myocyte (Jayasinghe et al., 2012). In turn, the SR is an intracellular membrane network that serves as a source for Ca2+ release during excitation–contraction coupling.
Progressive changes to the cardiomyocyte architecture have been documented during human fetal growth, although less information is available on the dynamic changes that occur postnatally. Early in fetal development (17–24 weeks), Kim et al. (1992) described cardiomyocytes that were smaller, more rounded and contained scattered myofibrils near the periphery of the cell (Fig. 1 and Table 2). Sarcomeres were identifiable but lacked some distinguishing features, and myofibrils were loosely packed, with limited alignment. Racca et al. (2016) reported similar observations in early fetal development (52 days of gestation), including wavy Z-bands, narrow myofibrils (0.36 μM) and low myofibril density. With continued fetal development (127 days of gestation), Racca et al. visualized Z-band alignment with adjacent myofibrils, which were noticeably wider (0.97 μM). Kim et al. also found more densely packed myofibrils with greater alignment at 32–40 weeks gestation, although some prominent features were still largely absent in sarcomeres (e.g. H zone, M line, crosslinking of thick filaments). In late gestation, T-tubules and the SR began to develop near sarcomere Z-lines, although these structures were immature in comparison to the adult myocardium (Crossman et al., 2011; Kim et al., 1992; Lyon et al., 2009). Wiegerinck et al. (2009) reported that T-tubules were still sparse in newborn hearts (<3 weeks old), but continued to develop throughout the first postnatal year (3–14 months).
Figure 1. Electron microscopy of fetal hearts.
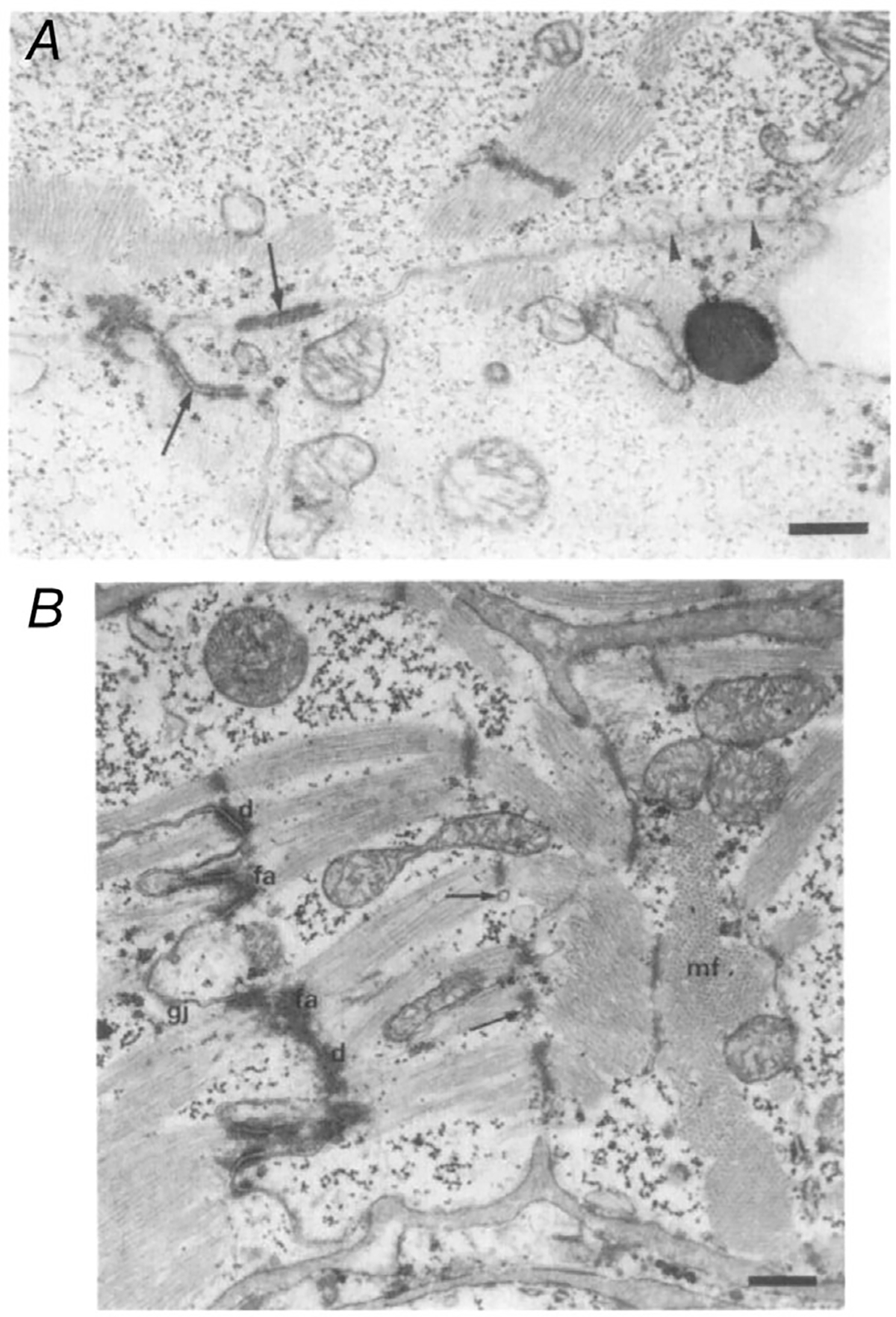
A, fetal hearts at 20 weeks of gestation, with some desmosomes forming at intercalated disc (arrows). Note scattered myofibrils. B, fetal heart at 30 weeks of gestation, with more abundant desmosomes at the intercalated disc and more densely packed myofibrils. Arrows indicate sarcoplasmic reticulum tubules at the Z-line, which were not present in the earlier period. Abbreviations: d, desmosomes; fa, fascia adherens; gj, gap junctions; mf, myofibrils. Scale bars: 0.53 μM. Adapted with permission from Kim et al. (1992).
Table 2.
Cardiomyocyte structural maturation
| Age range | Cardiomyocyte type | Key points | Citation |
|---|---|---|---|
|
| |||
| <0 weeks | Left and right ventricular |
Structural development continues after birth Increase in cell size, elongation and contractile force. Increase in myofibril organization, density and width. |
Kim et al. (1992) |
| <2 years | Left and right ventricular | Increase in SR formation, T-tubule formation and Z-band alignment | Wiegerinck et al. (2009) |
| <0–67 years | Left ventricular | Racca et al. (2016) | |
| <0–68 years | Left atrial, right and left ventricular | Myosin heavy chain expression, kinetics change with age (tissue specific) | Reiser et al. (2001) |
| <0–81 years | Left and right atrial, left and right ventricular | Decrease in α-MHC. Increase in β-MHC. Increase in myosin enzymatic activity |
Cummins and Lambert (1986) |
| 0–80 years | Left ventricular | Schier and Adelstein (1982) | |
| <0–11 years | Right ventricular | Myosin light chain isoform switching occurs during perinatal development | Elhamine et al. (2016) |
| <0–52 years | Atrial and ventricular | Increase in VLC-2. | Cummins et al. (1980) |
| <0–71 years | Left and right atrial, left and right ventricular | Increase in VLC-1. Decrease in ALC-1 |
Price et al. (1980) |
| <0–81 years | Right ventricular | Auckland et al. (1986) | |
| < 1 year | Atrial | Myofilament isoform switching occurs during perinatal development | Bhavsar et al. (1991) |
| <1 year | Atrial | Sasse et al. (1993) | |
| <0–11 years | Right ventricular | Decrease in number of cTnT isoforms. Decrease in ssTnI and cTnT1. |
Elhamine et al. (2016) |
| 0–35 years | Right atrial | Increase in cTnI and cTnT3 | Saba et al. (1996) |
| 0–58 years | Left ventricular | Anderson et al. (1991, 1995) | |
| 0-adult | Ventricular | Hunkeler et al. (1991) | |
The proteomic composition of the sarcomere also changes during development, as myofilament proteins, regulatory proteins and cytoskeletal proteins shift from fetal to adult isoforms, which change the inherent contractile properties of the cardiomyocyte. Muscle contraction occurs as myosin and actin myofilaments rachet or slide over one another, which results in sarcomere shortening. Myosin is comprised of light chains (MLCs) and heavy chains (MHCs); human cardiomyocytes express α-MHC and β-MHC, with the latter being associated with slower contractile kinetics (Bouvagnet et al., 1989; Mercadier et al., 1983). Reiser et al. (2001) found that fetal atrial samples (47–110 days) primarily express α-MHC (96–97%), whereas fetal ventricular samples express β-MHC (90–93%). In ventricular samples, the small amount of α-MHC expression declined even further between ~12 weeks of gestation and adulthood. In atrial samples, Cummins & Lambert (1986) reported an increase in β-MHC expression during gestation, reaching adult levels soon after birth. Other groups have detected only the β-MHC isoform in ventricular samples collected from fetal, paediatric and adult tissues, suggesting that any slight change in MHC isoform expression is likely to occur either before or near birth (Auckland et al., 1986; Elhamine et al., 2016; Racca et al., 2016; Schier & Adelstein, 1982). This is remarkably different from rodent models, wherein the fetal ventricle predominately expresses β-MHC, with α-MHC expression beginning to increase after birth, with nearly complete turnover to this isoform in young adult animals (Lompre et al., 1981; Lompré et al., 1984). This is one example of key species-specific differences, wherein larger animals with slower heart rates are more similar to humans and follow a similar pattern of MHC isoform switching (Hodges et al., 2021; Jann Hau, 2010; Janssen & Periasamy, 2007).
Although human cardiomyocytes have limited age-dependent MHC isoform switching, there is a significant developmental difference in the expression of MLCs in the human myocardium. Cummins et al. (1980) and Price et al. (1980) identified a unique MLC (ALC-1, atrial essential myosin light chain) expressed in fetal, but not adult ventricular tissue samples. Moreover, Schier and Adelstein (1982) reported that expression of this fetal isoform in infant tissue coincided with 50% less myosin enzymatic activity, in comparison to adults. Further study by Auckland et al. (1986) revealed a steady decline in ALC-1 expression, representing 46.5% of total MLC expression at 18–21 weeks of gestation and rapidly decreasing thereafter to 18.3% at birth, 5.5% in infants <2 years old, and negligible expression at older ages (only expressing VLC-1 and VLC-2, ventricular essential light chains). It is estimated that ALC-1 represents ~42% of total MLC expression in neonates after birth, declining monoexponentially with a half-time of 5.8 months that coincides with replacement by VLC-1 and VLC-2 (Elhamine et al., 2016). This isoform switch was associated with an age-dependent change in myofibrillar biomechanics, including contraction kinetics and maximal force. Conversely, infants and children with tetralogy of Fallot have persistently higher levels of ALC-1, probably owing to haemodynamic influences, which further highlights the effect of cardiac disease in postnatal maturation (Auckland et al., 1986).
Troponin I (TnI) is a regulatory protein that inhibits actin–myosin interactions at basal Ca2+ levels, and its isoforms are expressed in an age-dependent manner. Specifically, the slow isoform (ssTnI) is abundantly expressed in fetal and infant cardiomyocytes, with negligible amounts in adult tissue, which predominately expresses the cardiac isoform (cTnI) (Bhavsar et al., 1991; Elhamine et al., 2016; Hunkeler et al., 1991; Racca et al., 2016; Sasse et al., 1993). Bhavsar et al. (1991) reported that 8-week-old fetal atrial tissue expressed only ssTnI, whereas both ssTnI and the cTnI isoforms were readily detected later in gestation (33 weeks) and in the early postnatal period (15 days). Only cTnI was detected in atrial tissue from a 9-month-old specimen, suggesting that this switch occurs within the first year of life. Sasse et al. (1993) reported a similar temporal pattern in ventricular tissue, wherein ssTnI was the predominant isoform during fetal development (90% of total expression), with slightly reduced levels at birth (80%) and early postnatal development (60%), but undetectable levels by 9 months of age. As ssTnI declined, cTnI expression increased in an age-dependent manner, with a similar expression profile observed between 9 months of age and adulthood. In agreement, Elhamine et al. (2016) found that ssTnI declined monoexponentially within the first 10 months after birth and was replaced by the cTnI isoform with a half-time of 4.3 months, although there was variability between patients in this developmental decline, which might be attributed to the type of congenital heart disease. Racca et al. (2016) confirmed that ssTnI is expressed early in fetal development, which is associated with slower rates of force development and relaxation. A progressive increase in cTnI was seen with gestational age (up to 134 days), which coincided with increased force and faster activation/inactivation kinetics, probably owing to reduced Ca2+ sensitivity. Although maximal force increased 6-fold with increasing gestational age (7.8 mN mm−2 at 58 days vs. 49.9 mN mm−2 at 130 days of gestation), this was still considerably less than values reported for adult ventricular samples (90–110 mN mm−2; Piroddi et al., 2007). This is likely to be influenced by inefficient strain transmission caused by underdeveloped and unaligned myofibrils.
Developmental changes in cardiac troponin T (cTnT; a regulatory protein that binds the troponin complex to tropomyosin and modulates Ca2+ concentration-dependent ATPase activity) are largely limited to fetal development. Anderson et al. (1991, 1995) detected four cTnT isoforms in fetal ventricular tissue (14–15 weeks of gestation), but only two isoforms in adult ventricular tissue. Furthermore, myofibrillar ATPase activity was related to the ratio of cTnT isoform expression in adult tissue (healthy vs. heart failure), highlighting disease-associated changes. Similar to observations in adult heart failure tissue, Saba et al. (1996) reported an increase in cTnT4 expression in congenital heart disease patients with increased haemodynamic loading. In all atrial samples, cTnT3 was the predominant isoform (6 days to 35 years), but there was an age-dependent increase in cTnT3 expression as cTnT1 expression decreased. Racca et al. (2016) also found that cTnT3 was the predominant isoform in ventricular tissue samples, with increased protein density observed later in gestation (59–134 days). However, in samples collected postnatally (4 days to 11 years), Elhamine et al. (2016) did not find a significant difference in cTnT isoforms, suggesting that this age-dependent switch might occur around the time of birth. Additional work is needed to understand fully the dynamic shifts in myofilament, cytoskeletal and regulatory protein expression during postnatal development and the impact on myocardial stiffness and contractility. For example, Narolska et al. (2005) demonstrated that MHC isoform expression alters myocardial energetics and performance in adults with atrial fibrillation, whereas Bond et al. (2018) highlighted significant changes in the proteomic profile of the right ventricle in infants with cyanotic versus acyanotic heart disease. Specifically, acyanotic patients with a ventricular septal defect had increased ventricular stiffness and reduced expression of key sarcomeric proteins (e.g. tropomyosin, myomesin and obsecurin). Future studies are warranted to investigate differences in cardiomyocyte development and contractile function between healthy and diseased tissue.
