Abstract
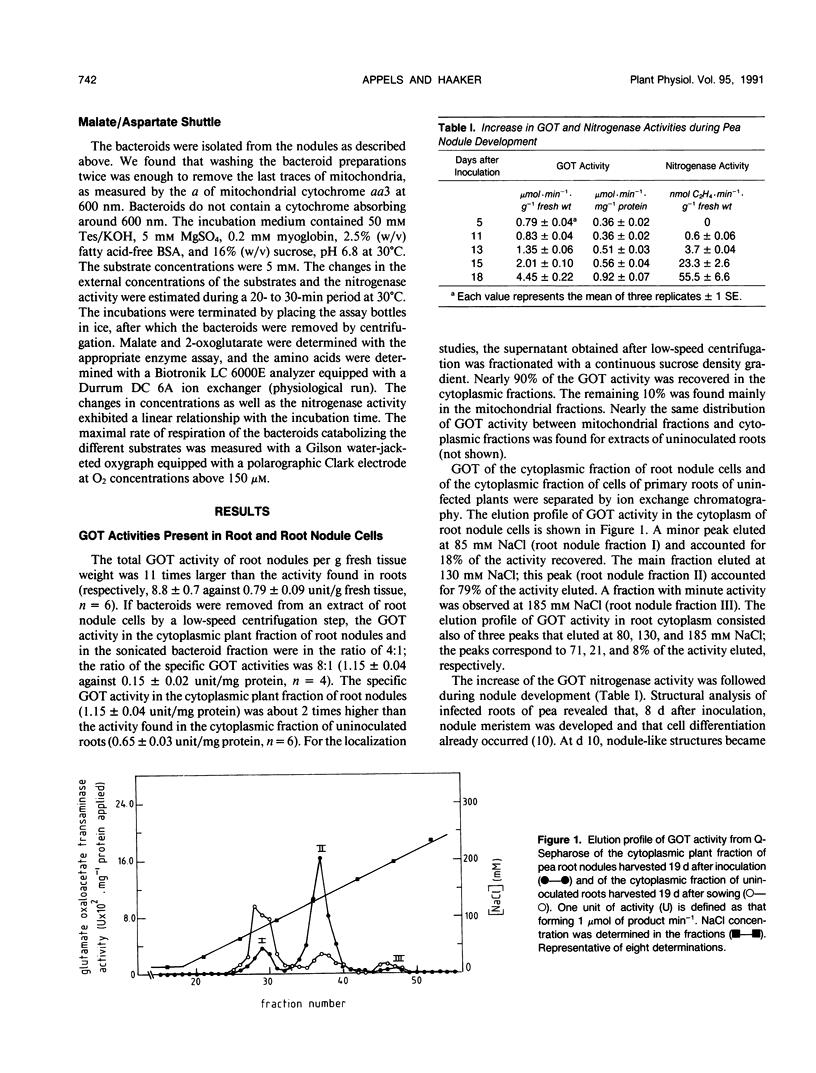
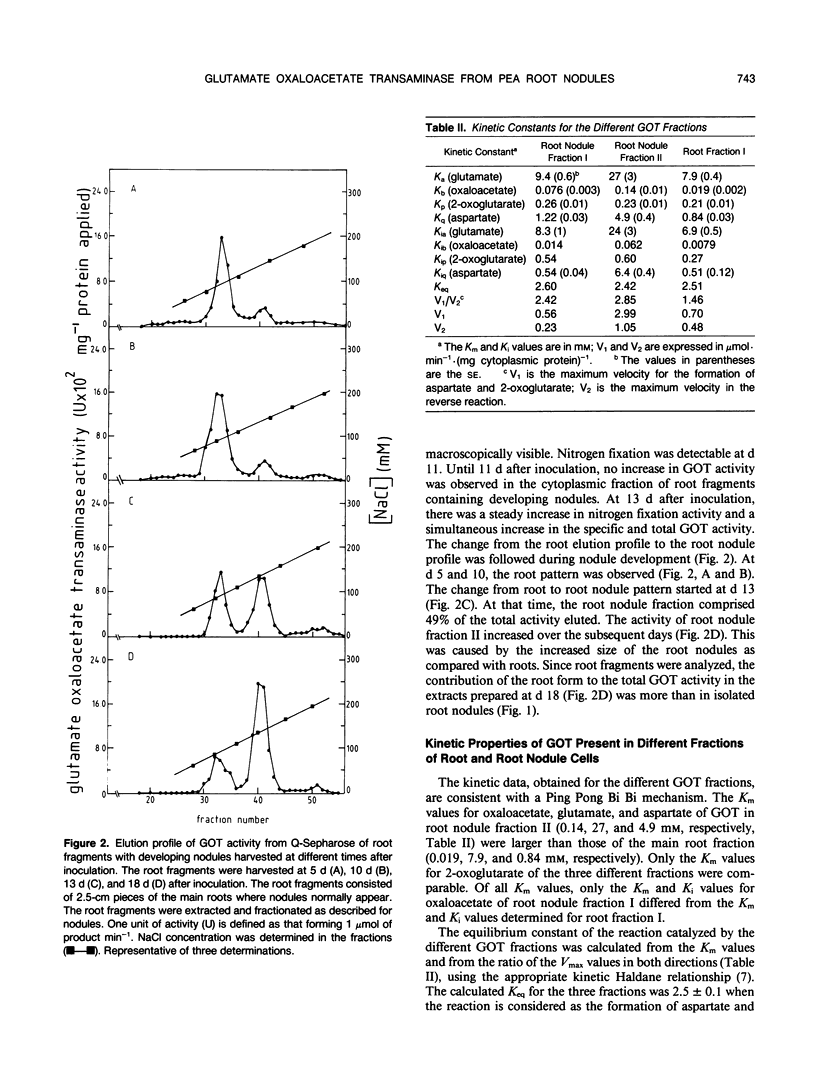
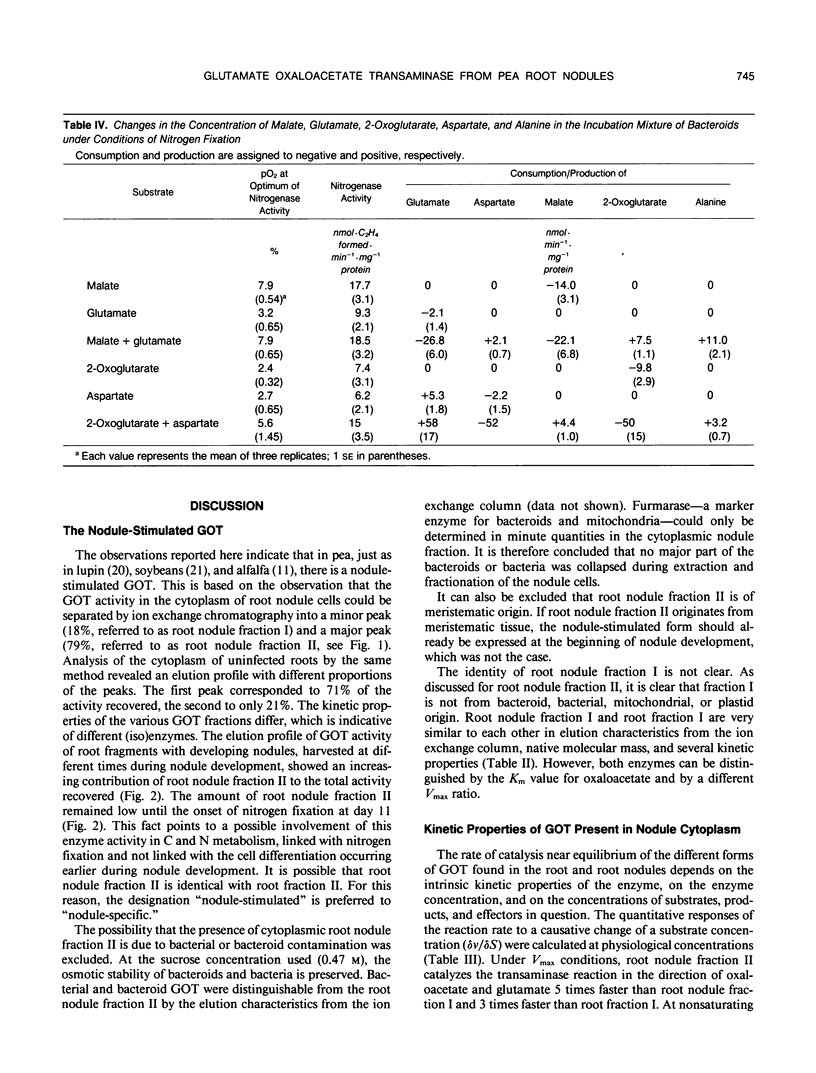
Glutamate oxaloacetate transaminase (l-glutamate: oxaloacetate aminotransferase, EC 2.6.1.1 [GOT]), a key enzyme in the flow of carbon between the organic acid and amino acid pools in pea (Pisum sativum L.) root nodules, was studied. By ion exchange chromatography, the presence of two forms of GOT in the cytoplasm of pea root nodule cells was established. The major root nodule form was present in only a small quantity in the cytoplasm of root cells. Fractionation of root nodule cell extracts demonstrated that the increase in the GOT activity during nodule development was due to the increase of the activity in the cytoplasm of the plant cells, and not to an increase in activity in the plastids or in the mitochondria. The kinetic properties of the different cytoplasmic forms of GOT were studied. Some of the Km values differed, but calculations indicated that not the kinetic properties but a high concentration of the major root nodule form caused the observed increase in GOT activity in the pea root nodules. It was found that the reactions of the malate/aspartate shuttle are catalyzed by intact bacteroids, and that