Abstract
Rationale
Xylazine has emerged in recent years as an adulterant in an increasing number of opioid-positive overdose deaths in the United States. Although its exact role in opioid-induced overdose deaths is largely unknown, xylazine is known to depress vital functions and cause hypotension, bradycardia, hypothermia, and respiratory depression.
Objectives
In this study, we examined the brain-specific hypothermic and hypoxic effects of xylazine and its mixtures with fentanyl and heroin in freely moving rats.
Results
In the temperature experiment, we found that intravenous xylazine at low, human-relevant doses (0.33, 1.0, 3.0 mg/kg) dose-dependently decreases locomotor activity and induces modest but prolonged brain and body hypothermia. In the electrochemical experiment, we found that xylazine at the same doses dose-dependently decreases nucleus accumbens oxygenation. In contrast to relatively weak and prolonged decreases induced by xylazine, intravenous fentanyl (20 μg/kg) and heroin (600 μg/kg) induce stronger biphasic brain oxygen responses, with the initial rapid and strong decrease, resulting from respiratory depression, followed by a slower, more prolonged increase reflecting a post-hypoxic compensatory phase, with fentanyl acting much quicker than heroin. The xylazine-fentanyl mixture eliminated the hyperoxic phase of oxygen response and prolonged brain hypoxia, suggesting xylazine-induced attenuation of the brain’s compensatory mechanisms to counteract brain hypoxia. The xylazine-heroin mixture strongly potentiated the initial oxygen decrease, and the pattern lacked the hyperoxic portion of the biphasic oxygen response, suggesting more robust and prolonged brain hypoxia.
Conclusions
These findings suggest that xylazine exacerbates the life-threatening effects of opioids, proposing worsened brain hypoxia as the mechanism contributing to xylazine-positive opioid-overdose deaths.
Keywords: fentanyl, heroin, brain hypoxia, brain hyperoxia, hypothermia, peripheral vasodilation, cerebral vasoconstriction
Introduction
A new player in the US opioid epidemic is xylazine, which is a non-controlled substance traditionally used as a veterinary tranquilizer and component of general anesthesia in animals (Reyes et al. 2012). Although xylazine is not approved for human use, a pattern of recreational use in the US has emerged in the past decade (Kariisa et al. 2021). Reports from the Drug Enforcement Administration show that xylazine-positive fatal overdoses have experienced a significant jump from 2020 to 2021, especially in the South region where the noted increase was 1127% (US Department of Justice 2021). More recently, in 2022, xylazine was found as an adulterant in a significant percentage of opioid-related overdose deaths, appearing in 25.8% of total overdose deaths in Philadelphia and 19.3% of total overdose deaths in Maryland (Friedman et al. 2022). Fentanyl was present in 98% and heroin in 23% of xylazine-involved deaths, revealing a dangerous connection between xylazine and these opioid drugs.
Xylazine’s exact role in opioid-induced overdose deaths is largely unknown. Similar to clonidine, xylazine is an agonist at the alpha-2 adrenergic receptors that induces sedation and muscle relaxation (Schwartz and Clark 1998). At higher doses, xylazine has been shown to significantly depress vital functions, causing strong hypotension, bradycardia, hypothermia, and respiratory depression (Caproro et al. 2001). When taken with opioids that share many common effects with xylazine, the risk of overdose and death may increase. However, the mechanisms underlying the effects of xylazine and its interaction with opioid drugs are still relatively unknown.
In this study, we focused on the effects of xylazine as an adulterant drug taken in combination with heroin and fentanyl. These two highly potent opioid drugs induce strong respiratory depression and robust brain hypoxia at low doses (Dahan et al. 2005; Solis et al. 2017, 2018; Yeadon and Kitchen 1989). To learn more about the basic physiological and behavioral effects of xylazine, we used multi-site thermorecording coupled with monitoring of locomotor activity. As temperature is an important homeostatic parameter, simultaneous recordings from the brain site, temporal muscle and subcutaneous space provides a valuable tool for clarifying the mechanisms underlying changes in brain temperature and assessing the metabolic and vascular effects of xylazine (Kiyatkin 2010).
Since xylazine is a CNS depressant that can inhibit respiration at high doses, we used oxygen sensors coupled with high-speed amperometry to examine the effects of xylazine on brain oxygenation. Although the hypoxic effects of drugs can be assessed by plethysmography (Dahan et al. 2005; Brown and Pleuvry 1981; Seckler et al. 2022) or pulse oximetry (Decker et al. 1989; Hedenqvist et al. 2000; Baby et al., 2021), it is unclear how changes in breathing activity or hemoglobin saturation in blood translate into changes in oxygen levels in the brain’s extracellular space—a functionally important parameter that affects the health and survival of neural cells. In contrast to these peripheral measures, oxygen sensors allow direct assessment of real-time fluctuations in brain oxygenation induced by natural stimuli, occurring during motivated behavior, and after exposure of different drugs in freely moving rats under physiologically relevant conditions (Kiyatkin 2010, 2019; Ledo et al, 2017; Kealy et al. 2013; Lowry and Fillenz 2001; Bolger et al. 2011).
After we established the basic physiological effects of xylazine, we examined how this drug at a moderate, human-relevant dose affects changes in brain oxygenation induced by fentanyl and heroin. In these tests we compared changes in brain oxygen levels induced by fentanyl and heroin with their combined administration with xylazine. Like in our previous studies, the nucleus accumbens (NAc), a deep brain structure involved in sensorimotor integration and functioning of the motivation-reinforcement circuits (Badiani et al. 2011; Mogenson et al. 1980; Wise and Bozarth 1987), was the recording site in both thermorecording and electrochemical experiments.
Materials and Methods
Subjects
15 adult male Long-Evans rats (Charles River Laboratories) weighing 450±50 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 hr light-dark cycle with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23). Maximal care was taken to minimize the number of experimental animals and any possible discomfort or suffering at all stages of the study.
Overview of the study
This study combines data obtained with two different technologies used in freely moving rats. By using multi-site thermorecording combined with monitoring of conventional locomotion, we assessed the basic behavioral, metabolic, and peripheral vascular effects of xylazine at doses within a range of possible human consumption. To assess drug-induced fluctuations in brain oxygenation, we used oxygen sensors coupled with high-speed amperometry. First, we examined the effects of xylazine at different doses on brain oxygenation. Second, we examined the changes in brain oxygenation induced by a fentanyl-xylazine mixture and compared them with the effects of fentanyl alone. To assess whether the effects of xylazine-fentanyl mixture are generalized to other opioid drugs, we examined the pattern of oxygen fluctuations induced by co-administration of xylazine with heroin and compared them with the effects of heroin alone.