Establishment of intercellular connections
The heart is composed of billions of individual cardiomyocytes, yet it functions as a syncytium, with synchronized contraction and relaxation. This coordination of electrical and mechanical activity is facilitated by specialized structures, called intercalated discs, which develop between neighbouring cardiomyocytes. Classically, the intercalated disc has been described to contain three main complexes: adherens junctions, desmosomes and gap junctions (Vermij et al., 2017). Desmosomes and adherens junctions are adhesion structures that tightly couple neighbouring cardiomyocytes. Desmosomes provide structural support through connections with intermediate filaments, whereas adherens junctions connect to the actin cytoskeleton and serve to anchor myofibrils. Gap junctions provide electrical continuity through intercellular connexon channels (composed of connexin proteins), which allow small molecules to flow freely between neighbouring myocytes. Nearly 200 different proteins have been found to localize to the intercalated disc (Estigoy et al., 2009), including the cardiac voltage-gated sodium channel (Nav1.5), which is responsible for the action potential upstroke and fast electrical propagation in atrial and ventricular cardiomyocytes.
Fully developed intercalated discs are absent at birth, but these specialized structures develop postnatally to maintain electromechanical function while cardiomyocytes undergo hypertrophy and remodelling. Across multiple species, mechanical junctions are established earlier in life than electrical junctions, although the timing varies by species (Hirschy et al., 2006; Vreeker et al., 2014). In a foundational study, Peters et al. (1994) monitored the postnatal development of intercalated discs in ventricular tissue specimens from paediatric patients (4 months to 15 years) (Table 3). In neonatal hearts, gap junction- (e.g. connexin-43) and adherens junction-specific proteins (e.g. N-cadherin) were punctuated indiscriminately throughout the myocyte surface until ~6 years of age, when these proteins co-localized and became confined to intercalated discs. This developmental time frame is in line with other age-related adaptations, including the maturation of heart rate and ventricular mechanics (Bratincsák et al., 2020; Colan et al., 1992). Likewise, Vreeker et al. (2014) reported age-dependent changes in the spatiotemporal localization of intercalated disc proteins in ventricular specimens (15 weeks to 11 years). Proteins found in adherens junctions (e.g. N-cadherin and Zonula occludens-1 (ZO-1)) and desmosomes (e.g. plakoglobin, desmoplakin and plakophilin-2) were diffuse with lateral labelling in fetal samples, but with postnatal development, these proteins moved progressively to the myocyte termini, displaying a mature, adult phenotype by ~1–2.5 years after birth. Notably, adherens junction and desmosome proteins were co-localized throughout all stages of fetal and postnatal development. Sodium channels (Nav1.5) followed a similar spatiotemporal pattern, with prominent detection at the intercalated disc at ~2.5 years old. In the study by Vreeker et al. (2014), connexins were scarce during fetal development, then diffuse with lateral localization ~5 months after birth. Connexin proteins did not co-localize with adherens junctions at the intercalated disc until much later in life (~7–11 years). In agreement, Salameh et al. (2014) reported an age-dependent change in connexin-43 distribution, whereby the ratio of polar/lateral localization to intercalated discs increased in children from 2.9 (0–2 years) to 8.5 (>12 years). Immunostaining revealed that connexins were largely confined to the intercalated disc ~8.5 years old, which is consistent with the described time line produced by Vreeker et al. (2014). However, it is important to note that these developmental time lines are only an approximation, because many of these human studies have a relatively small sample size. Further study is warranted, because developmental differences and interactions between cardiomyocyte size, cell–cell coupling and ion channel expression can contribute to arrhythmias with age-dependent penetrance (Nowak, Poelzing et al., 2021).
Table 3.
Maturation of intercellular connections
| Age range | Cardiomyocyte type | Key points | Citation |
|---|---|---|---|
|
| |||
| <0 years | Right and left ventricle |
Intercalated discs begin to form in utero Immature intercalated discs are identifiable by 20 weeks of gestation; sparse gap junctions are identifiable between neighbouring cells by 32 weeks of gestation |
Kim et al. (1992) |
| <1–15 years | Right ventricle |
Intercalated discs develop with postnatal age Punctuated connexin pattern in neonatal heart tissue shifts progressively to transverse terminals of the cardiomyocyte with age. Gap junctions and adherens junctions show a mature pattern by ~6–7 years of age |
Peters et al. (1994) |
| <0 years | Atria, ventricles and conduction tissue |
Regional specificity of connexin proteins begins in utero Fetal connexin-40, -43 and -45 expression varies across tissues |
Coppen et al. (2003) |
| <0–11 years | Left ventricle |
Intercalated discs develop with postnatal age Increased adherens junctions, desmosomes, gap junctions and sodium channel localization to the intercalated discs during paediatric development |
Vreeker et al. (2014) |
| <0–38 years | Atria, left and right ventricle |
Gap junction proteins develop with age Connexin content and distribution vary between fetal, paediatric and adult tissue |
Chen et al. ((1994) |
| <0–66 years | Right ventricle |
Density and localization of gap junction proteins develop with age Connexin-43 protein expression decreases, whereas localization to intercalated discs increases |
Salameh et al. (2014) |
It is important to note that human cardiomyocytes express multiple gap junction proteins (e.g. connexin-40, -43 and -45), each with distinct channel properties (e.g. voltage and chemical gating and permeability) that support cardiac electrophysiology in a chamber-related and age-dependent manner (Verheule & Kaese, 2013). In the adult heart, the faster-conducting bundle branches and Purkinje fibres have large gap junction plaques that contain a large quantity of connexin-43, -40 and -45, whereas slower-conducting nodal regions (sinus and atrioventricular) predominately express connexin-45 and -40 in lesser amounts (Chandler et al., 2009; Davis et al., 1995; Greener et al., 2011). All three major connexin isoforms are found in atrial and ventricular myocytes, although atria have a higher density of connexin-40, in comparison to connexin-43 in the ventricles (Davis et al., 1995; Greener et al., 2011; Vozzi et al., 1999). In the fetal heart (9 weeks of gestation), Coppen et al. (2003) detected connexin-40 and -43 most prominently in trabeculated ventricular tissue, with reduced expression in the compact myocardium. The authors also detected connexin-45 in developing conduction tissues, although this differs from a study by Chen et al. (1994), who reported that connexin-45 was inconspicuous in fetal hearts (74–122 days of gestation) but readily detected in cardiac tissue from older children (0.5 months to 3 years of age) and adults. In a study of patients with tetralogy of Fallot, Salameh et al. (2014) noted an age-dependent decline in connexin-43 protein expression between infants (0–2 years), older children (2–12 years) and those nearing adulthood (>12 years). This progressive decline in expression might be offset by dense connexin-43 localization to the intercalated discs in older children, wherein gap junctions facilitate fast electrical conduction along the fibre axis in the longitudinal direction. Collectively, the expression and spatial distribution of connexin proteins modulates electrical coupling within different regions of the heart, which changes dynamically during postnatal heart development (Nowak, Veeraraghavan et al., 2021; Spach et al., 2000).
Adaptations in myocardial metabolism
The heart has a high rate of energy production and utilization, with ~70% of ATP used for contraction and ~30% for specialized ion pumps (Gibbs, 1978; Suga, 1990). Given the hypoxic intrauterine environment, fetal cardiomyocytes rely heavily on glycolysis for energy production. Animal studies have shown that the transition to the extrauterine environment brings about sudden changes in circulating nutrients, oxygen levels and haemodynamics, which reshape cardiomyocyte bioenergetics (Girard et al., 1992; Makinde et al., 1998). At the cellular level, metabolic maturation includes alterations in mitochondrial content, membrane transporters and enzymatic activity to support the postnatal transition of the myocytes from glycolytic to oxidative metabolism (Bartelds et al., 1998; Lopaschuk & Jaswal, 2010; Lopaschuk et al., 1991; Mdaki et al., 2016). Metabolic remodelling becomes necessary to support the increased demand on the developing heart, as fatty acid oxidation yields more ATP (per unit substrate) compared with glycolysis. The time frame and mechanisms underlying this metabolic switch are underexplored in humans, but in rodent models, studies suggest that this process is influenced by the increase in ambient oxygen at birth and the subsequent reduction in hypoxia signalling via oxygen-sensitive transcription factors [e.g. hypoxia-inducible factor-1α (HIF1α) and HAND1; Liu et al., 2021; Neary et al., 2014]. Chronic hypoxic conditions have been shown to dysregulate metabolic remodelling in rodents and impair heart development in sheep by reducing the total number of cardiomyocytes (Botting et al., 2014; Smith et al., 2022; Song et al., 2021). In humans, cyanotic congenital heart disease can disrupt postnatal maturation, because chronic hypoxia results in elevated HIF1α and persistent glucose-dominated cardiac metabolism in paediatric patients (Horikoshi et al., 2019; Liu et al., 2021; Piccoli et al., 2017; Porter et al., 2011; Quing et al., 2007). Collectively, cardiomyocyte maturation and metabolic remodelling are likely to be influenced by a number of factors, including changes in oxygen levels, hormone levels and metabolic stress associated with intermittent feeding (Lock et al., 2018). Species-specific differences should also be considered, because larger mammals can use alternative energy sources (e.g. fatty acids and lactate) in utero (Bartelds et al., 1999, 2000).
Dramatic changes in mitochondrial morphology are observed throughout cardiomyocyte maturation, as these small, fragmented organelles expand and grow into larger networks with an increased density of cristae (Feric & Radisic, 2016; Horikoshi et al., 2019; Porter et al., 2011) and myofibril alignment (Kim et al., 1994; García-Pérez et al., 2008; Piquereau et al., 2010; Table 4). Kim et al. (1992) reported that human cardiomyocyte mitochondria are circular, scarce and scattered throughout the sarcoplasm during early fetal development (17–24 weeks of gestation). With further maturation (≥30 weeks gestation), mitochondria become more elongated, increase in number and begin to aggregate between myofibrils. Fetal cardiomyocytes also contain large pools of glycogen (stored carbohydrates), which are used for glycolytic metabolism. This is in agreement with mammalian studies, wherein glycogen occupies ~30% of the fetal myocyte cell volume and only ~2% of the adult cell volume (Shelley, 1961). As glycogen reserves become depleted, mitochondrial density increases to occupy ~22–25% of the human adult cardiomyocyte volume (Barth et al., 1992; Schaper et al., 1985). In an age comparison study, Marin-Garcia et al. (2000) reported a ~4-fold increase in mitochondrial DNA levels in human heart tissue between early gestation (45–65 days) and neonates (1–30 days). Furthermore, Pohjoismäki et al. (2013) reported a ~5-fold increase in mitochondrial DNA and ~2-fold increase in mitochondrial mass between fetal and adult cardiomyocytes. The impact of cyanotic heart conditions was highlighted in a study by Ye et al. (2020), wherein mitochondrial DNA was significantly less in tetralogy of Fallot patients with moderate versus mild hypoxia (75–85% vs. >85% arterial oxygen saturation).
Table 4.
Developmental changes in cardiomyocyte metabolism
| Age range | Cardiomyocyte type | Key points | Citation |
|---|---|---|---|
|
| |||
| <0 years | Right and left ventricle |
Mitochondrial mass increases throughout life Sparse, circular mitochondria in early fetal period; with variable shapes, increased size, increased mitochondrial density, aggregation near nuclei and myofibrils later in fetal development. |
Kim et al. (1992) |
| <0–90 years | Left ventricle | Mitochondrial mass and DNA content are increased in adult cardiomyocytes | Pohjoismaki et al. (2013) |
| 5–45 years | Left ventricle |
Correlation between mitochondrial density, heart rate and oxygen consumption Mitochondrial density increases, occupying ~25% of adult cardiomyocyte volume |
Schaper et al. (1985); Barth et al. (1992) |
| <0 years | Whole heart |
Metabolic genes upregulated throughout gestation Genes involved in fatty acid metabolism (FABP4, FABP2 and RBP) are upregulated between first and second trimester |
Iruretagoyena et al. ((2014a) |
| <0–22 years | Ventricles |
Increased mitochondrial activity during perinatal period Mitochondrial DNA content and mitochondrial enzymes (citrate synthase, complex IV, ATP synthase, cytochrome c oxidase) increase with gestational and postnatal age |
Marin-Garcia et al. (1989); Marin-Garcia et al. (2000) |
| <1 year | Right ventricle |
Age-dependent maturation of fatty acid oxidation Increase in proteins associated with fatty acid oxidation (malonyl coA decarboxylase); decrease in inhibitory regulators of fatty acid oxidation (acetyl CoA carboxylase, 5’-AMP-activated protein kinase) |
Yatscoff et al. (2008) |
| <2 years | Left atria |
Cyanotic congenital heart disease disturbs metabolic maturation Cyanotic heart samples have reduced protein synthesis and downregulation of aerobic energy metabolism, in comparison to acyanotic heart samples |
Dong et al. (2021) |
| 0–9 years | Right ventricle |
Age and disease impact metabolic maturation Younger patients had lower ATP/ADP ratio. Cyanotic heart samples had higher lactate levels |
Modi et al. (2004) |
Metabolic maturation is supported further by human developmental studies, which demonstrate age-dependent changes in membrane transporters, mitochondrial protein expression and enzymatic activity. With increased gestational age, human fetal cardiomyocytes gradually upregulate the expression of genes involved in fatty acid metabolism (Dai et al., 2017; Iruretagoyena et al., 2014). For example, Dai et al. (2017) reported a surge in mitochondrial activity between early (50–60 days) and late (100–115 days) gestation, with heightened respiratory complex activity (6.4-fold complex I, 3.6-fold complex II, 2.1-fold complex III and 3.1-fold complex IV). Respiratory complex activity was increased even further in adult tissue (3.4–11.3-fold) compared with early gestation. Marin-Garcia and colleagues also noted an age-dependent increase in key enzymes involved in the Krebs cycle and electron transport chain (Marin-Garcia & Baskin, 1989; Marin-Garcia et al., 2000). Between early gestation (45–65 days) and the neonatal period (1–30 days), a ~4-fold increase in citrate synthase, ~3-fold increase in complex IV and ~2-fold increase in ATP synthase was measured in ventricular tissue samples. ATP synthase was undetectable in cardiac tissue during early gestation, but increased ~3-fold between late gestation (85–110 days) and the neonatal period. Furthermore, cytochrome c oxidase content increased ~10-fold and oxidase activity ~8-fold across age groups (15–21 weeks of gestation vs. adults). Yatscoff et al. (2008) also reported an age-dependent (0.2–10 months) increase in proteins associated with fatty acid oxidation (e.g. malonyl CoA decarboxylase) and a decrease in inhibitory regulators of fatty acid oxidation (e.g. acetyl CoA carboxylase, 5′-AMP-activated protein kinase).
