Abstract
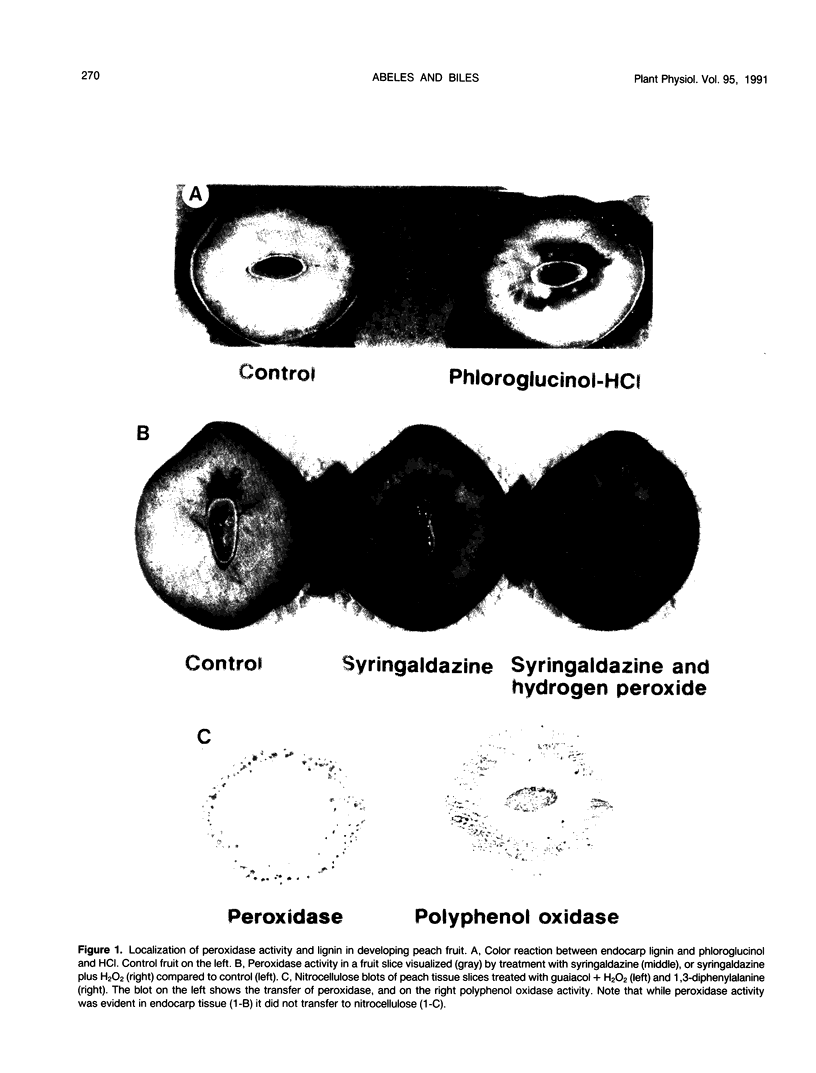
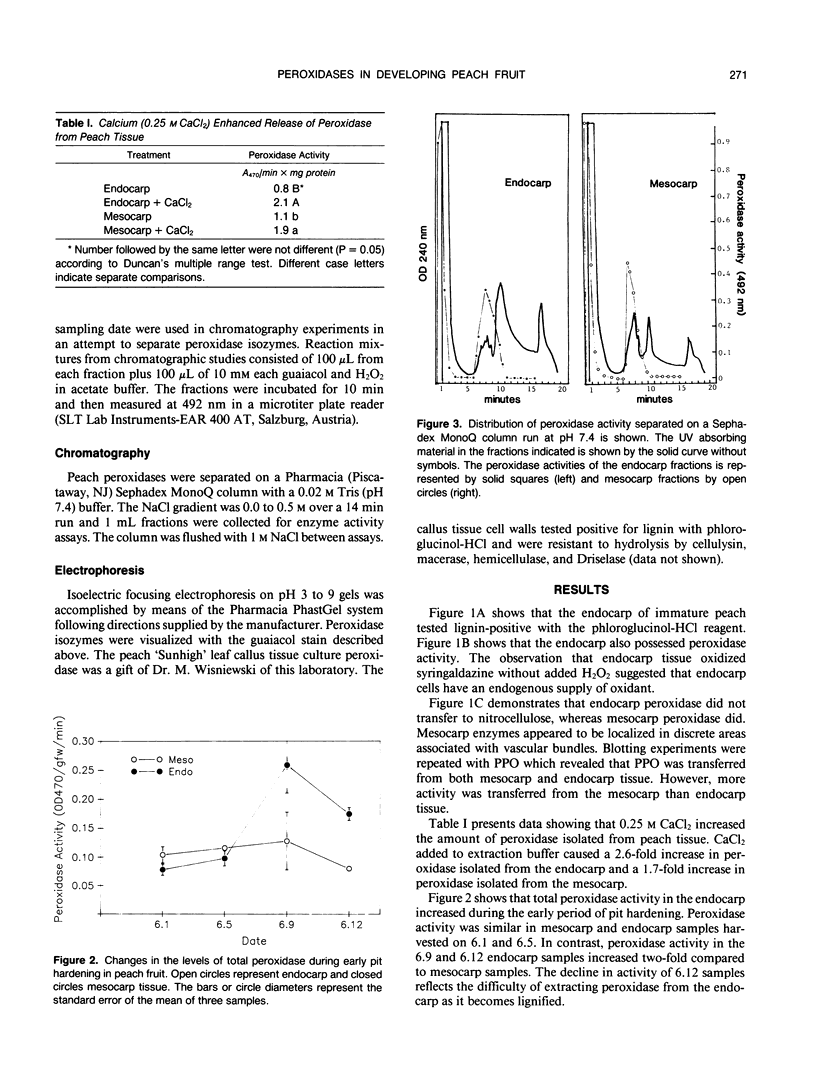
Developing peach (Prunus persica L. Batsch `Redskin') fruit were used to characterize the role of peroxidases in lignification. During development, the endocarp of these drupes becomes lignified while the mesocarp remains parenchymatous. Acidic peroxidase from lignifying endocarp were similar to those of the fleshy mesocarp. The endocarp had a larger amount and number of basic peroxidases than the mesocarp. Cultured peach leaf cells are thought to be lignified because their walls give a positive reaction with phloroglucinol-HCI. These cells also secreted a basic peroxidase. Peroxidases were difficult to extract from endocarp tissue as they lignified. This was also demonstrated by tissue printing on nitrocellulose. Flesh, but not endocarp peroxidase was evident in tissue prints. This suggests that tissue printing may fail to reveal the presence of enzymes which are firmly attached to the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAZAWA T., CONN E. E. The oxidation of reduced pyridine nucleotides by peroxidase. J Biol Chem. 1958 May;232(1):403–415. [PubMed] [Google Scholar]