Surgical preparations
In both thermorecording and electrochemical experiments, we used similar surgical preparations described in detail elsewhere (Kiyatkin and Brown 2005; Thomas et al. 2021). Under general anesthesia (ketamine 80 mg/kg + xylazine 8 mg/kg with subsequent dosing), each rat was implanted with a jugular catheter. For thermorecording experiments, the rat was implanted with three copper-constantan thermocouple sensers in the NAc shell, temporal muscle, and subcutaneously along the nasal ridge with the tip ~15 mm anterior to bregma. Target coordinates of the recordings in the right NAc shell were: AP +1.2 mm, ML ±0.8 mm, and DV +7.2–7.6 mm from the skull surface, according to coordinates of the rat brain atlas (Paxinos and Watson 1998). For electrochemical experiments, each rat was implanted in the same NAc location with a Pt-Ir oxygen sensor (Model 7002–02; Pinnacle Technology, Inc., Lawrence, KS, USA). The probes were secured with dental cement to the three stainless steel screws threaded into the skull. In both experiments, the jugular catheter ran subcutaneously to the head mount and was secured to the same head assembly. Rats were allowed a minimum of 5 days of post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment; jugular catheters were flushed daily with 0.2 ml heparinized saline to maintain patency.
Electrochemical detection of oxygen
Pinnacle oxygen sensors consist of an epoxy-sheathed disc electrode that is grounded to a fine surface using a diamond-lapping disc. These sensors are prepared from a Pt-Ir wire 180 μm in diameter, with a sensing area of 0.025 mm2 at the tip. The active electrode is incorporated with an integrated Ag/AgCl reference electrode. Dissolved oxygen is reduced on the active surface of these sensors, which is held at a stable potential of −0.6 V versus the reference electrode, producing an amperometric current. The current from the sensor is relayed to a computer via a potentiostat (Model 3104, Pinnacle Technology) and recorded at 1-s intervals, using PAL software utility (Version 1.5.0, Pinnacle Technology).
Oxygen sensors were calibrated at 37°C by the manufacturer (Pinnacle Technology) according to a standard protocol described elsewhere [20]. The sensors produced incremental current changes with increases in oxygen concentrations within the wide range of previously reported brain oxygen concentrations (0–40 μM). Substrate sensitivity of each sensor varied from 0.57–1.19 nA/1μM. Oxygen sensors were also tested by the manufacturer for their selectivity toward other electroactive substances, including dopamine (0.4 μM) and ascorbate (250 μM), none of which had significant effects on reduction currents.
Experimental procedures
All rats were habituated to the environment of future recording for 4–6 hrs over three days prior to surgery. A similar protocol was utilized in both thermorecording and electrochemical experiments. At the beginning of each experimental session, rats were minimally anesthetized (<2 min) with isoflurane and sensors (either thermocouple or oxygen) were connected via an electrically shielded flexible cable and a multi-channel electrical swivel to the recording instruments. The injection port of the jugular catheter on the head mount was connected to a plastic catheter extension that allowed stress- and cue-free drug delivery from outside the cage. When the rats received two different drugs within one recording session, two catheter extensions mounted on the recording cable were used to minimize any contamination of one drug by another drug. Testing began a minimum of 90 min after connecting the sensors to the recording instruments, when baseline values of temperature and electrochemical currents stabilized. For the next 4–6 hours, rats received one of three drug treatments. Upon completion of drug treatments, rats were removed from the cages and briefly anesthetized by isoflurane to disconnect them from the recording instruments. Then, catheters were flushed with heparinized saline before rats were returned to the animal colony. Temperature recordings were combined with monitoring of locomotor activity using 4 infrared motion detectors (Med Associates, Burlington, VT); measurements were conducted in a Plexiglass chamber (32 × 32 × 32 cm) under dim red light (Kiyatkin and Brown 2005). During the recording, animals were also directly observed to confirm drug-induced decreases in locomotion.
The recordings were conducted for several sessions (n=3–6), and the number of sessions in each experiment was determined by the quality of the recording and patency of the iv catheter over time. In the thermorecording experiment, we examined changes in temperatures and locomotion induced by iv xylazine (Xylazine hydrochloride, MP) at three doses (0.33, 1 and 3 mg/kg). These doses are lower than the generally accepted range of toxic effects of oral xylazine that vary in humans, between 40 and 2400 mg (or 0.6 and 34.3 mg /70 kg). The largest dose used (3 mg/kg) was well below the LD50 for iv xylazine in rats, which is between 22–43 mg/kg (Xylazine Hydrochloride, 1999). The drug was administered in an ascending order, with 60- and 90-min inter-injection intervals for the 0.33 and 1 mg/kg doses. Xylazine was delivered in 0.15–0.8 ml volumes of saline at a slow injection rate (0.2 ml/10 s).
Three types of tests were conducted in the electrochemical experiments. First, we mimicked the protocol of the thermorecording experiment and examined the effects of xylazine at the same three doses on NAc oxygenation. During the second treatment protocol, the rat received two iv injections of fentanyl (Fentanyl citrate injection 50 μg/mL; Fentanyl Citrate Injections; Hospira Inc.) at a 20 μg/kg dose both alone and as a mixture with xylazine at an effective dose determined in the first treatment protocol. As shown previously (Solis et al. 2018), 20 μg/kg is a modest dose that induces a biphasic brain oxygen response, with a rapid and strong decrease (to ~50% of baseline levels in drug-naïve rats) followed by more prolonged and weaker oxygen increase. In the third protocol, rats received two injections of heroin (Diamorphine Hydrochloride; obtained from NIDA-IRP Pharmacy) at a 600 μg/kg dose, both alone and as a mixture with xylazine at the same dose as in the second experiment. A 600 ug/kg dose is much larger than the optimal dose for heroin self-administration (75–100 μg/kg; (Gerber and Wise 1989)), but it induces a similar degree of decrease (~50% decrease) in NAc oxygen (Thomas et al. 2020). This dose difference is within the range of generally accepted differences in potencies of fentanyl and heroin to maintain iv self-administration behavior (ED50 2.5 ug/kg vs. 50 μg/kg or 1:20; (van Ree et al., 1978). In both the second and third treatment protocols, the second injection (xylazine-fentanyl or xylazine-heroin) was done 120 min after the first injection (fentanyl or heroin alone). The first and second drug protocols were conducted in the same rats and third protocol was conducted in a separate group of rats.