Collectively, these studies demonstrate an age-dependent shift in cardiac metabolism from glycolysis to fatty acid oxidation in humans, but the regulatory mechanisms and time line of this transition are unclear. Moreover, the impact of persistent hypoxia on this developmental process is understudied in humans. For example, metabolic maturation was impaired in patients with cyanotic heart conditions, because cardiac tissue samples had different metabolomic profiles (e.g. increased lactate and pyruvic acid, accumulation of Krebs cycle intermediates) compared with acyanotic myocardial samples (Dong et al., 2021). In a separate study, cyanotic patients also had higher myocardial lactate concentrations, and younger patients had a lower ATP/ADP ratio (Modi et al., 2004). Patient age and cyanosis are important factors affecting basal myocardial metabolism, which could also influence ischaemia–reperfusion injury and postoperative outcomes (Imura et al., 2001).
Age-dependent changes in electrophysiology
Transition from the intrauterine to extrauterine environment results in changes to blood circulation and myocardial maturation. These developmental adaptations are detected in the ECG, including changes in heart rate, the electrical axis, waveform morphology and ECG interval durations (Chan et al., 2008). During fetal development, blood is shunted away from the lungs, and the systemic circulation is driven by the right side of the heart. Accordingly, the electrical heart axis of the fetus is oriented to the right, owing to right ventricular dominance that is responsible for ~60% of cardiac output (Lempersz et al., 2021; Mielke & Benda, 2001). With birth, the placental circulation is halted, the ductus arteriosus closes, and venous return to the left atrium increases, culminating in an increase in left ventricular output (Agata et al., 1991). During infancy, the right ventricle remodels over 2–3 months, and the left ventricle increases in muscle mass, shifting the heart axis to the left (Jurko Jr, 2004). Normative data for paediatric ECG values are readily available, although ECG variables are heavily influenced by gestational age, sex and race (Bratincsák et al., 2020; Rijnbeek et al., 2001; Saarel et al., 2018; Schwartz et al., 2002; Sharieff & Rao, 2006). Broadly, postnatal age is associated with heart rate slowing from infancy (~150 beats min−1) to adulthood (~75 beats min−1), which is driven by changes in sinus node automaticity and increased vagal tone. Atrioventricular conduction is shorter in infants and children (~100 ms PR interval), probably owing to a smaller muscle mass, which lengthens during adolescence and adulthood (~150 ms). Likewise, ventricular depolarization (QRS complex) is also faster in infants and young children (~60 ms) compared with adults (~90 ms) (Bratincsák et al., 2020). The corrected QT interval, representing ventricular depolarization and repolarization, is longer in infants <6 months of age (upper limit of 490 ms) compared with other older age groups (upper limit 440 ms) (O’Connor et al., 2008).
Developmental changes in ECG variables are well documented (Bratincsák et al., 2020; O’Connor et al., 2008; Rijnbeek et al., 2001; Saarel et al., 2018; Schwartz et al., 2002; Sharieff & Rao, 2006), but the associated changes in action potential morphology and adaptations in cardiac ionic currents are vastly understudied in young (human) cells. Given that normal healthy myocardial tissue is unavailable, electrophysiological studies are performed using isolated cells from small pieces of atrial or ventricular tissue collected from patients undergoing palliative or corrective heart surgery. As such, patient age, disease and course of treatment are important confounding factors that can influence data interpretation. Although action potential recordings in neonatal paediatric human cardiomyocytes are limited (Escande et al., 1985; Cohen & Lederer, 1993; Schaffer et al., 1999; Wang et al., 2003; Table 5), a few distinguishing features have been noted; namely, young atrial or ventricular cardiomyocytes tend to have a more pronounced overshoot (peak amplitude at higher positive membrane potential), a less pronounced notch (early repolarization), and a plateau phase that persists at more positive membrane potentials, resulting in a longer action potential duration at 20–30% repolarization (Fig. 2).
Table 5.
Developmental changes in cardiomyocyte electrophysiology
| Age range | Cardiomyocyte type | Key points | Citation |
|---|---|---|---|
|
| |||
| <2 years | Right ventricular |
Age-dependent difference in IK1 IK1 density ~50% less than previously reported for adult cells |
Schaffer et al. (1999) |
| <1–68 years | Right atrial |
No age-dependent difference in IK1 IK1 density is comparable in paediatric and adult cells (room temperature) |
Crumb et al. (1995) |
| <2 years | Right ventricular | No age-dependent differences in If | Schaffer et al. (1999); |
| <1–53 years | Right and left ventricular | If detected in ~90% of cardiomyocytes; If density only slightly higher in paediatric cells versus values previously reported for adult cells | Zorn-Pauly et al. (2003) |
| <3.4–42.5 years 2–63.5 years |
Right atrial Ventricular |
Age-dependent difference in INa INa density is lower, with slower activation/inactivation; negative shift in voltage-dependent inactivation in paediatric versus adult cells |
Cai et al. (2011); Sakakibara et al. (1993) |
| <1–74 years | Right atrial |
Age-dependent difference in INa Faster depolarization in paediatric versus adult cells, suggests enhanced INa current |
Wang et al. (2003) |
| <1–54 years | Right atrial |
Age-dependent difference in transient outward current (Ito) Paediatric action potential has a smaller notch and more positive action potential plateau phase; suggests age-dependent increase in Ito |
Escande et al. (1985) |
| <1–57 years | Right atrial |
Ito is absent in children Ito is absent in cardiomyocytes from children aged 2–5 years. Samples were all dilated, which could also reduce Ito |
Mansourati and Le Grand (1993) |
| <1–68 years | Right atrial |
Age-dependent difference in Ito Ito is detected in 67% of paediatric cells and 100% of adult cells. Reduced Ito density, slower recovery time and frequency-dependent inhibition in paediatric cells versus adult cells |
Crumb et al. (1995) |
| <1–74 years | Right atrial |
Ito is present in neonatal cells, but properties are age dependent Greater Ito density, faster inactivation, slower recovery from inactivation, and frequency-dependent inhibition in neonatal versus adult cells; neonatal action potential has a less prominent notch, a more positive plateau phase and longer action potential duration at 30% compared with adult cells |
Wang et al. (2003) |
| <1–13 years | Right atrial |
Similar Ito properties across different age groups No difference in Ito density or recovery, but slightly slower inactivation in younger cells |
Gross et al. (1994) |
| <2 years | Right ventricular |
Frequency-dependent inhibition of Ito amplitude in young cells No observed age difference in Ito density, but measurements were variable even within same patient |
Schaffer et al. (1999) |
| <1–75 years | Right atrial and ventricular |
Age-dependent difference in action potential morphology Paediatric atrial myocytes have a prominent overshoot and longer action potential duration at 30–50% compared with adult cells. Paediatric ventricular myocytes have a less prominent notch, more positive plateau phase and longer action potential duration compared with adult cells |
Cohen and Lederer (1993) |
| <1–75 years | Right atrial and ventricular |
Age-dependent difference in ICa Reduced ICa density in paediatric versus adult ventricular myocytes (not atrial). Paediatric atrial and ventricular myocytes have a positive shift in steady-state activation and inactivation, resulting in a prominent calcium window current |
Cohen and Lederer (1993) |
| 1–8 years | Right ventricular |
Prominent calcium window current in paediatric cells Window current might contribute to longer action potentials and after-depolarizations in paediatric cells. Results might also be linked to the disease state of paediatric cells |
Pelzmann et al. (1998) |
Figure 2. Action potential morphology differs between neonatal and adult human atrial cardiomyocytes.
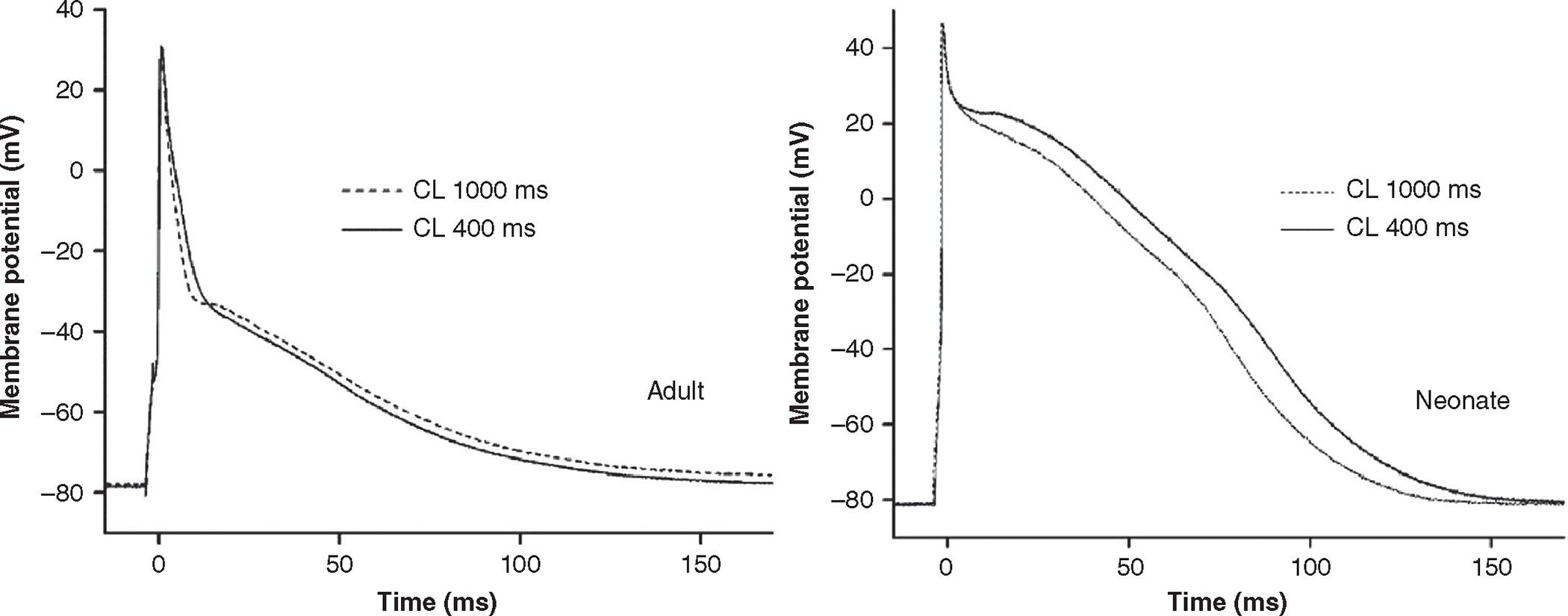
Action potential waveforms recorded at stimulation cycle lengths (CL) of 1000 and 400 ms. Adapted with permission from Wang et al. (2003).
Atrial and ventricular action potentials are characterized by a resting membrane potential that is stabilized by potassium currents, including IK1, which helps to suppress automaticity (phase 4 of the action potential; for a review, see Bartos et al., 2015). Across different species (e.g. rodents, hPSC-CM), neonatal and immature cardiomyocytes frequently display spontaneous activity that coincides with reduced IK1, increased If (pacemaker current) and/or increased (reverse-mode) Na+–Ca2+ exchanger (NCX) activity (Carmeliet, 2019; Cerbai et al., 1999; Giannetti et al., 2021; Goversen et al., 2018; Hoekstra et al., 2012; Kim et al., 2015; Masuda & Sperelakis, 1993; Vaidyanathan et al., 2016; Wahler, 1992; Wang et al., 2013). Experimental studies have reported similar resting membrane potentials, ranging from −71 to −85 mV in both paediatric and adult cardiomyocytes, isolated from either atrial or ventricular tissue (Cohen & Lederer, 1993; Escande et al., 1985; Schaffer et al., 1999; Wang et al., 2003). However, Schaffer et al. (1999) identified a barium-sensitive IK1 inward current in paediatric ventricular myocytes (16–91 months old), with a peak current density that was ~50% less (−22.8 pA pF−1) than the reported value for adult ventricular myocytes (−45 pA pF−1). This suggests an age-dependent difference in IK1 repolarizing current, which could alter the resting membrane potential and increase automaticity, akin to observations in immature hPSC-CM (Goversen et al., 2018; Hoekstra et al., 2012). In contrast, Crumb et al. (1995) found a comparable IK1 density in paediatric (−3.19 pA pF−1, 1 day to 2.5 years) and adult atrial cardiomyocytes (−2.5 pA pF−1, 11–68 years), with the caveat that these recordings at room temperature were significantly smaller in magnitude compared with the values reported by Schaffer et al. (1999) at physiological temperatures. Schaffer et al. (1999) also detected a slowly activating inward current in young ventricular myocytes that resembled the pacemaker current (If). In follow-up studies by the same group, Zorn-Pauly et al. (2003) detected this pacemaker current in 88% of paediatric right ventricular myocytes (mean age 15.4 months) but did not find an age-dependent difference in the current–voltage relationship or half-maximal activation, in comparison to adult left ventricular myocytes. Admittedly, the sample size for this study was small, and the If density in paediatric myocytes (−2.0 pA pF−1) was slightly larger than previously reported for adult myocytes (−1.18 pA pF−1; Hoppe et al., 1998). In experimental models, cardiomyocyte immaturity can alter If activation threshold, peak density and/or the expression of associated genes (Giannetti et al., 2021; Qu et al., 2001; Robinson et al., 1997), but additional studies are necessary to characterize postnatal developmental changes in human cardiomyocytes. Finally, spontaneous activity can be enhanced in immature cells through increased NCX expression and/or reverse-mode activity (Kim et al., 2015). NCX is localized to the cell periphery in neonatal human ventricular myocytes (preceding T-tubule formation), and NCX gene and/or protein expression is highest during the fetal–neonatal period (<2 weeks), declining thereafter with increasing postnatal age (Qu et al., 2000; Wiegerinck et al., 2009). In immature hPSC-CM, increased NCX activity enhances spontaneous activity (Kim et al., 2015), but the functional outcomes of NCX overexpression in human neonatal–infant cardiomyocytes have not been evaluated thoroughly.
