Abstract
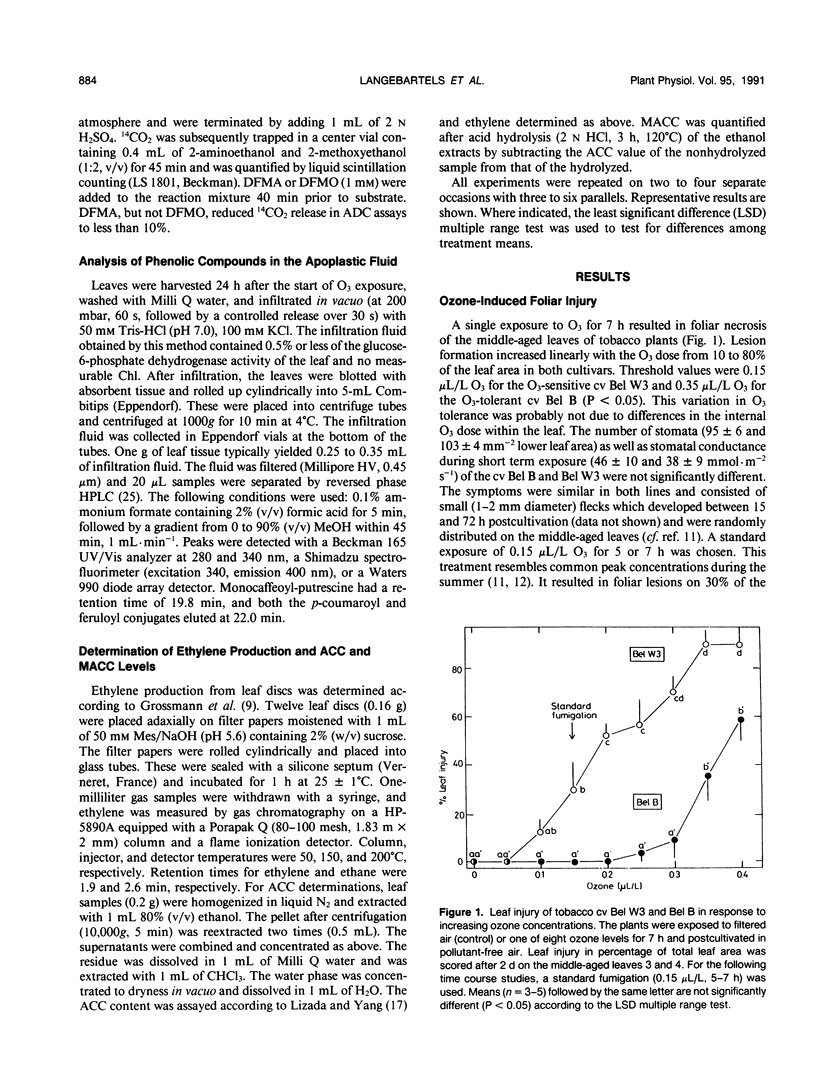
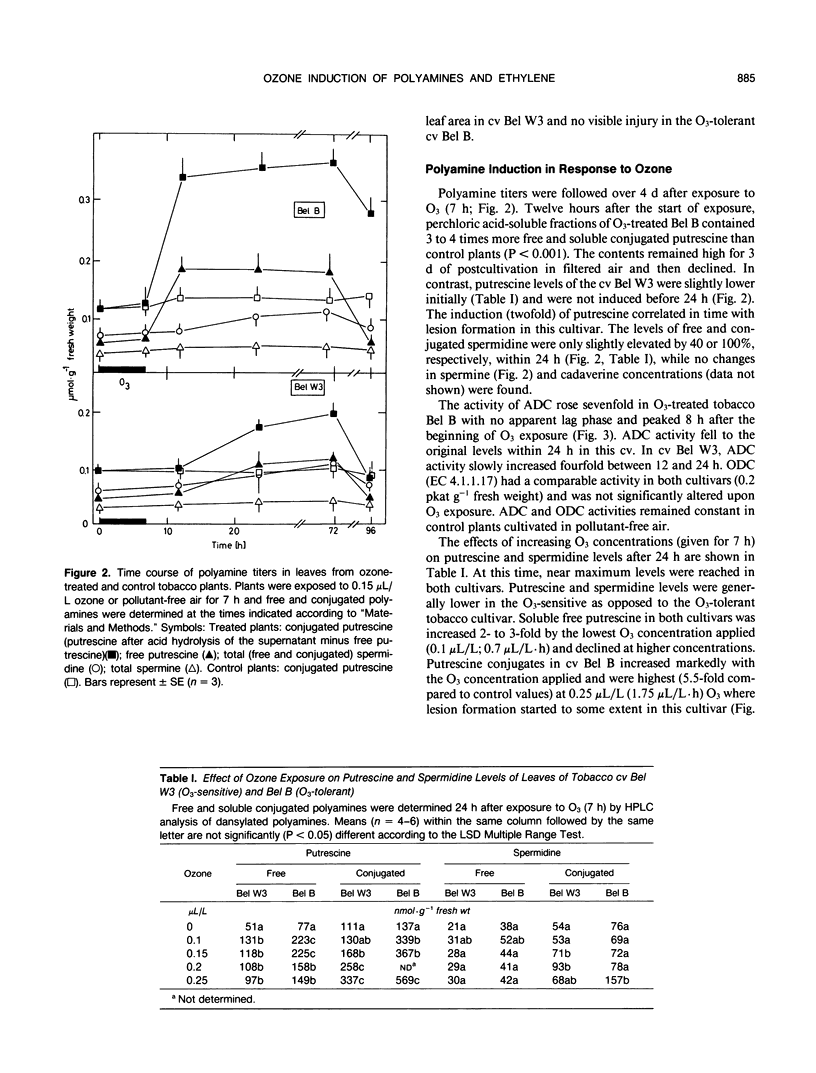
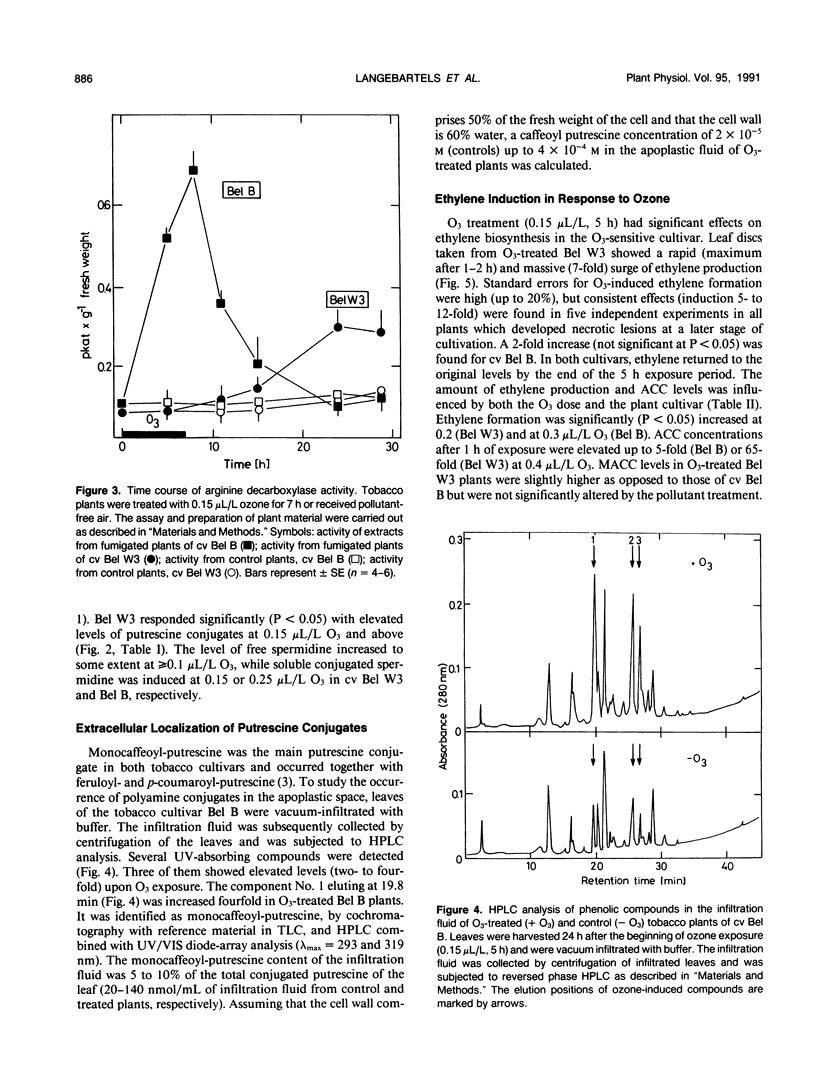
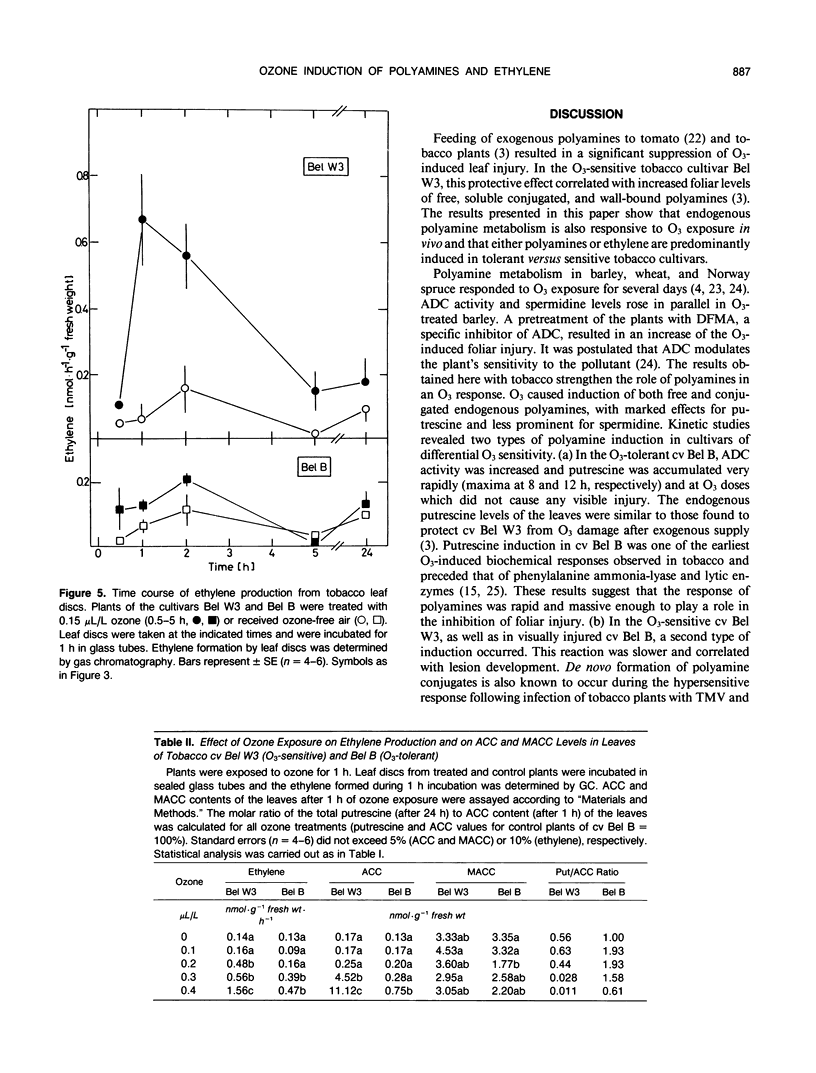
Polyamine metabolism was examined in tobacco (Nicotiana tabacum L.) exposed to a single ozone treatment (5 or 7 hours) and then postcultivated in pollutant-free air. The levels of free and conjugated putrescine were rapidly increased in the ozone-tolerant cultivar Bel B and remained high for 3 days. This accumulation was preceded by a transient rise of l-arginine decar-boxylase (ADC, EC 4.1.1.19) activity. The ozone-sensitive cultivar Bel W3 showed a rapid production of ethylene and high levels of 1-aminocyclopropane-1-carboxylic acid after 1 to 2 hours of exposure. Induction of putrescine levels and ADC activity was weak in this cultivar and was observed when necrotic lesions developed. Leaf injury occurred in both lines when the molar ratio of putrescine to 1-aminocyclopropane-1-carboxylic acid or ethylene fell short of a certain threshold