Abstract
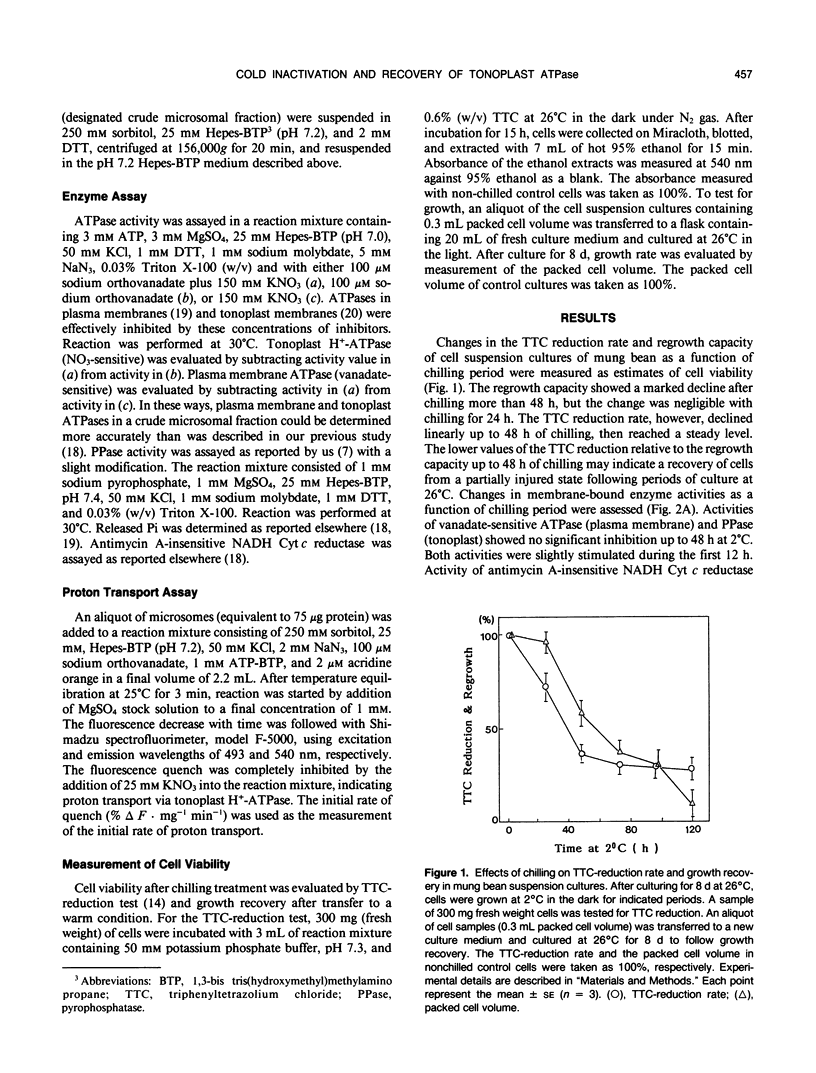
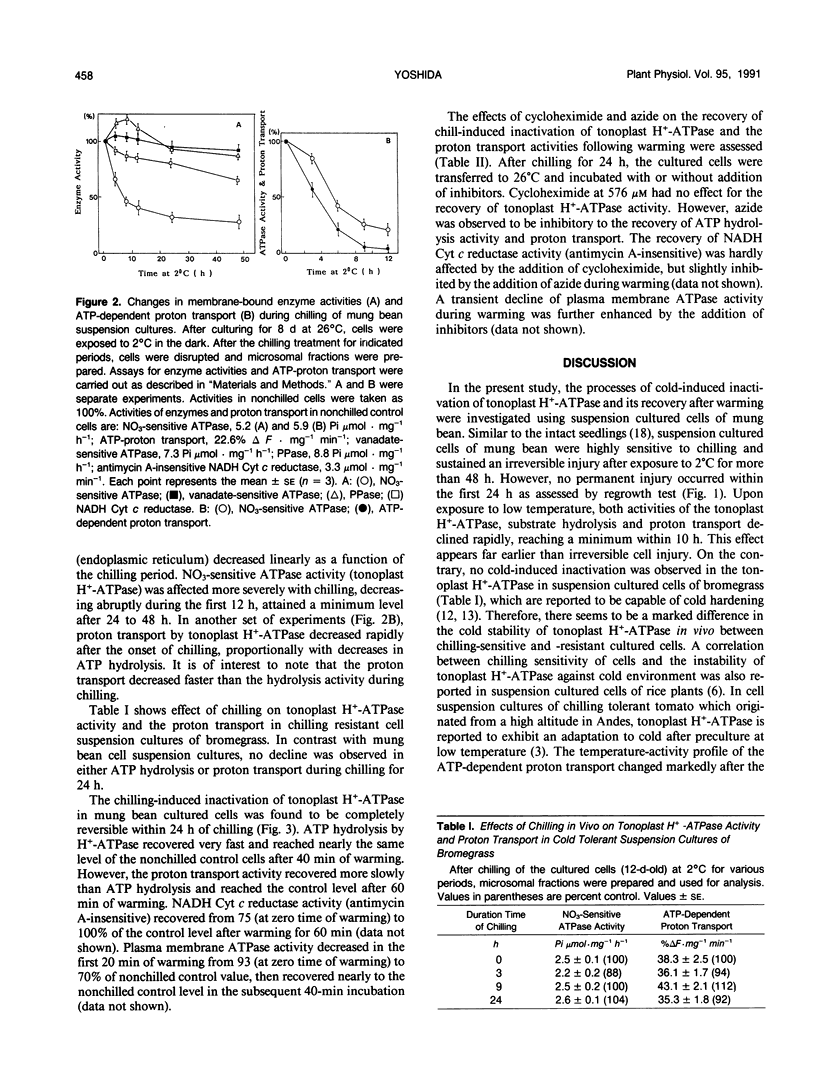
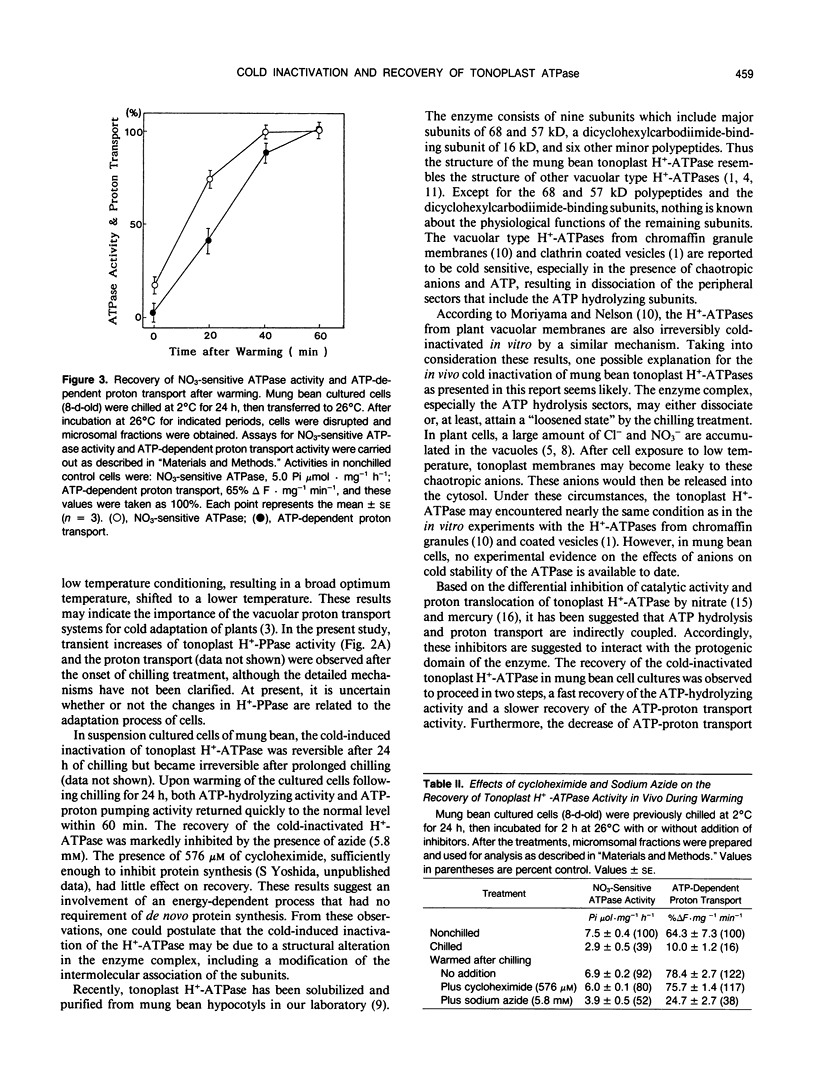
The processes involved in adaptation to cold temperature were examined by growing suspension cultured cells of mung bean (Vigna radiata [L.] Wilczek) at 2°C for various periods of time and assaying the activities of various membrane-bound enzymes in vitro. The tonoplast H+-ATPase activity and the ATP-proton transport extracted from cells incubated at 2°C declined rapidly and reached a minimum level after 10 hours. The inactivation was reversible within 24 hours of chilling. The recovery of the cold-inactivated H+-ATPase was found to proceed in two steps, a faster recovery of ATP hydrolysis activity and a slower recovery of the proton transport. The recovery was markedly inhibited by the presence of azide, but not affected by 0.578 millimolar cycloheximide. This suggested the involvement of an energy process that had no requirement for de novo synthesis of protein. The cold-induced inactivation of the H+-ATPase may be due to a structural alteration of the enzyme. The slower recovery of proton transport relative to ATP hydrolysis during warming suggests that the protogenic domains in the enzyme may be affected differently by chilling.