Histological verification of electrode placements
After completion of the experiments, rats were sacrificed, and their brains were extracted and placed in 10% formalin. Brains were sliced on a cryostat and analyzed for verification of the location of cerebral implants as well as possible tissue damage around the recording site.
Data analysis
Temperature data were sampled at 2-s time intervals and analyzed with 1-min time beans. They were presented as both absolute and relative changes with respect to the moment of drug administration. We also calculated NAc-muscle and skin-muscle temperature gradients that were used to determine the effects of xylazine on brain metabolic activity and tone of skin vasculature. Locomotor data were assessed with 1-min time resolution. Electrochemical data were sampled at 1 Hz using PAL software utility (Pinnacle Technology) and analyzed with 1-min and 10-s time resolutions. Electrochemical data were first analyzed as raw currents. Because each individual sensor differed slightly in background current and substrate sensitivity in vitro, currents were transformed into concentrations and represented as relative changes, with the pre-stimulus baseline set at 100%. One-way repeated measures ANOVAs (followed by Fisher LSD post-hoc tests) were used to evaluate statistical significance of drug-induced changes in temperature and brain oxygen changes. Two-way repeated-measure ANOVAs were used to analyze between-group differences in the effects of xylazine and its mixture with fentanyl and heroin.
Results
1. Behavioral and temperature effects of xylazine
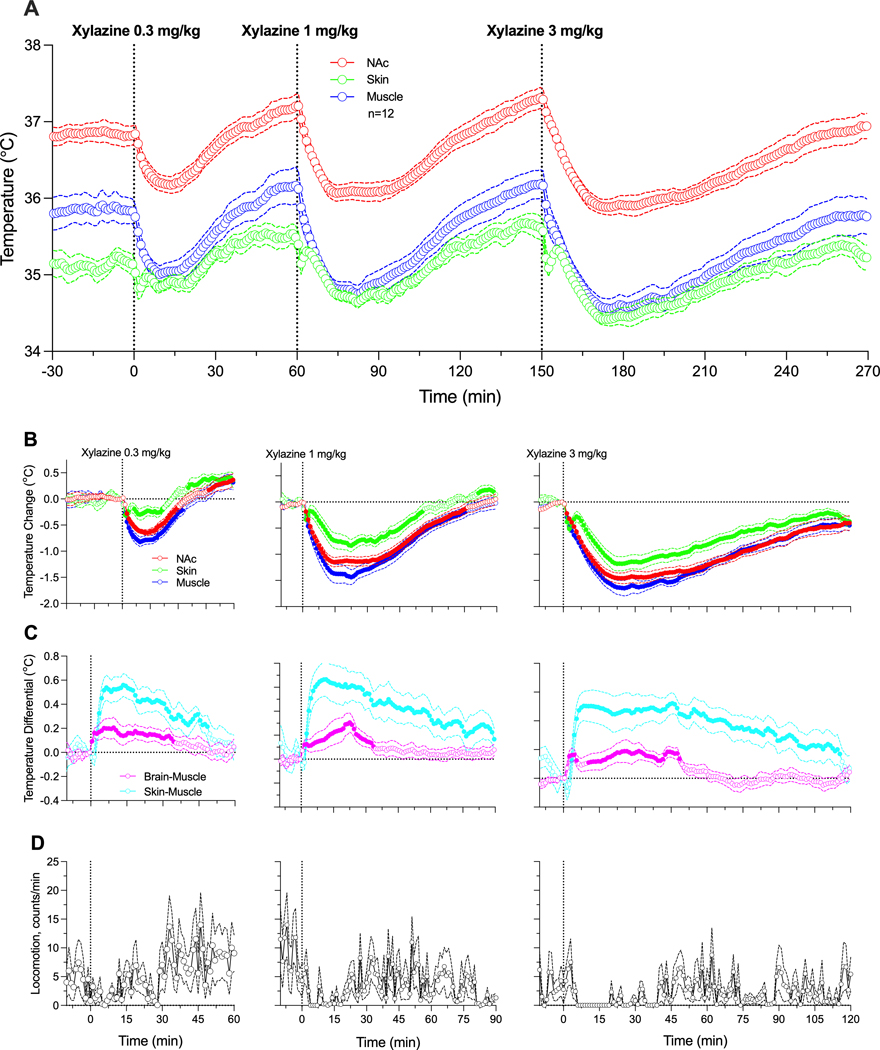
Xylazine, within the range of chosen doses, had sedative effects, decreasing temperature in all recording locations (Figure 1). Consistent with our previous studies [31], basal temperature was highest in the NAc, lower in temporal muscle, and lowest in the subcutaneous space (A). When analyzed as relative changes (B), temperature decreases were significant (see F values in Supplementary materials) and clearly dose-dependent. Despite parallel changes, the decrease was strongest in temporal muscle, weaker in the NAc, and lowest in the subcutaneous space. Due to these differences, NAc-muscle and skin-muscle temperature differentials significantly increased, with weaker changes for the former and much stronger changes for the latter (C). Figure 1D shows that xylazine tended to decrease locomotor activity; this effect was less evident at the lowest dose and more evident with higher drug doses.
Figure 1.
Changes in temperature induced by iv xylazine at different doses (0.33, 1.0 and 3.0 mg/kg in awake, freely moving rats. A = Absolute temperature changes. B = relative temperature changes; C = Brain-muscle and skin-muscle differentials. D = Locomotor activity. Filled symbols show values significantly different from pre-injection baseline.
