Abstract
A complete nodulin-35 (N-35) cDNA encoding nodule-specific uricase (EC 1.7.3.3) was isolated from a soybean (Glycine max L. var. Prize) nodule cDNA expression library using a previously isolated partial cDNA clone. The N-35 cDNA was expressed in Escherichia coli driven by the lacZ promoter and was found to be functionally active. The uricase activity was detected in the cytoplasmic fraction of E. coli with the same pH optimum and apparent Km values as that in the nodules. Because a stop codon is located 15 base pairs upstream of the N-35 initiation codon, it appears that a fusion protein with LacZ was not made, but reinitiation occurred due to the presence of a putative Shine-Dalgarno sequence in the appropriate region. The size of the N-35 polypeptide made in E. coli is identical to that present in soybean nodules and is able to assemble into a tetrameric holoenzyme with the same molecular weight as the native uricase. Thus, the presence of peroxisomes does not appear to be essential for the proper assembly of the holoenzyme in E. coli. These data also indicate that posttranslational modifications or membrane transport are not essential either for the assembly of N-35 into a holoenzyme or for the activity of uricase.
Full text
PDF

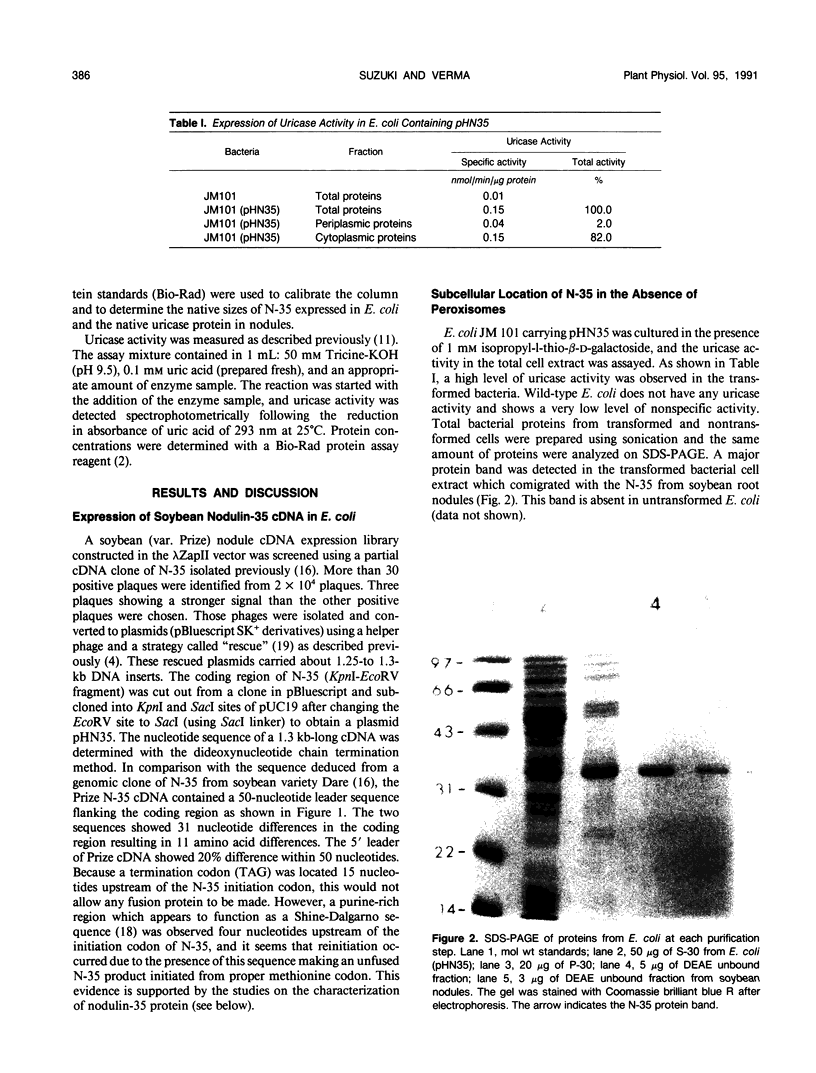
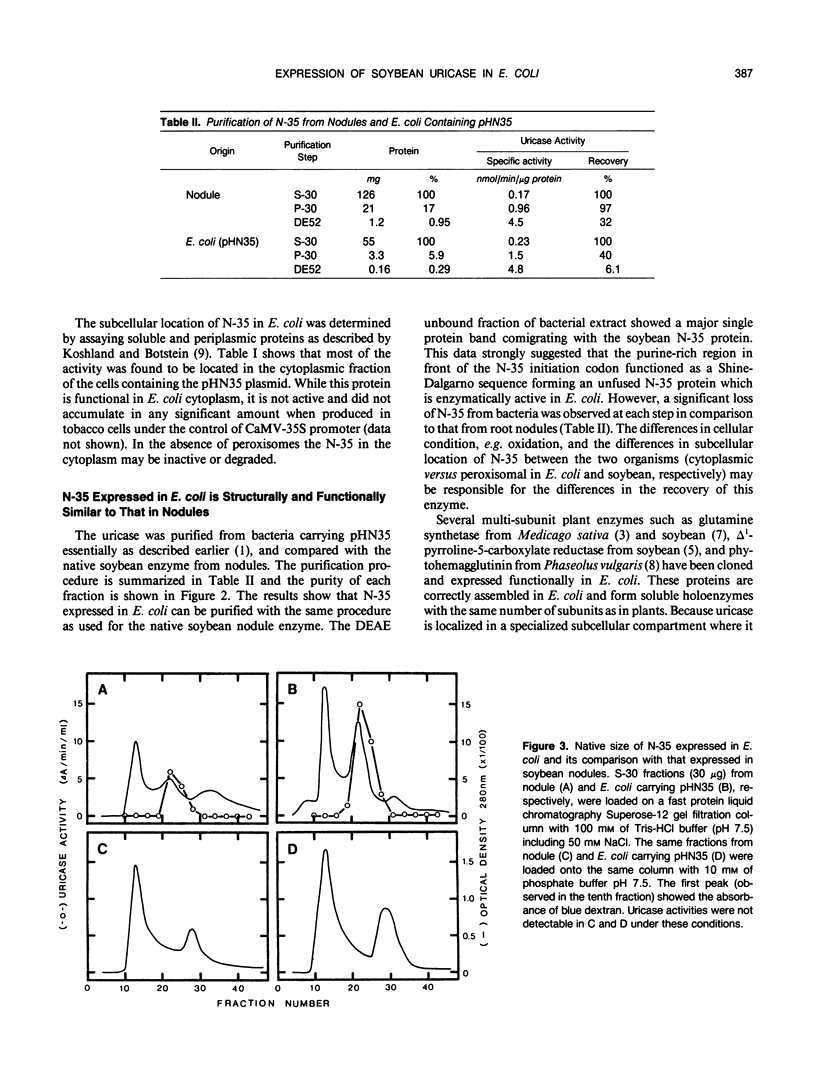
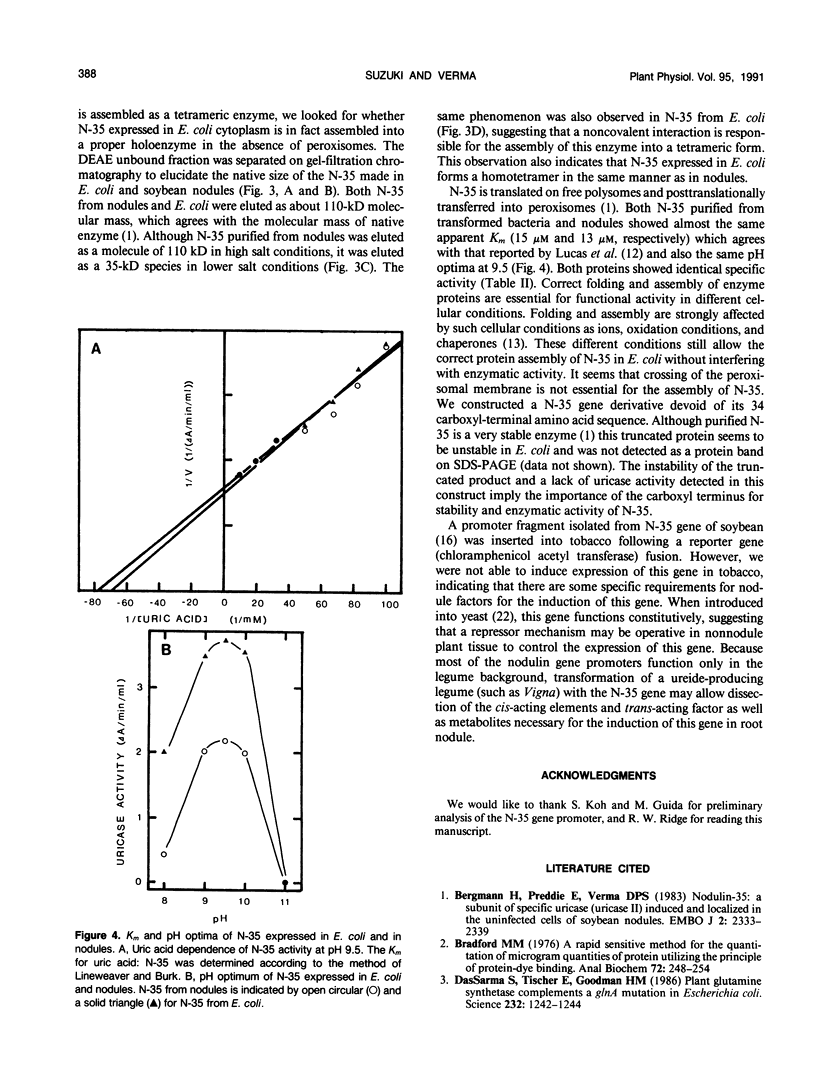

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann H., Preddie E., Verma D. P. Nodulin-35: a subunit of specific uricase (uricase II) induced and localized in the uninfected cells of soybean nodules. EMBO J. 1983;2(12):2333–2339. doi: 10.1002/j.1460-2075.1983.tb01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DasSarma S., Tischer E., Goodman H. M. Plant glutamine synthetase complements a glnA mutation in Escherichia coli. Science. 1986 Jun 6;232(4755):1242–1244. doi: 10.1126/science.2871626. [DOI] [PubMed] [Google Scholar]
- Delauney A. J., Verma D. P. A soybean gene encoding delta 1-pyrroline-5-carboxylate reductase was isolated by functional complementation in Escherichia coli and is found to be osmoregulated. Mol Gen Genet. 1990 May;221(3):299–305. doi: 10.1007/BF00259392. [DOI] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. A nodule-specific plant protein (nodulin-35) from soybean. Science. 1979 Jul 13;205(4402):190–193. doi: 10.1126/science.205.4402.190. [DOI] [PubMed] [Google Scholar]
- Lucas K., Boland M. J., Schubert K. R. Uricase from soybean root nodules: purification, properties, and comparison with the enzyme from cowpea. Arch Biochem Biophys. 1983 Oct 1;226(1):190–197. doi: 10.1016/0003-9861(83)90284-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T., Ohno K., Miura S., Fujiki Y. Peroxisome targeting signal of rat liver acyl-coenzyme A oxidase resides at the carboxy terminus. Mol Cell Biol. 1989 Jan;9(1):83–91. doi: 10.1128/mcb.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb E. H., Tandon S. R. Uninfected cells of soybean root nodules: ultrastructure suggests key role in ureide production. Science. 1981 Jun 19;212(4501):1394–1396. doi: 10.1126/science.212.4501.1394. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]