Full text
PDF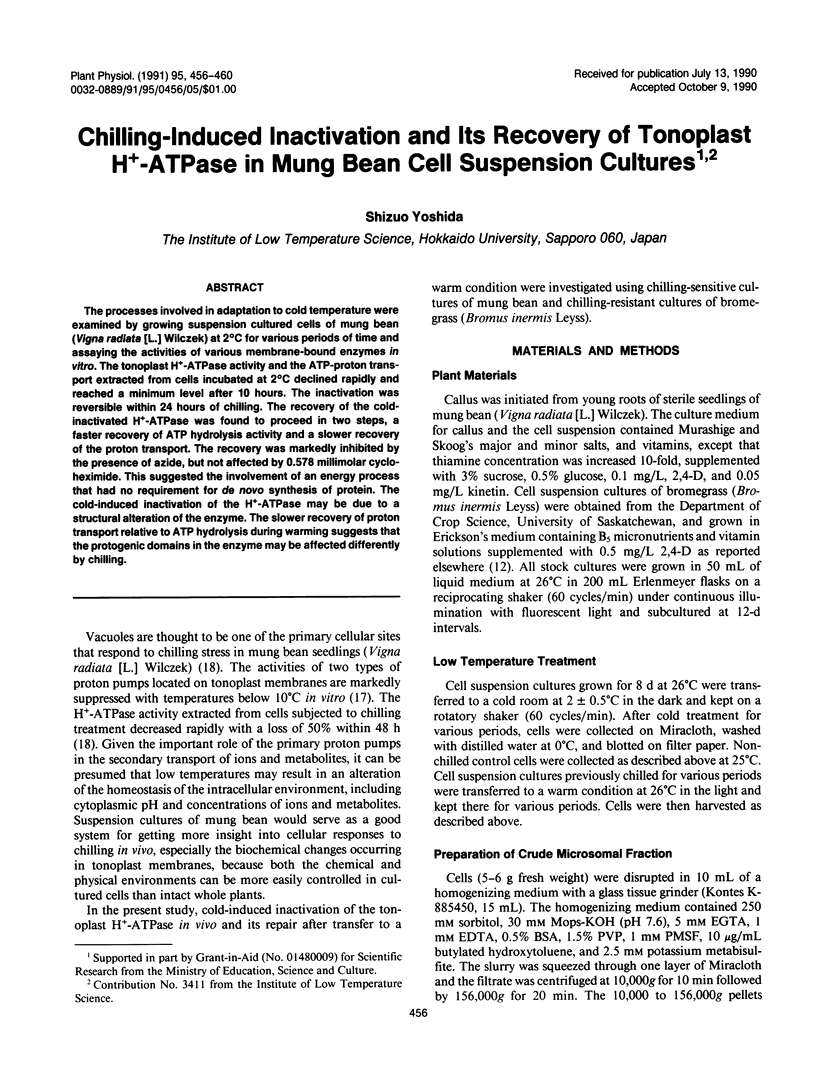

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Pink S., Forgac M. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 1989 Apr 4;28(7):3075–3082. doi: 10.1021/bi00433a051. [DOI] [PubMed] [Google Scholar]
- Arai H., Terres G., Pink S., Forgac M. Topography and subunit stoichiometry of the coated vesicle proton pump. J Biol Chem. 1988 Jun 25;263(18):8796–8802. [PubMed] [Google Scholar]
- Dupont F. M., Mudd J. B. Acclimation to low temperature by microsomal membranes from tomato cell cultures. Plant Physiol. 1985 Jan;77(1):74–78. doi: 10.1104/pp.77.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Immunoaffinity purification and characterization of vacuolar H+ATPase from bovine kidney. J Biol Chem. 1987 Nov 15;262(32):15780–15789. [PubMed] [Google Scholar]
- Granstedt R. C., Huffaker R. C. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiol. 1982 Aug;70(2):410–413. doi: 10.1104/pp.70.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M., Yoshida S. Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem. 1989 Nov 25;264(33):20068–20073. [PubMed] [Google Scholar]