Atrial and ventricular cardiomyocyte depolarization is driven by fast sodium current, INa (phase 0 of the action potential). Using isolated human atrial cardiomyocytes, Cai et al. (2011) measured a significantly smaller peak INa amplitude in paediatric cells (−31.8 pA pF−1; mean age 3.4 years old) compared with adult cells (−46.7 pA pF−1; mean age 42.5 years old). Furthermore, paediatric atrial myocytes had slower time-dependent properties (activation and inactivation time) and a shift in voltage-dependent inactivation towards a negative potential. A similar age-dependent shift in the voltage-dependence of INa inactivation was observed by Sakakibara et al. (1993) using paediatric and adult ventricular myocytes. Counterintuitive to the reduced INa peak amplitude observation by Cai et al. (2011), Wang et al. (2003) reported a significantly faster maximal rate of depolarization in paediatric (462 V s−1) versus adult (203 V s−1) atrial myocytes. A faster rate of depolarization is generally an indicator of cardiomyocyte maturation, because slower rates are commonly observed in neonatal myocytes or immature hPSC-CM (Agata et al., 1993; Hoekstra et al., 2012; Pacioretty & Gilmour, 1995).
Following rapid depolarization, early repolarization (or ‘notch’) is driven primarily by the transient outward current, Ito (phase 1 of the action potential). In an influential study, Escande et al. (1985) documented developmental changes in the atrial action potential of paediatric cells (2–22 months of age), with an immature repolarization pattern that suggested a lack of Ito. Paediatric atrial cells were less responsive to an Ito blocker (4-aminopyridine), and the shape of the paediatric action potential changed to become more comparable to an adult action potential after prolonged periods of rest, suggesting that Ito might be present but non-functional at physiological stimulation rates. Furthermore, paediatric atrial action potentials were more triangular, never displayed a prominent ‘notch’, and had a shorter (sometimes unidentifiable) plateau phase that hovered near a positive potential.
Follow-up studies strongly suggest a developmental change in Ito in the human atria, but details are inconsistent. Mansourati & le Grand (1993) reported that Ito was completely absent in atrial cells isolated from children (2 months to 5 years of age), but tissue samples were described as ‘clearly dilated and diseased’, which was found to reduce Ito markedly in adult cells. Using young atrial cells from haemodynamically normal children (1 day to 2.5 years of age), Crumb et al. (1995) detected Ito in only 67% of paediatric samples (compared with 100% of adult cells). Moreover, in younger atrial cells, Ito recovery was twice as slow (recovery time constant: 294 ms paediatric vs. 138 ms adult), with significant frequency-dependent inhibition and a significantly smaller peak current density (8 pA pF−1 young vs. 12.4 pA pF−1 adult). Notably, the greatest age-related change in peak current density occurred between the ages of 10 months and 2 years, suggesting that developmental adaptations occur early in life, within a narrow time frame. Wang et al. (2003) noted a similar action potential morphology in infant atrial cells (2.5–10.5 months of age), including a less prominent notch, more positive plateau phase, and a longer action potential duration at 30% repolarization (28.6 ms infant vs. 4 ms adult). However, in disagreement with earlier reports, Wang et al. (2003) found an increase in Ito peak density in infant atrial cells, but also noted faster inactivation, a slower recovery from inactivation (32 ms infant vs. 17 ms adult) and a more significant frequency-dependent inhibition of Ito peak current at physiological rates (23.7% infant vs. 8.9% adult inhibition at 3 Hz). Slower Ito inactivation in adult atrial cells would lead to more current during the early phase of depolarization, which shortens repolarization time at APD30. However, many of these findings were not observed in a study by Gross et al. (1994), in which the authors reported no discernible age-dependent differences in Ito density or recovery and only a slightly slower inactivation in younger atrial cells (59.3 ms, vs. 43.3 ms in older children). Measurements in paediatric ventricular cardiomyocytes are more limited. Cohen and Lederer (1993) reported that paediatric ventricular cardiomyocytes had a less prominent notch and a more positive plateau phase, consistent with many of the atrial myocyte findings described above. Paediatric action potentials also appeared significantly longer, at 30% or 50% repolarization, although average values were not reported in the study. Schaffer et al. (1999) detected Ito in paediatric ventricular myocytes (16–91 months of age), but peak density measurements were variable even within the same patient, ranging from 0.3 to 8.6 pA pF−1. Furthermore, the authors noted a rate-dependent reduction in Ito amplitude (11–65%) when ventricular myocytes were stimulated at 4 Hz, but with notable variations between individual cells.
As noted above, paediatric atrial and ventricular myocytes display a more positive plateau phase (phase 2 of the cardiac action potential), suggesting sustained Ca2+ influx and/or reduced K+ efflux (Cohen & Lederer, 1993; Escande et al., 1985; Wang et al., 2003). Cohen and Lederer (1993) reported a significant reduction in ICa density in paediatric (5.3 mA cm−2, 3–18 months of age) versus adult ventricular myocytes (10.1 mA cm−2), but did not find a comparable developmental difference in atrial cells. However, both paediatric atrial and ventricular myocytes displayed a shift in steady-state activation and inactivation to more positive potentials(+5to+17mVshift),resulting in a prominent ICa window current. This is consistent with the adult action potential plateau phase occurring at more negative potentials, in comparison to paediatric cells. Pelzmann et al. (1998) also observed a large Ca2+ window current between −45 and +40 mV in paediatric ventricular myocytes, which can prolong Ca2+ influx and increase the incidence of early after-depolarizations. Notably, two studies that used tissue samples from patients with tetralogy of Fallot recorded significantly longer action potential duration (APD) at 90% repolarization with a high incidence of afterdepolarizations [Pelzmann et al. (1998): 853 ± 167 ms; Schaffer et al. (1999): 794 ± 99 ms] compared with a mixed population of congenital heart disease patients [Cohen and Lederer (1993): 292 ± 84 ms]. Accordingly, it is difficult to discern whether developmental changes in ICa might be exaggerated further with specific pathologies. Minimal experimental data are available on the presence and kinetics of repolarizing K+ currents in phase 2 or 3 of the cardiac action potential. Schaffer et al. (1999) measured IK in ventricular myocytes from children with tetralogy of Fallot, but detected this current in only 2 of 34 myocytes. The time course of activation was fast (within 250 ms), suggesting that the current was probably attributable to the rapid component of the delayed rectifier (IKr) (Koumi et al., 1995). Developmental changes in cardiac delayed rectifier K+ current have been observed in other species (Obreztchikova et al., 2003; Wang et al., 1996), but postnatal adaptations remain underexplored in humans.
Maturation of excitation–contraction coupling
Excitation–contraction coupling is the process by which an action potential triggers contraction and subsequent relaxation. In adult ventricular cardiomyocytes, sarcolemmal L-type calcium channels (LTCCs) are localized to T-tubules in close proximity to RYRs on the junctional SR. These LTCCs open during phase 2 of the cardiac action potential, allowing Ca2+ ions to flow into the cardiomyocyte and increase local [Ca2+], which triggers Ca2+ release from the SR in a process known as calcium-induced calcium release (CICR). This increase in intracellular [Ca2+] saturates the troponin complex, resulting in a conformational change that permits actin–myosin interaction and contraction. Following contraction, cardiac relaxation requires a decline in intracellular [Ca2+], largely through the SR calcium-ATPase (SERCA) and NCX. This process is tightly regulated, both temporally and spatially, permitting fast, coordinated cycling of Ca2+ across the large-sized adult cardiomyocyte.
Developing cardiomyocytes have contractile activity, but anatomical immaturity limits excitation–contraction coupling efficiency in these cells. This is attributable, in part, to structural adaptations that are still missing during early postnatal life, because T-tubules are underdeveloped in the newborn human heart (<3 weeks) but are well developed later in infancy (3–14 months) (Crossman et al., 2011; Kim et al., 1992; Lyon et al., 2009; Wiegerinck et al., 2009). The T-tubules are extensions of the sarcolemma that penetrate into the cardiomyocyte; these structures are protein rich (e.g. LTCC and NCX), and subsections of the T-tubule network participate in dyadic coupling with the SR, which facilitates the CICR process. In rodents, T-tubules and dyads are also absent at birth, but begin to form and mature early in life between postnatal days 10 and 20 (Chen et al., 2013; Ziman et al., 2010). Consequently, fetal–neonatal rodent cardiomyocytes have fewer calcium sparks and less SR Ca2+ content and exhibit smaller calcium transients, with a slower upstroke and decay time (Seki et al., 2003; Ziman et al., 2010). As the cellular organization matures, excitation–contraction coupling becomes more efficient, with less dependence on trans-sarcolemmal Ca2+ influx (via NCX and T-type calcium channels), increased SR Ca2+ content and greater reliance on SERCA reuptake, resulting in prominent calcium transients (Bassani & Bassani, 2002; Cohen & Lederer, 1988; Escobar et al., 2004; Reppel et al., 2007; Swift et al., 2020).
Measurements of calcium require the use of live cardiomyocytes, and as a result, these studies are extremely limited in fetal, neonatal or infant human cardiomyocytes. Using single cells isolated from myocardial specimens obtained intraoperatively, Cohen and Lederer (1993) reported that paediatric ventricular myocytes (3–18 months of age) had reduced ICa density and a prominent Ca2+ window, in comparison to adults (Table 6). Likewise, Pelzmann et al. (1998) reported a large window current in paediatric ventricular myocytes (16–91 months of age), which favoured the occurrence of delayed after-depolarizations. A comparable shift in ICa steady-state inactivation and a prominent window current have also been observed in infant versus adult atrial myocytes (Cohen & Lederer, 1993). However, ICa density might be similar (Cohen & Lederer, 1993; Roca et al., 1996) or only slightly reduced in infant versus adult atrial cells (Hatem et al., 1995; Tipparaju et al., 2004). Tipparaju et al. (2004) reported that infant atrial myocytes had lower basal L-type calcium current amplitude (1.2 pA pF−1 at room temperature; 3–10 months of age) compared with adults (2.6 pA pF−1; >50 years of age). Additional work by Wagner et al. (2005) found that infant atrial myocytes (3–8 months old) had a shorter calcium transient amplitude when adult action potentials were applied as voltage-clamp waveforms, but the amplitude increased when infant action potentials were applied (with a longer early repolarization phase). This finding suggests that infant atrial cells are more dependent on trans-sarcolemmal Ca2+ influx for contraction, whereby a prolonged early repolarization phase helps to increase Ca2+ entry and intracellular Ca2+ levels (with perhaps, less dependence on CICR and SR Ca2+ release). Hatem et al. (1995) also reported age-dependent effects, whereby infant atrial cells (<1 month old) had a slower Ca2+ upstroke velocity compared with infant and adolescent cells (>1 month old), which is probably attributable to structural immaturity and inefficient coupling between LTCCs and RYRs. However, the authors did find evidence of CICR and SR Ca2+ release beginning at a young age (3 days to 4 years of age), because Ca2+ transient amplitudes in atrial myocytes were smaller when the SR Ca2+ load was depleted by caffeine or ryanodine.
Table 6.
Developmental changes in excitation-contraction coupling
| Age range | Cardiomyocyte type | Key points | Citation |
|
| |||
| <1–75 years | Right ventricular | Age-dependent increase in ICa density | Cohen and Lederer (1993) |
| <1–75 years 1–8 years | Right atrial and ventricular Right ventricular |
Age-dependent decrease in calcium window current | Cohen and Lederer (1993); Pelzmann et al. (1998) |
| <1–79 years | Right atria |
Age-dependent increase in ICa amplitude Lower basal L-type calcium current amplitude and increased expression of inhibitory G proteins is seen in infant atrial cells, compared with adults |
Tipparaju et al. (2004) |
| <1–79 years | Right atria |
No age-dependent difference in ICa No evidence for age-related changes in ICa density, steady-state inactivation or recovery from inactivation |
Roca et al. (1996) |
| <2 years <0–40 years |
Left and right ventricle Ventricular |
Age-dependent increase in calcium-handling proteins Age-dependent increase in NCX, SERCA, phospholamban and L-type calcium channel; age-dependent decrease in T-type calcium channel expression |
Wiegerinck et al. (2009); Qu et al. (2000) |
| <1–4 years | Right atria |
Excitation-contraction coupling matures with postnatal age Slower Ca2+ upstroke velocity in infant cells is likely to be attributable to structural immaturity and inefficient coupling between calcium channels and ryanodine receptors |
Hatem et al. (1995) |
| <1 year | Right atria |
Paediatric action potential waveform modulates excitation-contraction coupling Prolonged early repolarization in paediatric cells is correlated with increased dependence on trans-sarcolemmal Ca2+ influx for contraction |
Wagner et al. (2005) |
| <2 years | Left and right ventricle |
Age-dependent increase in force-frequency relationship At faster frequencies, newborn ventricular muscle strips have less developed force compared with slightly older infant muscle strips; results are likely to be linked to developmental changes in protein expression |
Wiegerinck et al. (2009) |
Age-dependent differences in excitation–contraction coupling are influenced by the variable expression of key calcium-handling proteins during development. Given that ICa density is reduced and CICR is inefficient in immature mammalian myocytes, these cells have a greater reliance on NCX for modulating intracellular [Ca2+] (Artman, 1992; Chin et al., 1997; Escobar et al., 2004). Age-dependent changes in NCX expression are also observed in the heart, wherein peak NCX expression is observed in the fetal heart, with reduced expression at birth or later in adulthood (Qu et al., 2000; Wiegerinck et al., 2009). The human heart also displays an age-dependent increase in LTCCs, SERCA and phospholamban expression and a decrease in T-type calcium channel expression at birth and later in adulthood, in comparison to early gestation (Qu & Boutjdir, 2001; Wiegerinck et al., 2009). It is unclear whether these developmental differences occur continuously after birth, because human tissue samples are scarce, and results are almost always compared with adult tissue. Accordingly, our knowledge of perinatal cardiomyocyte development (e.g. fetal, infant and adolescent) is extremely limited. One exception is a study by Wiegerinck et al. (2009), which found that newborn ventricular muscle strips (<2 weeks of age) had reduced phospholamban expression, reduced phospholamban:SERCA ratio and increased NCX expression compared with slightly older infants (3–14 months of age). In tandem, newborn muscle strips displayed a blunted force–frequency response, suggesting that the SR Ca2+ load was decreased, possibly through increased Ca2+ extrusion (via NCX) or reduced Ca2+ reuptake (via SERCA). However, a blunted force–frequency response might also be attributed to differences in troponin isoform expression and sensitivity (Bhavsar et al., 1991). Finally, the newborn myocardium was less responsive to adrenergic stimulation; immature atrial cells responded to isoprenaline application with increased ICa peak and Ca2+ transient amplitude, but relaxation was unchanged, which can be attributed to reduced troponin sensitivity, phospholamban activity and/or SERCA reuptake (Bhavsar et al., 1991; Hatem et al., 1995). Decreased sensitivity to isoprenaline stimulation has also been attributed to increased expression of inhibitory G proteins in infant atrial cells (Tipparaju et al., 2004), which is in agreement with age-dependent studies performed using rabbit neonatal and adult myocytes (Kumar et al., 1996). As noted in the electrophysiology section, slight discrepancies between studies might be attributable to differences in recording temperature, small sample size, variable age groups and/or underlying pathology. All the described studies were conducted using cells obtained intraoperatively from neonates or children with congenital heart defects. Similar studies using ‘normal’ cardiac tissue are not feasible.