2. Effects of xylazine on NAc oxygenation
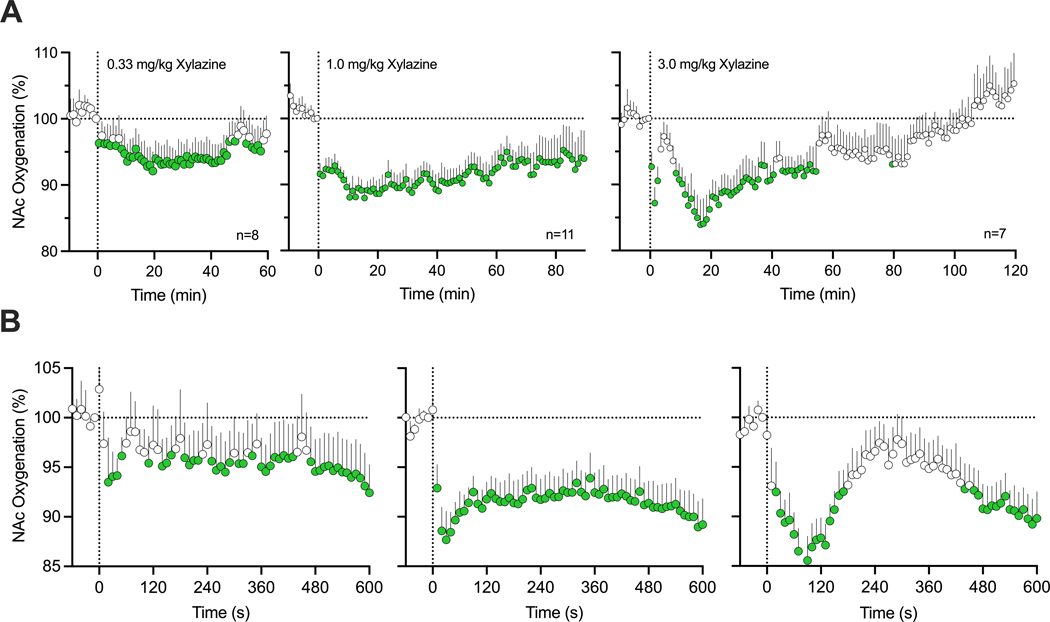
The effects of xylazine on NAc oxygenation were examined in 5 rats during 11 daily sessions. As shown in Figure 2A, xylazine at each dose rapidly decreased NAc oxygen levels, then followed with a slower ascent to baseline. Xylazine-induced oxygen responses were clearly dose-dependent. At the lowest dose (0.33 mg/kg), oxygen decrease was minimal in both its magnitude and duration, but stronger and more prolonged at higher drug doses (1.0 and 3.0 mg/kg). The rapidity of oxygen response was evident when data were analyzed with rapid, 10-s time resolution for 10 min post-injection (B). In this case, the largest drop in oxygen levels at each dose occurred within 10–30 s from the injection onset, i.e., within the duration of drug delivery.
Figure 2.
Relative changes in NAc oxygen levels induced by xylazine at different doses (0.3, 1.0 and 3.0) in freely moving rats. A = mean (±SEM) changes assessed with slow (1-min) time resolution. B = mean (±SEM) changes assessed with rapid (10-s) time resolution. Filled symbols show values significantly different from pre-injection baseline. n = numbers of averaged responses
3. Effects of xylazine on changes in NAc oxygenation induced by fentanyl
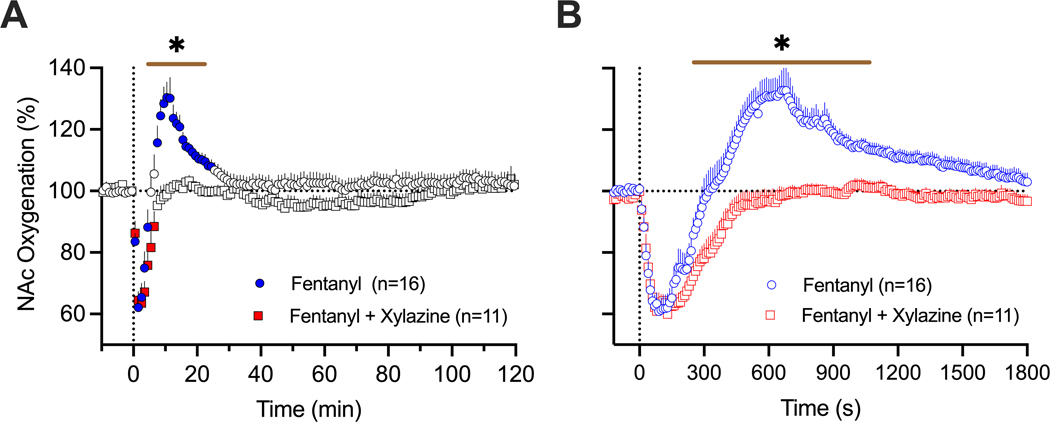
Next, we assessed the effects of a mixture of xylazine at a modest dose (1 mg/kg) on oxygen responses induced by fentanyl (20 μg/kg). These tests were conducted in 5 rats during 16 daily sessions. Consistent with our previous studies [9], fentanyl induced a biphasic NAc oxygen response (F15,1850=12.4, p<0.001) with a rapid and strong decrease (~62% below pre-injection baseline) followed by a more prolonged and weaker increase (Figure 3A).
Figure 3.
Mean (±SEM) changes in NAc oxygen levels induced by fentanyl (20 ug/kg) and its mixture with xylazine (20 ug/kg + 1 mg/kg) in freely moving rats. A = mean (±SEM) changes assessed with slow (1-min) time resolution. B = mean (±SEM) changes assessed with rapid (10-s) time resolution. Filled symbols show values significantly different from pre-injection baseline. n = numbers of averaged responses. Bold horizontal lines with asterisk show time intervals, during which between-group values were significant.
The xylazine-fentanyl mixture also induced an oxygen decrease (F10,1210=12.4, p<0.001), which mirrored the hypoxic response from administering fentanyl alone (~63% below the pre-injection baseline), but lacked the second phase of the oxygen response (Figure 3A). As shown by using a two-way ANOVA with repeated measures, between group-differences were significant from 4 to 22 min (Time x Treatment Interaction F130,3120=3.49, p<0.001). Between-group differences were especially evident when the data were analyzed with rapid, 10-s time resolution (Figure 3B). Due to disappearance of the second phase of oxygen response, the total duration of oxygen decrease was longer with the drug mixture than with the fentanyl alone (770 s vs. 330 s). Both fentanyl alone and its mixture with xylazine induced similar behavioral effects, including severe hypoactivity, muscle rigidity in the limbs, tail erection as well as decreases in rate and depth of respiration.