- Abeles F. B., Hershberger W. L., Dunn L. J. Hormonal Regulation, and Intracellular Localization of a 33-kD Cationic Peroxidase in Excised Cucumber Cotyledons. Plant Physiol. 1989 Feb;89(2):664–668. doi: 10.1104/pp.89.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. L., Galston A. W. 4-Coumarate:coenzyme A ligase and isoperoxidase expression in Zinnia mesophyll cells induced to differentiate into tracheary elements. Plant Physiol. 1988;88:679–684. doi: 10.1104/pp.88.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986 Jun;81(2):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D., Fabre M., Schmitt D. A., Garaud J. C., Pauly G., Tongio M. M., Mayer S., Cazenave J. P. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis "nonclassical" major histocompatibility complex class I molecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987 May;84(9):2901–2905. doi: 10.1073/pnas.84.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Terry M. E., Hoops P., Dauwalder M., Roux S. J. Production and characterization of monoclonal antibodies to wall-localized peroxidases from corn seedlings. Plant Physiol. 1988;88:1446–1453. doi: 10.1104/pp.88.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. G., Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer A., Bru R., Garcia-Carmona F. Novel procedure for extraction of a latent grape polyphenoloxidase using temperature-induced phase separation in triton x-114. Plant Physiol. 1989 Dec;91(4):1481–1487. doi: 10.1104/pp.91.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]