value. Monocaffeoyl-putrescine, an effective scavenger for oxyradicals, was detected in the apo-plastic fluid of the leaves of cv Bel B and increased upon exposure to ozone. This extracellular localization could allow scavenging of ozone-derived oxyradicals at the first site of their generation. Induction of either polyamine or ethylene pathways may represent a control mechanism for inhibition or promotion of lesion formation and thereby contribute to the disposition of plants for ozone tolerance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostol I., Heinstein P. F., Low P. S. Rapid Stimulation of an Oxidative Burst during Elicitation of Cultured Plant Cells : Role in Defense and Signal Transduction. Plant Physiol. 1989 May;90(1):109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen G. P., Koppers A., Langebartels C. Biochemical response of Norway spruce (Picea abies (L.) Karst.) towards 14-month exposure to ozone and acid mist: effects on amino acid, glutathione and polyamine titers. Environ Pollut. 1990;64(3-4):375–383. doi: 10.1016/0269-7491(90)90059-l. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Rowland-Bamford A. J., Borland A. M., Lea P. J., Mansfield T. A. The role of arginine decarboxylase in modulating the sensitivity of barley to ozone. Environ Pollut. 1989;61(2):95–106. doi: 10.1016/0269-7491(89)90030-4. [DOI] [PubMed] [Google Scholar]
- Tadolini B. Polyamine inhibition of lipoperoxidation. The influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads. Biochem J. 1988 Jan 1;249(1):33–36. doi: 10.1042/bj2490033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio A. F., Masdeu M. A., Dumortier F. M., Galston A. W. Polyamine metabolism and osmotic stress. I. Relation to protoplast viability. Plant Physiol. 1986;82:369–374. doi: 10.1104/pp.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey D. T., Standley C., Field R. W. Stress ethylene evolution: a measure of ozone effects on plants. Atmos Environ. 1976;10(11):969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]