these reactions can support nitrogen fixation. It is proposed that the main function of the nodule-stimulated cytoplasmic form of GOT is participation in this shuttle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appels M. A., Haaker H. Identification of cytoplasmic nodule-associated forms of malate dehydrogenase involved in the symbiosis between Rhizobium leguminosarum and Pisum sativum. Eur J Biochem. 1988 Feb 1;171(3):515–522. doi: 10.1111/j.1432-1033.1988.tb13820.x. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Robertson J. G., Wood E. A., Wells B., Larkins A. P., Galfre G., Butcher G. W. Monoclonal antibodies to antigens in the peribacteroid membrane from Rhizobium-induced root nodules of pea cross-react with plasma membranes and Golgi bodies. EMBO J. 1985 Mar;4(3):605–611. doi: 10.1002/j.1460-2075.1985.tb03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Govers F., Gloudemans T., Moerman M., van Kammen A., Bisseling T. Expression of plant genes during the development of pea root nodules. EMBO J. 1985 Apr;4(4):861–867. doi: 10.1002/j.1460-2075.1985.tb03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. M., Vance C. P. Aspartate aminotransferase in alfalfa root nodules : I. Purification and partial characterization. Plant Physiol. 1989 Aug;90(4):1622–1629. doi: 10.1104/pp.90.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- Laane C., Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Rhizobium leguminosarum bacteroids. Eur J Biochem. 1978 Jun 1;87(1):147–153. doi: 10.1111/j.1432-1033.1978.tb12361.x. [DOI] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Metabolism of C-labeled photosynthate and distribution of enzymes of glucose metabolism in soybean nodules. Plant Physiol. 1983 Jul;72(3):634–640. doi: 10.1104/pp.72.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S. O., Streeter J. G. Involvement of glutamate in the respiratory metabolism of Bradyrhizobium japonicum bacteroids. J Bacteriol. 1987 Feb;169(2):495–499. doi: 10.1128/jb.169.2.495-499.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Snapp S. S., Vance C. P. Asparagine Biosynthesis in Alfalfa (Medicago sativa L.) Root Nodules. Plant Physiol. 1986 Oct;82(2):390–395. doi: 10.1104/pp.82.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Kazazian V., Zogbi V., Bal A. K. Isolation and characterization of the membrane envelope enclosing the bacteroids in soybean root nodules. J Cell Biol. 1978 Sep;78(3):919–936. doi: 10.1083/jcb.78.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]