Conclusion
Here, we have highlighted key foundation studies that have informed our understanding of human cardiomyocyte maturation during postnatal development. Based on existing knowledge, it is evident that human atrial and ventricular myocytes undergo significant age-dependent changes in size, structure, electrophysiology and contractile phenotype as they transition from the intrauterine to extrauterine environment. Collectively, these studies suggest that human myocytes evolve rapidly within the first few months to 1 year, but do not reach a fully mature adult phenotype until the first decade of life (e.g. polyploidy and intracellular coupling). However, several limitations should be considered. Notably, human developmental studies rely on the availability of tissue biospecimens that are collected either post-mortem or as medical waste from patients undergoing palliative or corrective heart surgery. Biospecimens collected during surgery are dependent on clinical diagnosis; neonatal patients (<1 month of age) are likely to have more severe cyanotic heart conditions that require early intervention, whereas older infants or children are more likely to have acyanotic conditions. Furthermore, the type of tissue available will depend on the corrective surgery performed; atrial appendage tissue might be procured during cannulation for cardiopulmonary bypass, whereas ventricular tissue might be resected in select patient groups (e.g. tetralogy of Fallot, truncus arteriosus or patients receiving a ventricular assist device). As such, patient age, disease, course of treatment, medication use and the anatomical location of tissue samples are all confounding factors that can influence data interpretation. It is also important to note that most of the studies described in this review article estimate the timing of cardiomyocyte maturation based on a relatively small sample size, with a limited age distribution; therefore, temporal patterns are only a rough approximation. Experimental animal models and/or hPSC-CM can help to fill in knowledge gaps regarding postnatal maturation into adulthood, but this should be pursued with caution, because species-specific differences and/or the lack of maturity beyond a fetal/neonatal phenotype have been well documented.
Human cardiomyocyte maturation is a dynamic process that warrants further investigation for a variety of reasons. First, defining the normal developmental process can help us to understand better the cardiac diseases that develop during gestation or shortly after birth. Second, a number of cardiac medications have been designed for adults, with off-label use in neonates, infants and paediatric patients (Bates et al., 2012; Pasquali et al., 2008). A more thorough understanding of paediatric cardiomyocyte physiology can better inform clinical care decisions and the use of age-appropriate drug therapies for young patients. Third, human adult cardiomyocytes are considered non-proliferative, because cell-cycle arrest is observed within the first year of life. Identifying key regulators of cell-cycle arrest can identify new strategies to ‘restart’ cell proliferation, with a focus on regenerating and repairing injured muscle tissue (Patterson et al., 2017; Porrello et al., 2011; Puente et al., 2014; Velayutham et al., 2019). Finally, experimental animal or hPSC-CM models will continue to play a prominent role in cardiac research. By characterizing the temporal patterns of human cardiomyocyte maturation, we can optimize the use of these experimental models at developmentally appropriate stages and improve the applicability of our findings.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr Kazi Haq and Dr Charles Berul for helpful scientific discussions.
Funding
This work was supported by the US National Institutes of Health (R01HD108839 to N.G.P.), the Children’s National Research Institute and the Children’s National Heart Institute.
Biographies

Shatha Salameh is a PhD student in the Institute for Biomedical Sciences programme at The George Washington University School of Medicine and Health Sciences. She received her Master’s degree in Pharmacology from Georgetown University. Her research is focused on human postnatal cardiac maturation.

Nikki Gillum Posnack is an associate professor at the Children’s National Hospital, with faculty appointments in the Sheikh Zayed Institute for Pediatric Surgical Innovation and Children’s National Heart Institute. She also maintains academic appointments at The George Washington University School of Medicine and Health Sciences in the Department of Pediatrics and the Department of Pharmacology and Physiology. Researchers in Dr Posnack’s laboratory are studying the impact of age and disease on cardiac physiology.
Footnotes
Competing interests
The authors declare no competing interests.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the HTML view of the article. Supporting information files available:
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP283792#support-information-section).
References
- Abouleisa RRE, Salama ABM, Ou Q, Tang XL, Solanki M, Guo Y, Nong Y, McNally L, Lorkiewicz PK, Kassem KM, Ahern BM, Choudhary K, Thomas R, Huang Y, Juhardeen HR, Siddique A, Ifthikar Z, Hammad SK, Elbaz AS, … Mohamed TMA (2022). Transient cell cycle induction in cardiomyocytes to treat subacute ischemic heart failure. Circulation, 145(17), 1339–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler C (1975). Relationship between deoxyribonucleic acid content and nucleoli in human heart muscle cells and estimation of cell number during cardiac growth and hyperfunction – PubMed. Recent Advances in Studies of Cardiac Structure and Metabolism, 8, 373–386. [PubMed] [Google Scholar]
- Adler CP, & Costabel U (1975). Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent Advances in Studies on Cardiac Structure and Metabolism, 6, 343–355. [PubMed] [Google Scholar]
- Adler CP, & Costabel U (1980). Myocardial DNA and cell number under the influence of cytostatics – I. Post mortem investigations of human hearts. Virchows Archiv. B, Cell Pathology Including Molecular Pathology, 32(1), 109–125. [DOI] [PubMed] [Google Scholar]
- Adler CP, Friedburg H, Herget GW, Neuburger M, & Schwalb H (1996). Variability of cardiomyocyte DNA content, ploidy level and nuclear number in mammalian hearts. Virchows Archiv, 429, 159–164. [DOI] [PubMed] [Google Scholar]
- Agata N, Tanaka H, & Shigenobu K (1993). Developmental changes in action potential properties of the guinea-pig myocardium. Acta Physiologica Scandinavica, 149(3), 331–337. [DOI] [PubMed] [Google Scholar]
- Agata Y, Hiraishi S, Oguchi K, Misawa H, Horiguchi Y, Fujino N, Yashiro K, & Shimada N (1991). Changes in left ventricular output from fetal to early neonatal life. Journal of Pediatrics, 119(3), 441–445. [DOI] [PubMed] [Google Scholar]
- Anderson PAW, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, & Kay BK (1995). Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circulation Research, 76(4), 681–686. [DOI] [PubMed] [Google Scholar]
- Anderson PAW, Malouf NN, Oakeley AE, Pagani ED, & Allen PD (1991). Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circulation Research, 69(5), 1226–1233. [DOI] [PubMed] [Google Scholar]
- Ang KL, Shenje LT, Reuter S, Soonpaa MH, Rubart M, Field LJ, & Galiñanes M (2010). Limitations of conventional approaches to identify myocyte nuclei in histologic sections of the heart. American Journal of Physiology. Cell Physiology, 298(6), C1603–C1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artman M (1992). Sarcolemmal Na(+)-Ca2+ exchange activity and exchanger immunoreactivity in developing rabbit hearts. American Journal of Physiology, 263(5), H1506–H1513. [DOI] [PubMed] [Google Scholar]
- Auckland LM, Lambert SJ, & Cummins P (1986). Cardiac myosin light and heavy chain isotypes in tetralogy of Fallot. Cardiovascular Research, 20(11), 828–836. [DOI] [PubMed] [Google Scholar]
- Austin A, Fagan DG, & Mayhew TM (1995). A stereological method for estimating the total number of ventricular myocyte nuclei in fetal and postnatal hearts. Journal of Anatomy, 187, 641. [PMC free article] [PubMed] [Google Scholar]
- Ball AJ, & Levine F (2005). Telomere-independent cellular senescence in human fetal cardiomyocytes. Aging Cell, 4(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Bartelds B, Gratama JWC, Knoester H, Takens J, Smid GB, Aarnoudse JG, Heymans HSA, & Kuipers JRG (1998). Perinatal changes in myocardial supply and flux of fatty acids, carbohydrates, and ketone bodies in lambs. American Journal of Physiology, 274(6), H1962–H1969. [DOI] [PubMed] [Google Scholar]
- Bartelds B, Knoester H, Beaufort-Krol GCM, Smid GB, Takens J, Zijlstra WG, Heymans HSA, & Kuipers JRG (1999). Myocardial lactate metabolism in fetal and newborn lambs. Circulation, 99(14), 1892–1897. [DOI] [PubMed] [Google Scholar]
- Bartelds B, Knoester H, Smid GB, Takens J, Visser GH, Penninga L, van der Leij FR, Beaufort-Krol GCM, Zijlstra WG, Heymans HSA, & Kuipers JRG (2000). Perinatal changes in myocardial metabolism in lambs. Circulation, 102(8), 926–931. [DOI] [PubMed] [Google Scholar]
- Barth E, Stämmler G, Speiser B, & Schaper J (1992). Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. Journal of Molecular and Cellular Cardiology, 24(7), 669–681. [DOI] [PubMed] [Google Scholar]
- Bartos DC, Grandi E, & Ripplinger CM (2015). Ion Channels in the Heart. Compr Physiol, 5, 1423–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, & Bassani JWM (2002). Contribution of Ca(2+) transporters to relaxation in intact ventricular myocytes from developing rats. American Journal of Physiology. Heart and Circulatory Physiology, 282(6), H2406–H2413. [DOI] [PubMed] [Google Scholar]
- Bates KE, Vetter VL, Li JS, Cummins S, Aguel F, Almond C, Dubin AM, Elia J, Finkle J, Hausner EA, Joseph F, Karkowsky AM, Killeen M, Lemacks J, Mathis L, McMahon AW, Pinnow E, Rodriguez I, Stockbridge NL, … Krucoff MW (2012). Pediatric cardiovascular safety: Challenges in drug and device development and clinical application. American Heart Journal, 164(4), 481–492. [DOI] [PubMed] [Google Scholar]
- Bedada FB, Chan SSK, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, & Metzger JM (2014). Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports, 3(4), 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley JG, de Matteo R, Harding R, & Black MJ (2016). Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections. Scientific Reports, 6(1), 23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CW, Okawa S, Chuva De Sousa Lopes SM, van Iperen L, Passier R, Braam SR, Tertoolen LG, del Sol A, Davis RP, & Mummery CL (2015). Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development (Cambridge, England), 142, 3231–3238. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, & Frisén J (2015). Dynamics of Cell Generation and Turnover in the Human Heart. Cell, 161(7), 1566–1575. [DOI] [PubMed] [Google Scholar]
- Bhavsar PK, Dhoot GK, Cumming DVE, Butler-Browne GS, Yacoub MH, & Barton PJR (1991). Developmental expression of troponin I isoforms in fetal human heart. Febs Letters, 292, 5–8. [DOI] [PubMed] [Google Scholar]
- Bond AR, Iacobazzi D, Abdul-Ghani S, Ghorbel M, Heesom K, Wilson M, Gillett C, George SJ, Caputo M, Suleiman S, & Tulloh RMR (2018). Changes in contractile protein expression are linked to ventricular stiffness in infants with pulmonary hypertension or right ventricular hypertrophy due to congenital heart disease. Open Heart, 5(1), e000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting KJ, Caroline McMillen I, Forbes H, Nyengaard JR, & Morrison JL (2014). Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. Journal of the American Heart Association, 3(4), e000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botting KJ, Wang KCW, Padhee M, Mcmillen IC, Summers-Pearce B, Rattanatray L, Cutri N, Posterino GS, Brooks DA, & Morrison JL (2012). Early origins of heart disease: Low birth weight and determinants of cardiomyocyte endowment. Clinical and Experimental Pharmacology & Physiology, 39(9), 814–823. [DOI] [PubMed] [Google Scholar]
- Bouvagnet P, Mairhofer H, Leger JOC, Puech P, & Leger JJ (1989). Distribution pattern of alpha and beta myosin in normal and diseased human ventricular myocardium. Basic Research in Cardiology, 84(1), 91–102. [DOI] [PubMed] [Google Scholar]
- Bratincsák A, Kimata C, Limm-Chan BN, Vincent KP, Williams MR, & Perry JC (2020). Electrocardiogram Standards for Children and Young Adults Using Z-Scores. Circ Arrhythm Electrophysiol, 13(8), E008253. [DOI] [PubMed] [Google Scholar]
- Brodsky VY, Arefyeva AM, Gvasava IG, Sarkisov DS, & Panova NW (1994). Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Archiv, 424(4), 429–435. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, & Lumbers ER (2003). Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 274A(2), 952–961. [DOI] [PubMed] [Google Scholar]
- Cai B, Mu X, Gong D, Jiang S, Li J, Meng Q, Bai Y, Liu Y, Wang X, Tan X, Yang B, & Lu Y (2011). Difference of sodium currents between pediatric and adult human atrial myocytes: Evidence for developmental changes of sodium channels. Int J Biol Sci, 7(6), 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E (2019). Pacemaking in cardiac tissue. From IK2 to a coupled-clock system. Physiological Reports, 7(1), e13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini S, Verkerk AO, & Remme CA (2017). Human iPSC-derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: Strengths and limitations. Cardiovascular Drugs and Therapy, 31(3), 325–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Pino R, Sartiani L, & Mugelli A (1999). Influence of postnatal-development on I(f) occurrence and properties in neonatal rat ventricular myocytes. Cardiovascular Research, 42(2), 416–423. [DOI] [PubMed] [Google Scholar]
- Chan TC, Sharieff GQ, & Brady WJ (2008). Electrocardiographic Manifestations: Pediatric ECG. Journal of Emergency Medicine, 35(4), 421–430. [DOI] [PubMed] [Google Scholar]
- Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, & Dobrzynski H (2009). Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation, 119(12), 1562–1575. [DOI] [PubMed] [Google Scholar]
- Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XHT, & Song LS (2013). Critical roles of junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovascular Research, 100(1), 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen S-CC, Davis LM, Westphale EM, Beyer EC, Saffitz JE, & Saffitz JE (1994). Expression of multiple gap junction proteins in human fetal and infant hearts. Pediatric Research, 36(5), 561–566. [DOI] [PubMed] [Google Scholar]