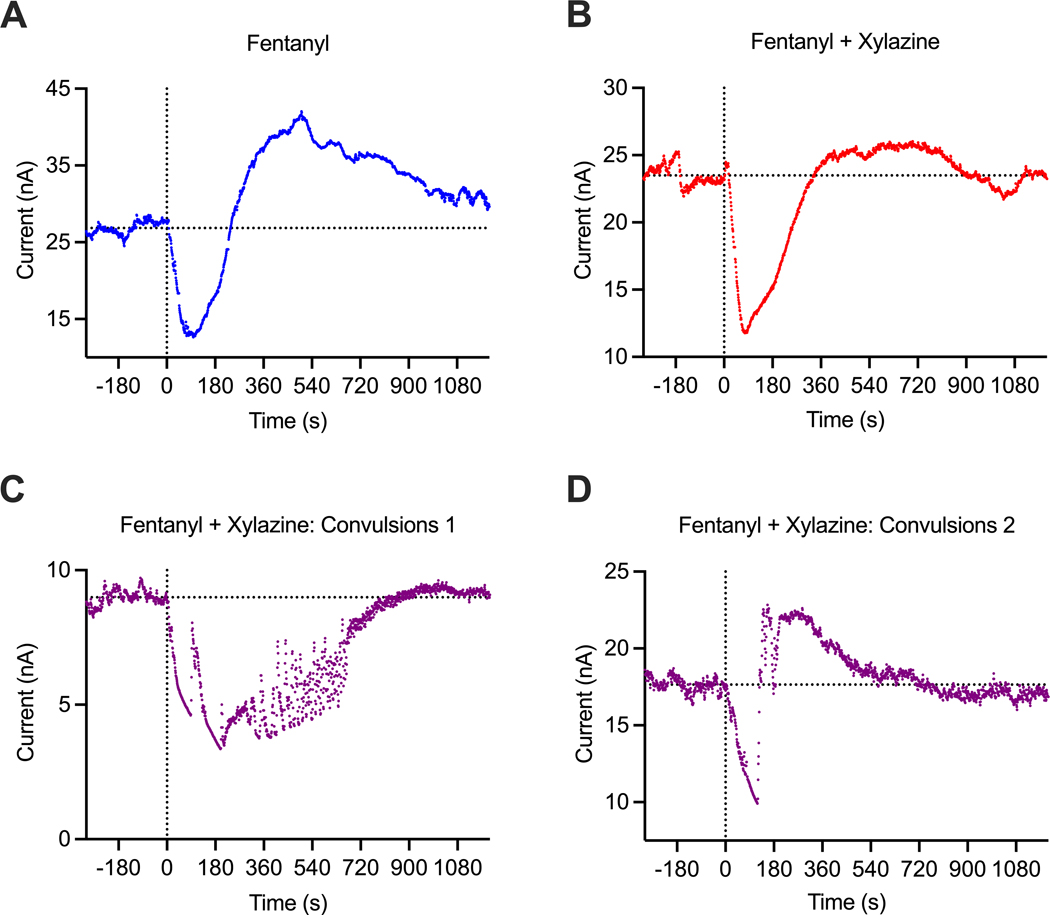
In most cases (10/13), the fentanyl-xylazine mixture induced similar, sedative behavioral effects and the same monophasic pattern of oxygen response (see original examples of oxygen changes induced by fentanyl alone and its mixture with xylazine in Figure 4A and B). However, in three cases (in 2 rats), the xylazine-fentanyl mixture produced convulsions within a couple of minutes post-injection. In this case, oxygen levels strongly decreased but robustly fluctuated in association with convulsion episodes (Figure 4C and D). These three cases were analyzed separately from the main data set.
Figure 4.
Primary data examples of changes in electrochemical currents (nA) induced by fentanyl and fentanyl-xylazine mixture in freely moving rats. A = fentanyl alone (20 ug/kg), B = fentanyl (20 ug/kg)+xylazine (1 mg/kg), typical example; C and D = unusual changes induced by fentanyl-xylazine mixture with convulsions. Values of reduction current are shown with original (1-s) time resolution, and they were inverted. Since basal reduction currents widely varied between sensors, data were analyzed as the change relative to basal value=100%. Convulsions were never seen after fentanyl alone, but they occurred in 3 cases (in 2 rats) after injections of fentanyl-xylazine mixture.
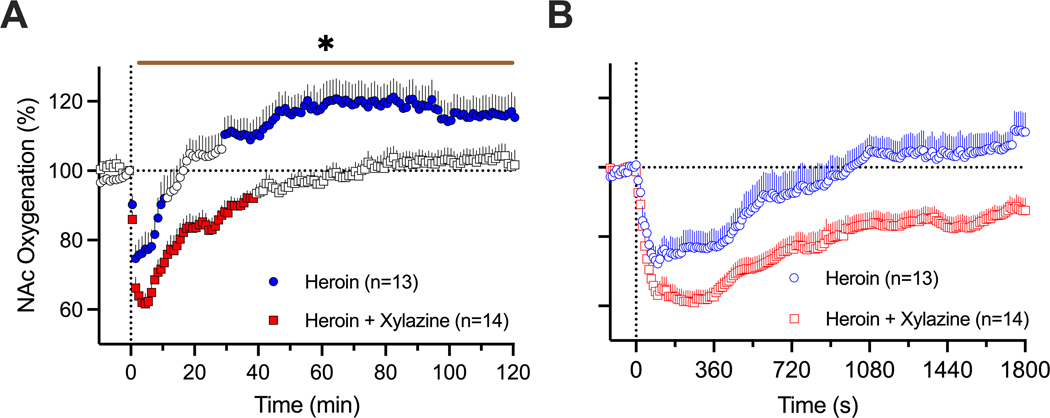
4. Effects of xylazine on changes in NAc oxygenation induced by heroin
The effects of xylazine on NAc oxygenation were tested in 6 rats during 14 daily sessions. As shown in Figure 5, heroin (600 ug/kg) administered alone induced a robust and prolonged decrease in NAc oxygen levels (75.0±3.9 % of baseline for ~17 min) followed by a weaker, more prolonged oxygen increase. The xylazine-heroin mixture also strongly decreased brain oxygenation and this decrease was clearly stronger (61.6±3.0 %; t=2.06, p<0.05) and more prolonged (~72 min) than with heroin alone. As shown by used a two-way ANOVA with repeated measures, the between-group difference was significant (Time x Treatment Interaction F130,3250=2.55, p<0.001). Between-group differences in oxygen response were especially evident when we analyzed the areas under the curve for oxygen decrease (4461.1±138.1 vs. 1124±153.6, t=16.2; p<0.0001).
Figure 5.
Mean (±SEM) changes in NAc oxygen levels induced by heroin (600 ug/kg) and its mixture with xylazine (600 ug/kg + 1 mg/kg) in freely moving rats. A = mean (±SEM) changes assessed with slow (1-min) time resolution. B = mean (±SEM) changes assessed with rapid (10-s) time resolution. Filled symbols show values significantly different from pre-injection baseline. n = numbers of averaged responses. Bold horizontal lines with asterisk show time intervals, during which between-group values were significant.