- Matsuura-Endo C., Maeshima M., Yoshida S. Subunit composition of vacuolar membrane H(+)-ATPase from mung bean. Eur J Biochem. 1990 Feb 14;187(3):745–751. doi: 10.1111/j.1432-1033.1990.tb15362.x. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989 Feb 25;264(6):3577–3582. [PubMed] [Google Scholar]
- Percy J. M., Pryde J. G., Apps D. K. Isolation of ATPase I, the proton pump of chromaffin-granule membranes. Biochem J. 1985 Nov 1;231(3):557–564. doi: 10.1042/bj2310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney M. J., Gusta L. V. Factors Influencing the Induction of Freezing Tolerance by Abscisic Acid in Cell Suspension Cultures of Bromus inermis Leyss and Medicago sativa L. Plant Physiol. 1987 Feb;83(2):423–427. doi: 10.1104/pp.83.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. J., Gusta L. V., Reaney M. J., Ishikawa M. Protein Synthesis in Bromegrass (Bromus inermis Leyss) Cultured Cells during the Induction of Frost Tolerance by Abscisic Acid or Low Temperature. Plant Physiol. 1987 Aug;84(4):1331–1336. doi: 10.1104/pp.84.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Lanphear F. O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967 Oct;42(10):1423–1426. doi: 10.1104/pp.42.10.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S. I., Brouillette J. N., Nagahashi G., Brauer D., Nungesser E. Temperature dependence and mercury inhibition of tonoplast-type H+-ATPase. Arch Biochem Biophys. 1988 Oct;266(1):289–297. doi: 10.1016/0003-9861(88)90261-5. [DOI] [PubMed] [Google Scholar]
- Tu S. I., Nagahashi G., Brouillette J. N. Proton pumping kinetics and origin of nitrate inhibition of tonoplast-type H+-ATPase. Arch Biochem Biophys. 1987 Aug 1;256(2):625–637. doi: 10.1016/0003-9861(87)90620-5. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Isolation and Characterization of Tonoplast from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):161–166. doi: 10.1104/pp.80.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kawata T., Uemura M., Niki T. Properties of Plasma Membrane Isolated from Chilling-Sensitive Etiolated Seedlings of Vigna radiata L. Plant Physiol. 1986 Jan;80(1):152–160. doi: 10.1104/pp.80.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Matsuura C., Etani S. Impairment of Tonoplast H-ATPase as an Initial Physiological Response of Cells to Chilling in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1989 Feb;89(2):634–642. doi: 10.1104/pp.89.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]