- Chin TK, Christiansen GA, Caldwell JG, & Thorburn J (1997). Contribution of the sodium-calcium exchanger to contractions in immature rabbit ventricular myocytes. Pediatric Research, 41(4), 480–485. [DOI] [PubMed] [Google Scholar]
- Clubb FJ, & Bishop SP (1984). Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Laboratory Investigation, 50, 571–577. [PubMed] [Google Scholar]
- Cohen N, & Lederer W (1993). Calcium Current in Single Human Cardiac Myocytes. Journal of Cardiovascular Electrophysiology, 4(4), 422–437. [DOI] [PubMed] [Google Scholar]
- Cohen NM, & Lederer WJ (1988). Changes in the calcium current of rat heart ventricular myocytes during development. The Journal of Physiology, 406(1), 115–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colan SD, Parness IA, Spevak PJ, & Sanders SP (1992). Developmental modulation of myocardial mechanics: Age- and growth-related alterations in after-load and contractility. Journal of the American College of Cardiology, 19(3), 619–629. [DOI] [PubMed] [Google Scholar]
- Coppen SR, Kaba RA, Halliday D, Dupont E, Skepper JN, Elneil S, & Severs NJ (2003). Comparison of connexin expression patterns in the developing mouse heart and human foetal heart. Molecular and Cellular Biochemistry, 242(1/2), 121–127. [PubMed] [Google Scholar]
- Crossman DJ, Ruygrok PR, Soeller C, & Cannell MB (2011). Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS ONE, 6(3), e17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumb WJ, Pigott JD, & Clarkson CW (1995). Comparison of I(to) in young and adult human atrial myocytes: Evidence for developmental changes. American Journal of Physiology. Heart and Circulatory Physiology, 268(3), H1335–H1342. [DOI] [PubMed] [Google Scholar]
- Cummins P, & Lambert SJ (1986). Myosin transitions in the bovine and human heart. A developmental and anatomical study of heavy and light chain subunits in the atrium and ventricle. Circulation Research, 58(6), 846–858. [DOI] [PubMed] [Google Scholar]
- Cummins P, Price KM, & Littler WA (1980). Foetal myosin light chain in human ventricle. Journal of Muscle Research and Cell Motility, 1(3), 357–366. [DOI] [PubMed] [Google Scholar]
- Dai DF, Danoviz ME, Wiczer B, Laflamme MA, & Tian R (2017). Mitochondrial Maturation in Human Pluripotent Stem Cell Derived Cardiomyocytes. Stem Cells Int, 2017, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LM, Rodefeld ME, Green K, Beyer EC, & Saffitz JE (1995). Gap junction protein phenotypes of the human heart and conduction system. Journal of Cardiovascular Electrophysiology, 6(10), 813–822. [DOI] [PubMed] [Google Scholar]
- DeLaughter DM, Bick AG, Wakimoto H, McKean D, Gorham JM, Kathiriya IS, Hinson JT, Homsy J, Gray J, Pu W, Bruneau BG, Seidman JG, & Seidman CE (2016). Single-cell resolution of temporal gene expression during heart development. Developmental Cell, 39(4), 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning C, Borgdorff V, Crutchley J, Firth KSA, George V, Kalra S, Kondrashov A, Hoang MD, Mosqueira D, Patel A, Prodanov L, Rajamohan D, Skarnes WC, Smith JGW, & Young LE (2016). Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochimica Et Biophysica Acta, 1863(7), 1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Wu L, Duan Y, Cui H, Chen K, Chen X, Sun Y, Du C, Ren J, Shu S, Yan X, Wan X, Song J, Yan J, Ya X, Wan X, Song J, & Yan J (2021). Metabolic profile of heart tissue in cyanotic congenital heart disease. American journal of translational research, 13, 4224. [PMC free article] [PubMed] [Google Scholar]
- Edwards AG, & Louch WE (2017). Species-dependent mechanisms of cardiac arrhythmia: A cellular focus. Clin Med Insights Cardiol, 11, 117954681668606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E (2016). Cardiac cytoarchitecture – why the “hardware” is important for heart function! Biochimica Et Biophysica Acta, 1863(7), 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein R, & Wied GL (1970). Myocardial DNA and protein in maturing and hypertrophied human hearts. Proceedings of the Society for Experimental Biology and Medicine, 133(1), 176–179. [DOI] [PubMed] [Google Scholar]
- Elhamine F, Iorga B, Krüger M, Hunger M, Eckhardt J, Sreeram N, Bennink G, Brockmeier K, Pfitzer G, & Stehle R (2016). Postnatal Development of Right Ventricular Myofibrillar Biomechanics in Relation to the Sarcomeric Protein Phenotype in Pediatric Patients with Conotruncal Heart Defects. Journal of the American Heart Association, 5(6), e003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D, Loisance D, Planche C, & Coraboeuf E (1985). Age-related changes of action potential plateau shape in isolated human atrial fibers. American Journal of Physiology. Heart and Circulatory Physiology, 249(4), H843–H850. [DOI] [PubMed] [Google Scholar]
- Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Pérez CG, & Mejía-Alvarez R (2004). Developmental changes of intracellular Ca2+ transients in beating rat hearts. American Journal of Physiology. Heart and Circulatory Physiology, 286(3), H971–H978. [DOI] [PubMed] [Google Scholar]
- Estigoy CB, Pontén F, Odeberg J, Herbert B, Guilhaus M, Charleston M, Ho JWK, Cameron D, & dos Remedios CG (2009). Intercalated discs: Multiple proteins perform multiple functions in non-failing and failing human hearts. Biophysical Reviews, 1(1), 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric NT, & Radisic M (2016). Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Advanced Drug Delivery Reviews, 96, 110–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez C, Hajnóczky G, & Csordás G (2008). Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. Journal of Biological Chemistry, 283(47), 32771–32780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M, & Djinović-Carugo K (2016). The sarcomeric cytoskeleton: From molecules to motion. Journal of Experimental Biology, 219(2), 135–145. [DOI] [PubMed] [Google Scholar]
- Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, & Schocken DD (1992). Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation, 86(2), 426–430. [DOI] [PubMed] [Google Scholar]
- Giannetti F, Benzoni P, Campostrini G, Milanesi R, Bucchi A, Baruscotti M, Dell’Era P, Rossini A, & Barbuti A (2021). A detailed characterization of the hyperpolarization-activated “funny” current (If) in human-induced pluripotent stem cell (iPSC)-derived cardiomyocytes with pacemaker activity. Pflugers Archiv: European journal of physiology, 473(7), 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CL (1978). Cardiac energetics. Physiological Reviews, 58(1), 174–254. [DOI] [PubMed] [Google Scholar]
- Girard J, Ferre P, Pegorier JP, & Duee PH (1992). Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiological Reviews, 72(2), 507–562. [DOI] [PubMed] [Google Scholar]
- Goversen B, van der Heyden MAG, van Veen TAB, & de Boer TP (2018). The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on IK1. Pharmacology & Therapeutics, 183, 127–136. [DOI] [PubMed] [Google Scholar]
- Grandi E, Navedo MF, Saucerman JJ, Bers DM, Chiamvimonvat N, Dixon RE, Dobrev D, Gomez AM, Harraz OF, Hegyi B, Jones DK, Krogh-Madsen T, Murfee WL, Nystoriak MA, Posnack NG, Ripplinger CM, Veeraraghavan R, & Weinberg S (2023). Diversity of cells and signals in the cardiovascular system. The Journal of Physiology. Advance online publication. 10.1113/JP284011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener ID, Monfredi O, Inada S, Chandler NJ, Tellez JO, Atkinson A, Taube MA, Billeter R, Anderson RH, Efimov IR, Molenaar P, Sigg DC, Sharma V, Boyett MR, & Dobrzynski H (2011). Molecular architecture of the human specialised atrioventricular conduction axis. Journal of Molecular and Cellular Cardiology, 50(4), 642–651. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Burke RP, & Castle NA (1994). Characterisation of transient outward current in young human atrial myocytes. Cardiovascular Research, 28, 112–117. [PubMed] [Google Scholar]
- Günthel M, Barnett P, & Christoffels VM (2018). Development, proliferation, and growth of the mammalian heart. Molecular Therapy, 26(7), 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem SN, Sweeten T, Vetter V, & Morad M (1995). Evidence for presence of Ca2+ channel-gated Ca2+ stores in neonatal human atrial myocytes. American Journal of Physiology. Heart and Circulatory Physiology, 268(3), H1195–H1201. [DOI] [PubMed] [Google Scholar]
- Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, & Penninger JM (2016). Functional recovery of a human neonatal heart after severe myocardial infarction. Circulation Research, 118(2), 216–221. [DOI] [PubMed] [Google Scholar]
- Henderson CA, Gomez CG, Novak SM, Mi-Mi L, & Gregorio CC (2017). Overview of the muscle cytoskeleton. Compr Physiol, 7, 891–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschy A, Schatzmann F, Ehler E, & Perriard JC (2006). Establishment of cardiac cytoarchitecture in the developing mouse heart. Developmental Biology, 289(2), 430–441. [DOI] [PubMed] [Google Scholar]
- Hodges MM, Zgheib C, & Liechty KW (2021). A large mammalian model of myocardial regeneration after myocardial infarction in fetal sheep. Adv Wound Care (New Rochelle), 10(4), 174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M, Mummery CL, Wilde AAM, Bezzina CR, & Verkerk AO (2012). Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front Physiol, 3, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe UC, Jansen E, Südkamp M, & Beuckelmann DJ (1998). Hyperpolarization-activated inward current in ventricular myocytes from normal and failing human hearts. Circulation, 97(1), 55–65. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, & Bai X (2019). Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cells, 8(9), 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Chen YC, Yeh HI, & Chen SA (2012). Mononucleated and binucleated cardiomyocytes in left atrium and pulmonary vein have different electrical activity and calcium dynamics. Progress in Biophysics and Molecular Biology, 108(1–2), 64–73. [DOI] [PubMed] [Google Scholar]
- Hunkeler NM, Kullman J, & Murphy AM (1991). Troponin I isoform expression in human heart. Circulation Research, 69(5), 1409–1414. [DOI] [PubMed] [Google Scholar]
- Huttenbach Y, Ostrowski ML, Thaller D, & Kim HS (2001). Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovascular Pathology, 10(3), 119–123. [DOI] [PubMed] [Google Scholar]
- Imura H, Caputo M, Parry A, Pawade A, Angelini GD, & Suleiman MS (2001). Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation, 103(11), 1551–1556. [DOI] [PubMed] [Google Scholar]
- Iruretagoyena JI, Davis W, Bird C, Olsen J, Radue R, Teo Broman A, Kendziorski C, Splinter BonDurant S, Golos T, Bird I, & Shah D (2014). Metabolic gene profile in early human fetal heart development. Molecular Human Reproduction, 20(7), 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann Hau SJS (2010). Handbook of laboratory animal science, volume I, third edition: Essential principles and practices. CRC Press. [Accessed November 7, 2022]. https://www.crcpress.com/Handbook-of-Laboratory-Animal-Science-Volume-I-Third-Edition-Essential/Hau-Schapiro/9781420084559 [Google Scholar]
- Janssen PML, & Periasamy M (2007). Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. Journal of Molecular and Cellular Cardiology, 43(5), 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe ID, Crossman DJ, Soeller C, & Cannell MB (2012). Comparison of the organization of T-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clinical and Experimental Pharmacology & Physiology, 39(5), 469–476. [DOI] [PubMed] [Google Scholar]
- St John Sutton MG, Marier DL, & Oldershaw PJ (1982). Effect of age related changes in chamber size, wall thickness, and heart rate on left ventricular function in normal children. British Heart Journal, 48, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, & Giraud GD (2007). Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 292(2), R913–R919. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Kamna D, LoTurco D, Kailey J, & Brown LD (2018). IUGR impairs cardiomyocyte growth and maturation in fetal sheep. Journal of Endocrinology, 239(2), 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, & Louey S (2016). Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment. Journal of Endocrinology, 228(1), R1–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Louey S, Giraud GD, Thornburg KL, & Faber JJ (2015). Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. Faseb Journal, 29(10), 4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Louey S, & Roselli CE (2018). Cardiac myocyte proliferation and maturation near term is inhibited by early gestation maternal testosterone exposure. American Journal of Physiology. Heart and Circulatory Physiology, 315(5), H1393–H1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, & Faber JJ (2007). Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. Journal of Applied Physiology, 102(3), 1130–1142. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Martí M, Raya A, & Belmonte JCI (2010). Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature, 464(7288), 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurko Jr A. (2004). Echocardiographic evaluation of left ventricle postnatal growth in newborns and infants – PubMed. Bratislavske Lekarske Listy, 105(2), 78–85. [PubMed] [Google Scholar]
- Kannan S, Farid M, Lin BL, Miyamoto M, & Kwon C (2021). Transcriptomic entropy benchmarks stem cell-derived cardiomyocyte maturation against endogenous tissue at single cell level. Plos Computational Biology, 17(9), e1009305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, & Kwon C (2020). Regulation of cardiomyocyte maturation during critical perinatal window. The Journal of Physiology, 598(14), 2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-D, Kim CH, Rah B-J, Chung H-I, & Shim T-S (1994). Quantitative study on the relation between structural and functional properties of the hearts from three different mammals. Anatomical Record, 238(2), 199–206. [DOI] [PubMed] [Google Scholar]
- Kim H-D, Kim D-J, Lee I-J, Rah B-J, Sawa Y, & Schaper J (1992). Human fetal heart development after mid-term: Morphometry and ultrastructural study. Journal of Molecular and Cellular Cardiology, 24(9), 949–965. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yang L, Lin B, Zhu X, Sun B, Kaplan AD, Bett GCL, Rasmusson RL, London B, & Salama G (2015). Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. Journal of Molecular and Cellular Cardiology, 81, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CW, Chen S, Geng L, Shum AMY, Sun D, & Li RA (2017). Increasing the physical size and nucleation status of human pluripotent stem cell-derived ventricular cardiomyocytes by cell fusion. Stem Cell Res, 19, 76–81. [DOI] [PubMed] [Google Scholar]