Discussion
We examined the effects of xylazine as an adulterant to highly potent opioids fentanyl and heroin. Since brain hypoxia following respiratory depression is the most dangerous effect of opioid drugs (Dahan et al. 2005; Jaffe et al. 1997; Pattinson 2008; Yeadon and Kitchen 1989), we employed oxygen sensors coupled with high-speed amperometry to examine how xylazine at relatively low, human-relevant doses affects brain oxygenation and whether the addition of xylazine affects the hypoxic effects of fentanyl and heroin, the two drugs largely implicated in overdose-related health complications and death.
Alpha-2 adrenoceptors, the primary substrate for xylazine action, are expressed in both the central and peripheral nervous systems (Drew 1976; Kobinger 1978; Greene and Thurmon 1988). By preferential presynaptic location on central neurons, stimulation of these receptors inhibits the release of norepinephrine, decreasing sympathetic activity and inducing CNS depression. These receptors are also located on smooth muscle cells in blood vessels, and their stimulation induces muscular atonia and decreases vascular tone. Stimulation of these multiple receptors appears to be responsible for a plethora of physiological effects of xylazine, which also depend on the dose and route of administration. In contrast to the relatively large doses of xylazine used during general anesthesia in animals (~10 mg/kg with ip administration in rats), doses used by humans are highly variable and the drug is delivered orally, subcutaneously, and intravenously. The range of toxic doses in humans also greatly varies from 40 to 2400 mg (or 0.6–34.3 mg/70 kg). Although human data with other routes of drug administration are limited, based on intoxication in non-fatal cases, the ranges for iv or im routes are 1 mg/kg and 0.6 to 9 mg/kg, respectively (Ruiz-Colon et al. 2014). Since xylazine is rarely used alone and its dosage as adulterant to other more potent drugs is typically low, we chose to test the effects of this drug at relatively low iv doses (0.33–3.0 mg/kg), much lower than the LD50 for iv injections in rats (22–43 mg/kg) [Xylazine hydrochloride 1999).
Despite this low-dose exposure, xylazine induced evident sedation, muscle relaxation, and hypothermia—known effects of the drug (Hsu 1981; Livingston et al. 1984). In contrast to older studies employing rectal temperature measurements, we used chronically implanted thermocouple sensors, stress-free drug delivery, and high-resolution data analyses to reveal differences in xylazine-induced temperature changes in the brain, temporal muscle, and skin. Although basal temperature in the brain was larger than in temporal muscle, the xylazine-induced decrease was stronger in the muscle than in the brain. This pattern is unusual since temperature changes induced by natural arousing stimuli are typically more rapid and stronger in the brain than in the temporal muscle, suggesting an increase in intra-brain heat production, a sequence of metabolic neural activation (Kiyatkin 2010). While we could not fully exclude that xylazine may increase metabolic brain activity, stronger temperature decreases in temporal muscle likely result from muscular atonia and atonia-related decreases in heat production (Lomo et al. 2020). The xylazine-induced temperature decrease in the subcutaneous space was weaker than in the muscle, resulting in a strong increase in the skin-muscle differential. While this change indicates skin vasodilation as a primary reason for heat loss and resulting brain and body hypothermia, we cannot exclude the possibility of an atonia-related decrease in muscular heat production.
Xylazine at large doses can induce respiratory depression and life-threatening hypoxia in animals (Thurmon and Benson 1986; Sanford and Colby 1980). Our data revealed that low doses of xylazine delivered iv decreases oxygen levels in the brain, a central effect that may result from respiratory depression. While the effect was weak (93%, 88%, and 82% of baseline levels for 0.33, 1.0, and 3 mg/kg doses), it was prolonged and dose-dependent. This tonic decrease in brain oxygen levels differed from the much stronger but transient decreases induced by fentanyl and heroin, suggesting different underlying mechanisms. These moderate decreases in brain oxygenation may also result from drug-induced decreases in sympathetic activity due to alpha-2 agonism and subsequent tonic decreases in respiratory activity. Alternatively, the weak hypoxic effect of xylazine may be independent of respiratory depression, resulting from cerebral vasoconstriction due to the stimulation of alpha-2 adrenoceptors on cerebral vessels (Kanawati et al. 1986).
The known physiological effects of xylazine and its prevalence in opioid-related overdose deaths led us to study the effects of this drug in combination with opioids. The potentiating effect of xylazine and other alpha-2 agonists has been found in early studies on the analgesic effects of opioid drugs, including fentanyl (Meert and De Kock 1994). However, these studies use larger doses of xylazine, and it is still under debate whether the weakening of nociceptive responses reflects the enhancement of opioid analgesia or reinforced sedation by xylazine. To test for the same potentiation effect, we examined xylazine effects at a relatively low, human-relevant dose (1.0 mg/kg) on brain oxygen responses induced by fentanyl and heroin.
We found that the addition of xylazine dramatically changes the pattern of brain oxygen response induced by fentanyl. In contrast to the biphasic effect of fentanyl, with a rapid and strong oxygen decrease followed by its rebound-like increase, the fentanyl-xylazine mixture resulted in elimination of the secondary increase, prolonging the duration of the initial decrease (Fig. 3). Stronger potentiating effects of xylazine were found on decreases in brain oxygenation induced by heroin at a relatively large dose (600 ug/kg; 30:1 ratio vs. fentanyl). Like fentanyl, heroin also induced biphasic oxygen responses, but both the initial decreases and subsequent increases were much more prolonged than with fentanyl. In contrast to heroin alone, the mixture with xylazine induced stronger and more prolonged decreases in brain oxygenation and eliminated the second hyperoxic phase of brain oxygen response.
To determine the possible mechanisms underlying xylazine’s ability to potentiate hypoxic effects of opioid drugs, we need to understand the mechanisms underlying the biphasic changes in oxygen induced by these drugs. In contrast to peripheral tissues, where heroin and fentanyl induce monophasic and relatively prolonged oxygen decreases, the brain hosts two-part oxygen responses with a strong, transient oxygen decrease followed by a weaker, prolonged oxygen increase (Thomas et al. 2021; Curay et al, 2023). While the initial decrease results from respiratory depression and subsequent drop in oxygen in the blood, the subsequent increase may result from cerebral vasodilation and increased vertebral blood flow due to post-hypoxic accumulation of CO2, a powerful vasoconstrictor, in the brain (Battisti-Charbonney et al. 2011; Schmidt and Kety 1947; Kontos 1981). Another factor that mediates cerebral vasodilation induced by opioid drugs is peripheral vasoconstriction that results in redistribution of arterial blood from the periphery to the brain and heart (Kiyatkin 2021). These two factors likely explain the cerebral vasodilation and increased cerebral blood flow that enhances oxygen entry into brain tissue despite its drop in arterial blood. This is an adaptive mechanism to counteract severe brain hypoxia following insufficient oxygen delivery to the brain from arterial blood.
While the exact mechanisms responsible for prolongation of fentanyl- and heroin-induced hypoxia and disappearance of post-hypoxic hyperoxia induced by xylazine still remain hypothetical, numerous studies point to the vascular effects of this drug in the brain, specifically the blockade of adaptive cerebral vasodilation due to known cerebral vasoconstrictive effects of xylazine and other alpha2 adrenergic agonists (Busija and Leffler 1987; Skarby 1984; Lee et al. 1997). Xylazine-induced peripheral vasodilation, which diminishes blood inflow to the brain, and decrease in arterial blood pressure due to xylazine-induced decreased sympathetic outflow may also contribute to the brain-specific hypoxic effects of this drug.
One finding of this study was unexpected. While administration of the fentanyl-xylazine mixture induced sedation in rats during most sessions, the mixture rarely induced convulsions associated with robust fluctuations in brain oxygen levels (Fig. 4). This finding suggests that the effects of the combined use of fentanyl and xylazine are not limited to potentiation of hypoxia and may induce other life-threatening complications. Although the mechanisms underlying this effect of drug combination remain unclear and require further investigation, it is known that convulsions result in robust cerebral vasodilation/increased cerebral blood flow (Penfield et al 1939; Ferlini et al. 2021), which may counteract the cerebral vasoconstriction induced by xylazine. This explanation remains speculative and require further investigation.
Conclusions
Overall, our findings deepen the understanding of the involvement of xylazine adulterants in opioid overdose-induced health complications. We highlight the damaging physiological effects of xylazine as it pertains to substance abuse when taken in tandem with opioids by showing the effects of drug mixtures on brain oxygenation and brain temperature. We found that xylazine addition to fentanyl results in elimination of the brain’s compensatory mechanisms to counteract rapid brain hypoxia, and that xylazine addition to heroin potentiates the rapid opioid-induced brain hypoxia in addition to elimination of the following compensatory mechanisms. Our results imply that xylazine can exacerbate the life-threatening potential of opioid use, positing worsened brain hypoxia as a potential cause of death. As xylazine and fentanyl or heroin have different receptor targets, clinicians should suspect the presence of xylazine when opioid overdoses are less responsive to treatment with naloxone. Experiments with naloxone and xylazine antagonists are currently underway.
Supplementary Material
Acknowledgements:
The study was supported by the Intramural Research Program of the NIH, NIDA (# 1ZIA DA000566-13 for EAK).
Funding:
The study was supported by the Intramural Research Program of the NIH, NIDA (NIH Grant 1ZIADA000566-12 for Dr. Eugene A. Kiyatkin).
Abbreviations:
- ANOVA
analysis of variance
- iv
intravenous
- NAc
nucleus accumbens
- ip
intraperitoneal
Footnotes
Competing interests: The authors have nothing to disclose.
Conflict of interest: The authors report no conflict of interests.
Data availability:
Raw data and the results of their primary analyses are available on request from Dr. Eugene A. Kiyatkin (NIDA-IRP, NIH; ekiyatki@intra.nida.nih.gov).
References
- Baby SM, Discala JF, Gruber R, Getsy P, Cheng F et al. Tempol reverses the negative effects of morphine on arterial blood-gas chemistry and tissue oxygen saturation in freely-moving rats. Front Pharmacol. 2021; 12:749084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011; 12(11):685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti-Charbonney A, Fisher J, Duffin J. The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011; 589(Pt 12):3039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger FB, Bennett R, Lowry JP. An in vitro characterization comparing carbon paste and Pt microelectrodes for real-time detection of brain tissue oxygen. Analyst. 2011; 136(19):4028–35. [DOI] [PubMed] [Google Scholar]
- Brown JH, Pleuvry BJ. Antagonism of the respiratory effects of alfentanil and fentanyl by naloxone in the conscious rabbit. Br J Anaesth. 1981; 53(10):1033–7. [DOI] [PubMed] [Google Scholar]
- Busija DW, Leffler CW. Postjunctional alpha 2-adrenoceptors in pial arteries of anesthetized newborn pigs. Dev Pharmacol Ther. 1987; 10(1):36–46. [DOI] [PubMed] [Google Scholar]
- Capraro AJ, Wley JF, Tucker JR. Severe intoxication from xylazine inhalation. Pediatr Emerg Care. 2001; 17(6):447–8. [DOI] [PubMed] [Google Scholar]
- Curay CM, Irwin MR, Kiyatkin EA. The pattern of brain oxygen response induced by intravenous fentanyl limits the time window of therapeutic efficacy of naloxone. Neuropharmacology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth. 2005; 94(6):825–34. [DOI] [PubMed] [Google Scholar]
- Decker MJ, Conrad KP, Strohl KP. Noninvasive oximetry in the rat. Biomed Instrum Technol. 1989; 23(3):222–8. [PubMed] [Google Scholar]
- Drew GM. Effects of alpha-adrenergic agonists and antagonists on pre- and postsynaptically located alpha-adrenoceptors. Eur J Pharmacol. 1976; 36(2):313–20. [DOI] [PubMed] [Google Scholar]
- Ferlini L, Su F, Creteur J, Taccone FS, Gaspard N. Cerebral and systemic hemodynamic effect of recurring seizures. Sci Rep. 2021; 11(1), 22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Montero F, Bourgois P, Wahbi R, Dye D et al. Xylazine spreads across the US: A growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022; 233:109380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber CJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: A variable dose paradigm. Pharmacol Biochem Behav. 1989; 32(2):527–31. [DOI] [PubMed] [Google Scholar]
- Greene SA, Thurmon JC. Xylazine—a review of its pharmacology and use in veterinary medicine. J Vet Pharmacol Ther. 1988; 11(4):295–313. [DOI] [PubMed] [Google Scholar]
- Hedenqvist P, Roughan JV, Flecknell PA. Sufentanil and medetomidine anesthesia in the rat and its reversal with atipamezole, and butorphanol. Lab Anim 2000; 34(3):244–51. [DOI] [PubMed] [Google Scholar]
- Hsu WH. Xylazine-induced depression and its antagonism by alpha adrenergic blocking agents. J Pharmacol Exp Ther. 1981; 218(1):188–92. [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, Ciraulo DA, 1997. Opiates: Clinical Aspects. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse, Third edn. Baltimore: Williams & Wilkins,. p. 158–66. [Google Scholar]