- Koumi SI, Backer CL, & Arentzen CE (1995). Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation, 92(2), 164–174. [DOI] [PubMed] [Google Scholar]
- Kumar R, Akita T, & Joyner RW (1996). Adenosine and carbachol are not equivalent in their effects on L-type calcium current in rabbit ventricular cells. Journal of Molecular and Cellular Cardiology, 28(2), 403–415. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, & Murry CE (2011). Heart regeneration. Nature, 473(7347), 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempersz C, Noben L, Clur SAB, van HE, Zhan Z, Haak M, Guid Oei S, Vullings R, & van Laar J (2021). The electrical heart axis of the fetus between 18 and 24 weeks of gestation: A cohort study. PLoS ONE, 16(12), e0256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski J, Rozwadowska N, Kolanowski TJ, Malcher A, Zimna A, Rugowska A, Fiedorowicz K, Łabędź W, Kubaszewski Ł, Chojnacka K, Bednarek-Rajewska K, Majewski P, & Kurpisz M (2018). The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin. Cell Transplantation, 27(7), 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, & Gerdes AM (1996). Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. Journal of Molecular and Cellular Cardiology, 28(8), 1737–1746. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, & Gerdes AM (1997). Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation. Journal of Molecular and Cellular Cardiology, 29(6), 1553–1565. [DOI] [PubMed] [Google Scholar]
- Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, & Wu JC (2013). Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation, 127(16), 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, Nadelmann ER, Roberts K, Tuck L, Fasouli ES, DeLaughter DM, McDonough B, Wakimoto H, Gorham JM, Samari S, … Teichmann SA (2020). Cells of the adult human heart. Nature, 588(7838), 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Luo Q, Su Z, Xing J, Wu J, Xiang L, Huang Y, Pan H, Wu X, Zhang X, Li J, Yan F, & Zhang H (2021). Suppression of myocardial hypoxia-inducible factor-1α compromises metabolic adaptation and impairs cardiac function in patients with cyanotic congenital heart disease during puberty. Circulation, 143(23), 2254–2272. [DOI] [PubMed] [Google Scholar]
- Lock MC, Tellam RL, Botting KJ, Wang KCW, Selvanayagam JB, Brooks DA, Seed M, & Morrison JL (2018). The role of miRNA regulation in fetal cardiomyocytes, cardiac maturation and the risk of heart disease in adults. The Journal of Physiology, 596(23), 5625–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompre AM, Mercadier JJ, Wisnewsky C, Bouveret P, Pantaloni C, D’Albis A, & Schwartz K (1981). Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Developmental Biology, 84(2), 286–290. [DOI] [PubMed] [Google Scholar]
- Lompré AM, Nadal-Ginard B, & Mahdavi V (1984). Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. Journal of Biological Chemistry, 259(10), 6437–6446. [PubMed] [Google Scholar]
- Lopaschuk GD, & Jaswal JS (2010). Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. Journal of Cardiovascular Pharmacology, 56(2), 130–140. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Spafford MA, & Marsh DR (1991). Glycolysis is predominant source of myocardial ATP production immediately after birth. American Journal of Physiology, 261(6), H1698–H1705. [DOI] [PubMed] [Google Scholar]
- Lundy SD, Zhu WZ, Regnier M, & Laflamme MA (2013). Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells and Development, 22(14), 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, & Gorelik J (2009). Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. PNAS, 106(16), 6854–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde AO, Kantor PF, & Lopaschuk GD (1998). Maturation of fatty acid and carbohydrate metabolism in the newborn heart – PubMed. Molecular and Cellular Biochemistry, 188(1/2), 49–56. [PubMed] [Google Scholar]
- Mandarim-de-Lacerda CA, Barbosa dos Santos M, le Floch-Prigent P, & Narcy F (1997). Stereology of the myocardium in human foetuses. Early Human Development, 48(3), 249–259. [DOI] [PubMed] [Google Scholar]
- Mansourati J, & le Grand B (1993). Transient outward current in young and adult diseased human atria. American Journal of Physiology. Heart and Circulatory Physiology, 265(4), H1466–H1470. [DOI] [PubMed] [Google Scholar]
- Marin-Garcia J, Ananthakrishnan R, & Goldenthal MJ (2000). Heart mitochondrial DNA and enzyme changes during early human development. Molecular and Cellular Biochemistry, 210(1/2), 47–52. [DOI] [PubMed] [Google Scholar]
- Marin-Garcia J, & Baskin LS (1989). Human cytochrome c oxidase during cardiac growth and development. Pediatric Cardiology, 10(4), 212–215. [DOI] [PubMed] [Google Scholar]
- Masuda H, & Sperelakis N (1993). Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. American Journal of Physiology, 265(4), H1107–H1111. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gregson C, Pharaoh A, & Fagan DG (1998). Numbers of nuclei in different tissue compartments of fetal ventricular myocardium from 16 to 35 weeks of gestation. Virchows Archiv, 433(2), 167–172. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Pharaoh A, Austin A, & Fagan DG (1997). Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. Journal of Anatomy, 191(Pt 1), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdaki KS, Larsen TD, Weaver LJ, & Baack ML (2016). Age related bioenergetics profiles in isolated rat cardiomyocytes using extracellular flux analyses. PLoS ONE, 11(2), e0149002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadier JJ, Bouveret P, Gorza L, Schiaffino S, Clark WA, Zak R, Swynghedauw B, & Schwartz K (1983). Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circulation Research, 53(1), 52–62. [DOI] [PubMed] [Google Scholar]
- Mielke G, & Benda N (2001). Cardiac output and central distribution of blood flow in the human fetus. Circulation, 103(12), 1662–1668. [DOI] [PubMed] [Google Scholar]
- Milani-Nejad N, & Janssen PML (2014). Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacology & Therapeutics, 141(3), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi P, Suleiman MS, Reeves BC, Pawade A, Parry AJ, Angelini GD, & Caputo M (2004). Basal metabolic state of hearts of patients with congenital heart disease: The effects of cyanosis, age, and pathology. Annals of Thoracic Surgery, 78(5), 1710–1716. [DOI] [PubMed] [Google Scholar]
- Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S-YY, Silberstein LE, dos Remedios CG, Graham D, Colan S, & Kühn B (2013). Cardiomyocyte proliferation contributes to heart growth in young humans. Proceedings of the National Academy of Sciences, 110(4), 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Miyamoto M, Kervadec A, Kannan S, Tampakakis E, Kambhampati S, Lin BL, Paek S, Andersen P, Lee DI, Zhu R, An SS, Kass DA, Uosaki H, Colas AR, & Kwon C (2021). PGC1/PPAR drive cardiomyocyte maturation at single cell level via YAP1 and SF3B2. Nature Communications, 12(1), 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DIK, Lefer DJ, Graham RM, & Husain A (2014). A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell, 157(4), 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narolska NA, Eiras S, van Loon RB, Boontje NM, Zaremba R, Spiegelen Berg SR, Stooker W, Huybregts M, Visser FC, van der Velden J, & Stienen GJM (2005). Myosin heavy chain composition and the economy of contraction in healthy and diseased human myocardium. Journal of Muscle Research and Cell Motility, 26(1), 39–48. [DOI] [PubMed] [Google Scholar]
- Neary MT, Ng KE, Ludtmann MHR, Hall AR, Piotrowska I, Ong SB, Hausenloy DJ, Mohun TJ, Abramov AY, & Breckenridge RA (2014). Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. Journal of Molecular and Cellular Cardiology, 74(100), 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noujaim SF, Lucca E, Muñoz V, Persaud D, Berenfeld O, Meijler FL, & Jalife J (2004). From mouse to whale: A universal scaling relation for the PR Interval of the electrocardiogram of mammals. Circulation, 110(18), 2802–2808. [DOI] [PubMed] [Google Scholar]
- Nowak MB, Poelzing S, & Weinberg SH (2021). Mechanisms underlying age-associated manifestation of cardiac sodium channel gain-of-function. Journal of Molecular and Cellular Cardiology, 153, 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MB, Veeraraghavan R, Poelzing S, & Weinberg SH (2021). Cellular size, gap junctions, and sodium channel properties govern developmental changes in cardiac conduction. Front Physiol, 12, 731025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obreztchikova MN, Sosunov EA, Plotnikov A, Anyukhovsky EP, Gainullin RZ, Danilo P, Yeom ZH, Robinson RB, & Rosen MR (2003). Developmental changes in IKr and IKs contribute to age-related expression of dofetilide effects on repolarization and proarrhythmia. Cardiovascular Research, 59(2), 339–350. [DOI] [PubMed] [Google Scholar]
- O’Connor M, McDaniel N, & Brady WJ (2008). The pediatric electrocardiogram. Part I: Age-related interpretation. American Journal of Emergency Medicine, 26(4), 221–228. [DOI] [PubMed] [Google Scholar]
- Odening KE, Gomez AM, Dobrev D, Fabritz L, Heinzel FR, Mangoni ME, Molina CE, Sacconi L, Smith G, Stengl M, Thomas D, Zaza A, Remme CA, & Heijman J (2021). ESC working group on cardiac cellular electrophysiology position paper: Relevance, opportunities, and limitations of experimental models for cardiac electrophysiology research. Europace, 23(11), 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara CC, Wamstad JA, Gladstone RA, Fomovsky GM, Butty VL, Shrikumar A, Gannon JB, Boyer LA, & Lee RT (2015). Transcriptional reversion of cardiac myocyte fate during mammalian cardiac regeneration. Circulation Research, 116(5), 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacioretty LM, & Gilmour RF (1995). Developmental changes of action potential configuration and I(to) in canine epicardium. American Journal of Physiology, 268(6), H2513–H2521. [DOI] [PubMed] [Google Scholar]
- Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tønnessen T, Kryshtal DO, Louch WE, & Knollmann BC (2017). Thyroid and glucocorticoid hormones promote functional t-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circulation Research, 121(12), 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali SK, Hall M, Slonim AD, Jenkins KJ, Marino BS, Cohen MS, & Shah SS (2008). Off-label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circ Cardiovasc Qual Outcomes, 1(2), 74–83. [DOI] [PubMed] [Google Scholar]
- Patterson M, Barske L, van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR, & Sucov HM (2017). Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nature Genetics, 49(9), 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzmann B, Schaffer P, Bernhart E, Lang P, Mächler H, Rigler B, & Koidl B (1998). L-type calcium current in human ventricular myocytes at a physiological temperature from children with tetralogy of Fallot. Cardiovascular Research, 38(2), 424–432. [DOI] [PubMed] [Google Scholar]
- Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, & Green CR (1994). Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation, 90(2), 713–725. [DOI] [PubMed] [Google Scholar]
- Piccoli M, Conforti E, Varrica A, Ghiroldi A, Cirillo F, Resmini G, Pluchinotta F, Tettamanti G, Giamberti A, Frigiola A, & Anastasia L (2017). NEU3 sialidase role in activating HIF-1α in response to chronic hypoxia in cyanotic congenital heart patients. International Journal of Cardiology, 230, 6–13. [DOI] [PubMed] [Google Scholar]
- Piquereau J, Novotova M, Fortin D, Garnier A, Ventura-Clapier R, Veksler V, & Joubert F (2010). Postnatal development of mouse heart: Formation of energetic microdomains. The Journal of Physiology, 588(13), 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroddi N, Belus A, Scellini B, Tesi C, Giunti G, Cerbai E, Mugelli A, & Poggesi C (2007). Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflugers Archiv: European journal of physiology, 454(1), 63–73. [DOI] [PubMed] [Google Scholar]
- Pohjoismäki JLO, Krüger M, Al-Furoukh N, Lagerstedt A, Karhunen PJ, & Braun T (2013). Postnatal cardiomyocyte growth and mitochondrial reorganization cause multiple changes in the proteome of human cardiomyocytes. Molecular Biosystems, 9(6), 1210–1219. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, & Sadek HA (2011). Transient regenerative potential of the neonatal mouse heart. Science, 331(6020), 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, & Sadek HA (2013). Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. PNAS, 110(1), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA, Hom JR, Hoffman DL, Quintanilla RA, de Bentley KL M, & Sheu SS (2011). Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog Pediatr Cardiol, 31(2), 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KM, Littler WA, & Cummins P (1980). Human atrial and ventricular myosin light-chains subunits in the adult and during development. Biochemical Journal, 191(2), 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, … Sadek HA (2014). The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell, 157(3), 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, & Robinson RB (2001). HCN2 overexpression in newborn and adult ventricular myocytes: Distinct effects on gating and excitability. Circulation Research, 89(1), E8–14. [DOI] [PubMed] [Google Scholar]
- Qu Y, & Boutjdir M (2001). Gene expression of SERCA2a and L- and T-type Ca channels during human heart development. Pediatric Research, 50(5), 569–574. [DOI] [PubMed] [Google Scholar]
- Qu Y, Ghatpande A, El-Sherif N, & Boutjdir M (2000). Gene expression of Na+/Ca2+ exchanger during development in human heart. Cardiovascular Research, 45(4), 866–873. [DOI] [PubMed] [Google Scholar]
- Quing M, Görlach A, Schumacher K, Wötje M, Vazquez-Jimenez JF, Hess J, & Seghaye MC (2007). The hypoxia-inducible factor HIF-1 promotes intra-myocardial expression of VEGF in infants with congenital cardiac defects. Basic Research in Cardiology, 102(3), 224–232. [DOI] [PubMed] [Google Scholar]
- Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi-Harami F, Bamshad MJ, & Regnier M (2016). Contractile properties of developing human fetal cardiac muscle. The Journal of Physiology, 594(2), 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Portman MA, Ning XH, & Moravec CS (2001). Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American Journal of Physiology. Heart and Circulatory Physiology, 280(4), H1814–H1820. [DOI] [PubMed] [Google Scholar]