- Kanawati S, Yaksh TL, Anderson RE, Marsh. Effects of clonidine on cerebral blood flow and the response to arterial CO2. J Cereb Blood Flow Metab. 1986; 6(3):358–65. [DOI] [PubMed] [Google Scholar]
- Kariisa M, Patel P, Smith H, Bitting J. Xylazine detection and involvement in drug overdose deaths—United States, 2019. MMWR Morb Mortal Wkly Rep. 2021; 70(37):1300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Bennett R, Lowry JP. Simultaneous recording of hippocampal oxygen and glucose in real time using constant potential amperometry in the freely-moving rat. J Neurosci Methods. 2013; 215(1):110–20. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, 2019. Physiological and drug-induced fluctuations in brain oxygen and glucose assessed by substrate-sensitive sensors coupled with high-speed amperometry. In: Wilson GS and Michael AC, editors. Compendium of in vivo monitoring in real-time molecular neuroscience. Volume 3: Probing brain functions, disease and Injury with enhanced optical and electrochemical sensors. World Scientific; 2019. p. 219–50. [Google Scholar]
- Kiyatkin EA, Brown PL. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speed in rats. Psychopharmacology (Berl). 2005; 181(2): 299–308. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. 2021. Functional role of peripheral vasoconstriction: not only thermoregulation but much more. J Integr Neurosci. 2021; 20(3):755–64. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed). 2010; 15(1):73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger W. Central alpha-adrenergic systems as target for hypotensive drugs. Rev Physiol Biochem Pharmacol. 1978; 81:39–100. [DOI] [PubMed] [Google Scholar]
- Kontos HA. Regulation of the cerebral circulation. Annu Rev Physiol. 1981; l 43:397–407. [DOI] [PubMed] [Google Scholar]
- Ledo A, Lourenco C, Laranjinha J, Gerhardt GA. Barbosa RM. Combined in vivo amperometric oximetry and electrophysiology in a single sensor: a tool for epilepsy research. Anal Chem. 2017; 89(22):12383–90. [DOI] [PubMed] [Google Scholar]
- Lee HW, Caldwell JE, Dodson B, Talke P, Howley J. The effect of clonidine on cerebral blood flow velocity, carbon dioxide cerebral vasoreactivity, and response to increased arterial pressure in human volunteers. Anesthesiology 1997; 87(3):553–8. [DOI] [PubMed] [Google Scholar]
- Livingston A, Low J, Morris B. Effects of clonidine and xylazine on body temperature in the rat. Br J Pharmacol. 1984; 81(1):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T, Eken T, Rein EB, Nja A. Body temperature control in rats by muscle tone during rest or sleep. Acta Physiol (Oxf). 2020; 228(2):e13348. [DOI] [PubMed] [Google Scholar]
- Lowry JP, Fillenz M. Real-time monitoring of brain energy metabolism in vivo using microelectrochemical sensors: the effects of anesthesia. Bioelectrochemistry. 2001; 54(1):39–47. [DOI] [PubMed] [Google Scholar]
- Meert TF, De Kock M. Potentiation of the analgesic properties of fentanyl-like opioids with alpha 2 adrenoceptor agonists in rats. Anesthesiology. 1994; 81(3):677–88. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980; 14(2–3):69–97. [DOI] [PubMed] [Google Scholar]
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008; 100(6):747–58. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates, Academic Press: San Diego. [DOI] [PubMed] [Google Scholar]
- Penfield W, von Sántha K, Cipriani A. Cerebral blood flow during induced epileptiform seizures in animals and man. J Neurophysiol 1939; 2:257–67. [Google Scholar]
- Reyes JC, Negron JL, Colob HM, Padilla AM, Millan MY et al. The emerging of xylazine as a new drug of abuse and its health consequences among drug users in Puerto Rico . J Urban Health. 2012; 89(3):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Colon K, Chavez-Arias C, Diaz-Alcala J, and Martinez MA. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: A comprehensive review of the literature. Forensic Sci Int. 2014; 240:1–8. [DOI] [PubMed] [Google Scholar]
- Sanford TD, Colby ED. Effect of xylazine and ketamine on blood pressure, heart rate and respiratory rate in rabbits. Lab Anim Sci. 1980; 30(3):519–23. [PubMed] [Google Scholar]
- Schmidt CF, Kety SS. Recent studies of cerebral blood flow and cerebral metabolism in man. Trans Assoc Am Physicians. 1947; 60(1):52–58. [PubMed] [Google Scholar]
- Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J Vet Pharmacol Ther. 1998; 21:107–11. [DOI] [PubMed] [Google Scholar]
- Seckler JM, Grossfield A, May WJ, Getsy PM, Lewis SJ. Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats. Biomed Pharmacother. 2022; 146:112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro. 2017; 4(3) ENEURO.0151–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skärby T. Pharmacological properties of prejunctional alpha-adrenoceptors in isolated feline middle cerebral arteries; comparison with the postjunctional alpha-adrenoceptors. Acta Physiol Scand. 1984; 122(2):165–74. [DOI] [PubMed] [Google Scholar]
- Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology. 2018; 43(4):810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Curay CM, Kiyatkin EA. Relationships between oxygen changes in the brain and periphery following physiological activation and the action of heroin and cocaine. Sci Rep. 2021; 11(1):6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Perekopskiy D, Kiyatkin EA. Cocaine added to heroin fails to affect heroin-induced hypoxia. Brain Res. 2020; 1746:147008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmon JC, Benson GJ. Anesthesia in ruminants and swine. In Anderson DE, Rings DM, editors. The veterinary clinics of North America: food animal practice. Philadelphia: W. B. Saunders Company; 2009. pp. 51–71. [Google Scholar]
- U.S. Department of Justice. Drug Enforcement Administration. The growing threat of xylazine and its mixture with illicit drugs. DEA Joint Intelligence Report. 2021. [Google Scholar]
- van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. J. Pharmacol Exp Ther. 1978; 204(3):547–57. [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987; 94(4):469–92. [PubMed] [Google Scholar]
- Xylazine Hydrochloride: Summary report. The European Agency for the Evaluation of Medicinal Products. 1999. [Google Scholar]
- Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol. 1989; 33(1):1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data and the results of their primary analyses are available on request from Dr. Eugene A. Kiyatkin (NIDA-IRP, NIH; ekiyatki@intra.nida.nih.gov).