- Reppel M, Sasse P, Malan D, Nguemo F, Reuter H, Bloch W, Hescheler J, & Fleischmann BK (2007). Functional expression of the Na+/Ca2+ exchanger in the embryonic mouse heart. Journal of Molecular and Cellular Cardiology, 42(1), 121–132. [DOI] [PubMed] [Google Scholar]
- Ribeiro AJS, Ang YS, Fu JD, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, & Pruitt BL (2015). Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. PNAS, 112(41), 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, Trafford AW, & Dibb KM (2011). Transverse tubules are a common feature in large mammalian atrial myocytes including human. American Journal of Physiology. Heart and Circulatory Physiology, 301(5), H1996–H2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnbeek PR, Witsenburg M, Schrama E, Hess J, & Kors JA (2001). New normal limits for the paediatric electrocardiogram. European Heart Journal, 22(8), 702–711. [DOI] [PubMed] [Google Scholar]
- Ripplinger CM, Glukhov AV, Kay MW, Boukens BJ, Chiamvimonvat N, Delisle BP, Fabritz L, Hund TJ, Knollmann BC, Li N, Murray KT, Poelzing S, Quinn TA, Remme CA, Rentschler SL, Rose RA, & Posnack NG (2022). Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals. American Journal of Physiology. Heart and Circulatory Physiology, 323(6), H1137–H1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Yu H, Chang F, & Cohen IS (1997). Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflugers Archiv: European Journal of Physiology, 433(4), 533–535. [DOI] [PubMed] [Google Scholar]
- Roca TP, Pigott JD, Clarkson CW, & Crumb WJ (1996). L-type calcium current in pediatric and adult human atrial myocytes: Evidence for developmental changes in channel inactivation. Pediatric Research, 40(3), 462–468. [DOI] [PubMed] [Google Scholar]
- Rudolph AM (2009). Congenital diseases of the heart: Clinical-physiological considerations. Wiley-Blackwell. [Google Scholar]
- Rumyantsev P (1991). Growth and hyperplasia of cardiac muscle cells. Taylor & Francis. [Google Scholar]
- Saarel EV, Granger S, Kaltman JR, Minich LL, Tristani-Firouzi M, Kim JJ, Ash K, Tsao SS, Berul CI, Stephenson EA, Gamboa DG, Trachtenberg F, Fischbach P, Vetter VL, Czosek RJ, Johnson TR, Salerno JC, Cain NB, & Pass RH, Pediatric Heart Network Investigators (2018). Electrocardiograms in healthy North American children in the digital age. Circ Arrhythm Electrophysiol, 11(7), e005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba Z, Nassar R, Ungerleider RM, Oakeley AE, & Anderson PAW (1996). Cardiac troponin T isoform expression correlates with pathophysiological descriptors in patients who underwent corrective surgery for congenital heart disease. Circulation, 94(3), 472–476. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, & Wasserstrom JA (1993). Sodium current in isolated human ventricular myocytes. American Journal of Physiology, 265(4), H1301–H1309. [DOI] [PubMed] [Google Scholar]
- Salameh A, Haunschild J, Bräuchle P, Peim O, Seidel T, Reitmann M, Kostelka M, Bakhtiary F, Dhein S, & Dähnert I (2014). On the role of the gap junction protein Cx43 (GJA1) in human cardiac malformations with fallot-pathology. A study on paediatric cardiac specimen. PLoS ONE, 9(4), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, & Barton PJR (1993). Troponin I gene expression during human cardiac development and in end-stage heart failure. Circulation Research, 72(5), 932–938. [DOI] [PubMed] [Google Scholar]
- Schaffer P, Pelzmann B, Bernhart E, Lang P, Mächler H, Rigler B, & Koidl B (1999). Repolarizing currents in ventricular myocytes from young patients with tetralogy of Fallot. Cardiovascular Research, 43(2), 332–343. [DOI] [PubMed] [Google Scholar]
- Schaper J, Meiser E, & Stammler G (1985). Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circulation Research, 56(3), 377–391. [DOI] [PubMed] [Google Scholar]
- Schier JJ, & Adelstein RS (1982). Structural and enzymatic comparison of human cardiac muscle myosins isolated from infants, adults, and patients with hypertrophic cardiomyopathy. Journal of Clinical Investigation, 69(4), 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz DG, Kitzman DW, Hagen PT, Ilstrup DM, & Edwards WD (1988). Age-related changes in normal human hearts during the first 10 decades of life. Part I (Growth): A quantitative anatomic study of 200 specimens from subjects from birth to 19 years old. Mayo Clinic Proceedings, 63(2), 126–136. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Garson A, Paul T, Stramba-Badiale M, Vetter VL, Villain E, & Wren C (2002). Guidelines for the interpretation of the neonatal electrocardiogram. A task force of the European Society of Cardiology. European Heart Journal, 23(17), 1329–1344. [DOI] [PubMed] [Google Scholar]
- Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, & Tohse N (2003). Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovascular Research, 58(3), 535–548. [DOI] [PubMed] [Google Scholar]
- Sharieff GQ, & Rao SO (2006). The pediatric ECG. Emergency Medicine Clinics of North America, 24(1), 195–208. [DOI] [PubMed] [Google Scholar]
- Shelley H (1961). Glycogen reserves and their changes at birth and in anoxia. British Medical Bulletin, 17(2), 137–143. [Google Scholar]
- De Simone G, Devereux RB, Kimball TR, Mureddu GF, Roman MJ, Contaldo F, & Daniels SR (1998). Interaction between body size and cardiac workload: Influence on left ventricular mass during body growth and adulthood. Hypertension, 31(5), 1077–1082. [DOI] [PubMed] [Google Scholar]
- Smith KLM, Swiderska A, Lock MC, Graham L, Iswari W, Choudhary T, Thomas D, Kowash HM, Desforges M, Cottrell EC, Trafford AW, Giussani DA, & Galli GLJ (2022). Chronic developmental hypoxia alters mitochondrial oxidative capacity and reactive oxygen species production in the fetal rat heart in a sex-dependent manner. Journal of Pineal Research, 73(3), e12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Polster BM, & Thompson LP (2021). Chronic hypoxia alters cardiac mitochondrial complex protein expression and activity in fetal guinea pigs in a sex-selective manner. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 321(6), R912–R924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonpaa MH, & Field LJ (1997). Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. American Journal of Physiology, 272(1), H220–H226. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, & Field LJ (1998). Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circulation Research, 83(1), 15–26. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Kim KK, Pajak L, Franklin M, & Field LJ (1996). Cardiomyocyte DNA synthesis and binucleation during murine development. American Journal of Physiology, 271(5), H2183–H2189. [DOI] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Dolber PC, & Barr RC (2000). Electrophysiological effects of remodeling cardiac gap junctions and cell size: Experimental and model studies of normal cardiac growth. Circulation Research, 86(3), 302–311. [DOI] [PubMed] [Google Scholar]
- Suga H (1990). Ventricular energetics. Physiological Reviews, 70(2), 247–277. [DOI] [PubMed] [Google Scholar]
- Suryawanshi H, Clancy R, Morozov P, Halushka MK, Buyon JP, & Tuschl T (2020). Cell atlas of the foetal human heart and implications for autoimmune-mediated congenital heart block. Cardiovascular Research, 116(8), 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift LM, Burke M, Guerrelli D, Reilly M, Ramadan M, McCullough D, Prudencio T, Mulvany C, Chaluvadi A, Jaimes R, & Posnack NG (2020). Age-dependent changes in electrophysiology and calcium handling: Implications for pediatric cardiac research. American Journal of Physiology. Heart and Circulatory Physiology, 318(2), H354–H365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T, Nakanishi K, Fukuda M, & Fujita S (1983). Cytoflurometric nuclear DNA-determinations in infant, adolescent, adult and aging human hearts. Histochemistry, 77(4), 485–494. [DOI] [PubMed] [Google Scholar]
- Tan CMJ, & Lewandowski AJ (2020). The transitional heart: From early embryonic and fetal development to neonatal life. Fetal Diagnosis and Therapy, 47(5), 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipparaju SM, Kumar R, Wang Y, Joyner RW, & Wagner MB (2004). Developmental differences in L-type calcium current of human atrial myocytes. American Journal of Physiology. Heart and Circulatory Physiology, 286(5), H1963–H1969. [DOI] [PubMed] [Google Scholar]
- Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Akkad AD, Herndon CN, Arduini A, Papangeli I, Roselli C, Aguet F, Choi SH, Ardlie KG, Babadi M, Margulies KB, Stegmann CM, & Ellinor PT (2020). Transcriptional and cellular diversity of the human heart. Circulation, 142(5), 466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Markandeya YS, Kamp TJ, Makielski JC, January CT, & Eckhardt LL (2016). IK1-enhanced human-induced pluripotent stem cell-derived cardiomyocytes: An improved cardiomyocyte model to investigate inherited arrhythmia syndromes. American Journal of Physiology. Heart and Circulatory Physiology, 310(11), H1611–H1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayutham N, Agnew EJ, & Yutzey KE (2019). Postnatal cardiac development and regenerative potential in large mammals. Pediatric Cardiology, 40(7), 1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayutham N, Alfieri CM, Agnew EJ, Riggs KW, Baker RS, Ponny SR, Zafar F, & Yutzey KE (2020). Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine. Journal of Molecular and Cellular Cardiology, 146, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheule S, & Kaese S (2013). Connexin diversity in the heart: Insights from transgenic mouse models. Frontiers in Pharmacology, 4, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermij SH, Abriel H, & van Veen TAB (2017). Refining the molecular organization of the cardiac intercalated disc. Cardiovascular Research, 113, 259–275. [DOI] [PubMed] [Google Scholar]
- Vozzi C, Dupont E, Coppen SR, Hung IY, & Severs NJ (1999). Chamber-related differences in connexin expression in the human heart. Journal of Molecular and Cellular Cardiology, 31(5), 991–1003. [DOI] [PubMed] [Google Scholar]
- Vreeker A, van Stuijvenberg L, Hund TJ, Mohler PJ, Nikkels PGJ, & van Veen TAB (2014). Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS ONE, 9(4), e94722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MB, Wang Y, Kumar R, Tipparaju SM, & Joyner RW (2005). Calcium transients in infant human atrial myocytes. Pediatric Research, 57(1), 28–34. [DOI] [PubMed] [Google Scholar]
- Wahler GM (1992). Developmental increases in the inwardly rectifying potassium current of rat ventricular myocytes. American Journal of Physiology, 262(5), C1266–C1272. [DOI] [PubMed] [Google Scholar]
- Wang L, Feng ZP, Kondo CS, Sheldon RS, & Duff HJ (1996). Developmental changes in the delayed rectifier K+ channels in mouse heart. Circulation Research, 79(1), 79–85. [DOI] [PubMed] [Google Scholar]; Wang P, Tang M, Gao L, Luo H, Wang G, Ma X, & Duan Y. (2013). Roles of I(f) and intracellular Ca2+ release in spontaneous activity of ventricular cardiomyocytes during murine embryonic development. Journal of Cellular Biochemistry, 114(8), 1852–1862. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, & Joyner RW (2003). Differences in transient outward current properties between neonatal and adult human atrial myocytes. Journal of Molecular and Cellular Cardiology, 35(9), 1083–1092. [DOI] [PubMed] [Google Scholar]
- West GB, Brown JH, & Enquist BJ (1997). A general model for the origin of allometric scaling laws in biology. Science (1979), 276(5309), 122–126. [DOI] [PubMed] [Google Scholar]
- Wiegerinck RF, Cojoc A, Zeidenweber CM, Ding G, Shen M, Joyner RW, Fernandez JD, Kanter KR, Kirshbom PM, Kogon BE, & Wagner MB (2009). Force frequency relationship of the human ventricle increases during early postnatal development. Pediatric Re, 65(4), 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, Ritterhoff J, Zhao L, Kolwicz SC, Pabon L, Reinecke H, Sniadecki NJ, Tian R, Ruohola-Baker H, Xu H, & Murry CE (2019). Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports, 13(4), 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatscoff MA, Jaswal JS, Grant MR, Greenwood R, Lukat T, Beker DL, Rebeyka IM, & Lopaschuk GD (2008). Myocardial hypertrophy and the maturation of fatty acid oxidation in the newborn human heart. Pediatric Research, 64(6), 643–647. [DOI] [PubMed] [Google Scholar]
- Ye L, Qiu L, Feng B, Jiang C, Huang Y, Zhang H, Zhang H, Hong H, & Liu J (2020). Role of blood oxygen saturation during post-natal human cardiomyocyte cell cycle activities. JACC: Basic to Translational Science, 5(5), 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Qiu L, Zhang H, Chen H, Jiang C, Hong H, & Liu J (2016). Cardiomyocytes in young infants with congenital heart disease: A three-month window of proliferation. Scientific Reports, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K, & Sawa Y (2018). Maturation of human induced pluripotent stem cell-derived cardiomyocytes by soluble factors from human mesenchymal stem cells. Molecular Therapy, 26(11), 2681–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgheib C, Allukian MW, Xu J, Morris MW, Caskey RC, Herdrich BJ, Hu J, Gorman JH, Gorman RC, & Liechty KW (2014). Mammalian fetal cardiac regeneration after myocardial infarction is associated with differential gene expression compared with the adult. Annals of Thoracic Surgery, 97(5), 1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, & Pu WT (2016). Recounting cardiac cellular composition. Circulation Research, 118(3), 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Do VD, Richards AM, & Foo R (2021). What we know about cardiomyocyte dedifferentiation. Journal of Molecular and Cellular Cardiology, 152, 80–91. [DOI] [PubMed] [Google Scholar]
- Ziman AP, Gómez-Viquez NL, Bloch RJ, & Lederer WJ (2010). Excitation-contraction coupling changes during postnatal cardiac development. Journal of Molecular and Cellular Cardiology, 48(2), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn-Pauly K, Schaffer P, Pelzmann B, Bernhart E, Lang P, Zink M, Mächler H, Rigler B, & Koidl B (2003). A hyperpolarization activated inward current (If) is present in infant ventricular myocytes. Basic Research in Cardiology, 98(6), 362–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


