Abstract
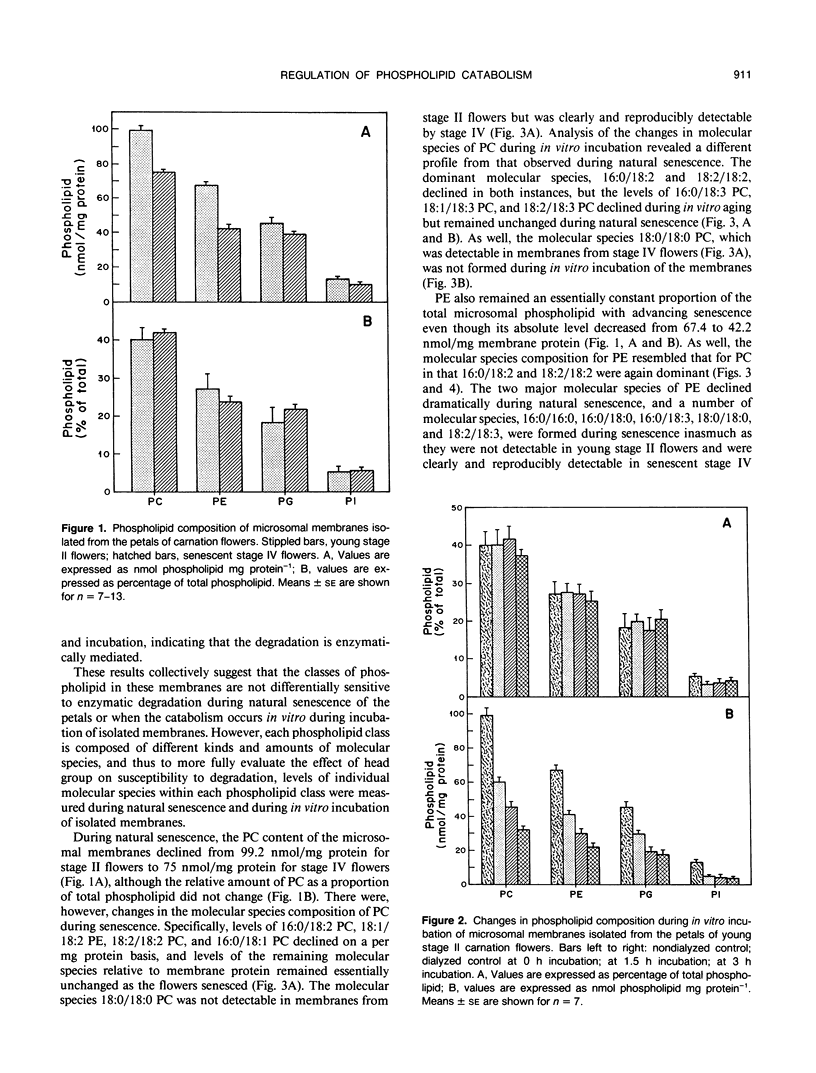
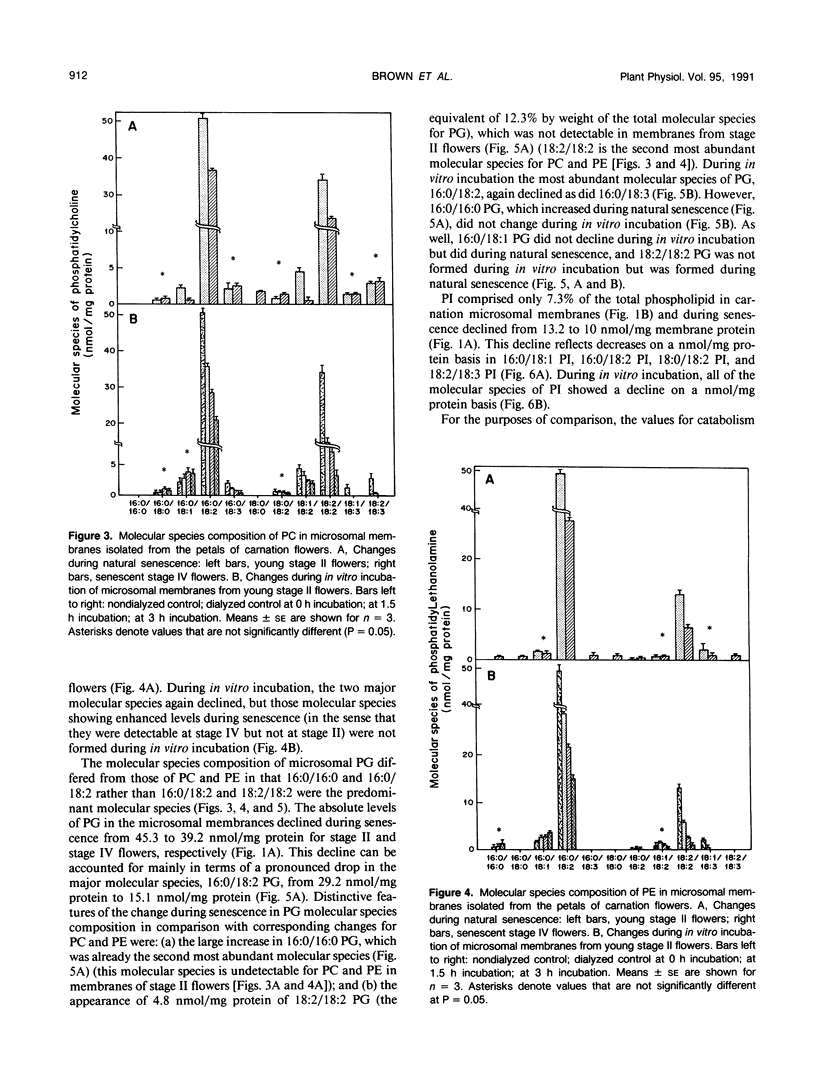
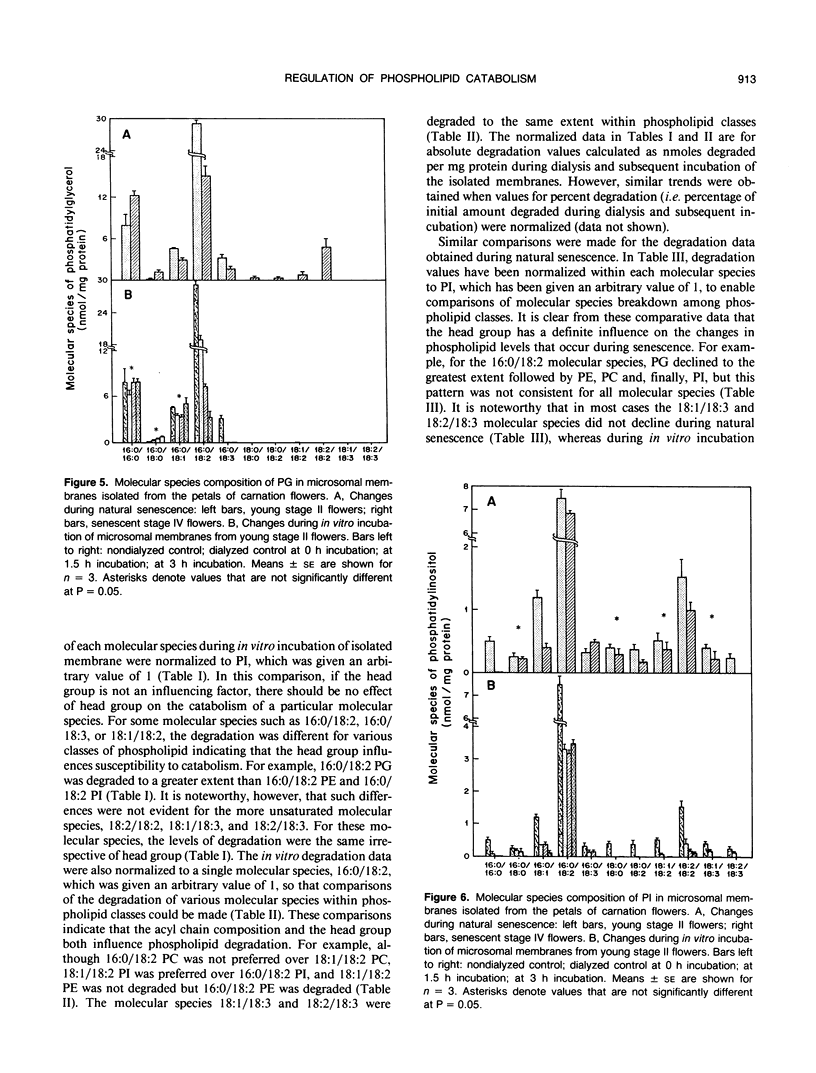
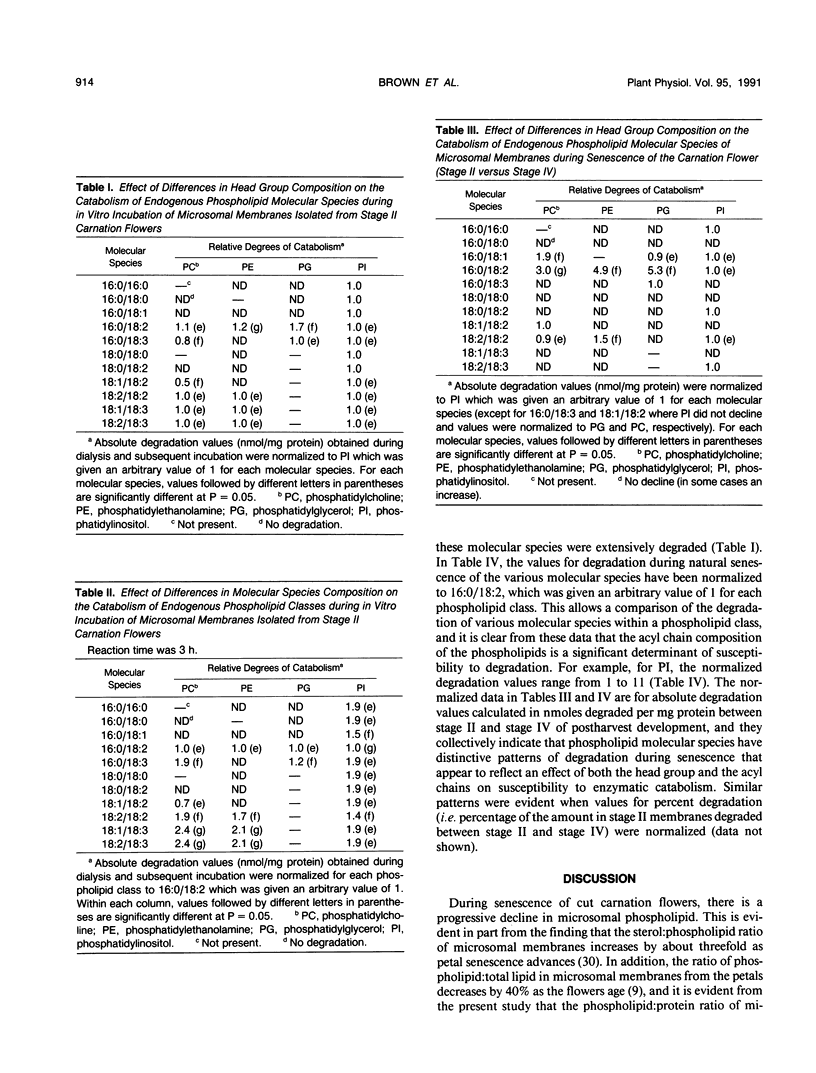
Microsomal membranes from the petals of senescing carnation (Dianthus caryophyllus L.) flowers contain phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylinositol. These phospholipid classes decline essentially in parallel during natural senescence of the flower and when microsomal membranes isolated from young flowers are aged in vitro. However, measurements of changes in the endogenous molecular species composition of microsomal phospholipids during natural senescence of the flower petals and during in vitro aging of isolated membranes have indicated that the various molecular species of phospholipids have quite different susceptibilities to catabolism. Acyl chain composition and the nature of the head group are both determinants of their susceptibility to catabolism. As well, a comparison of the phospholipid catabolism data for naturally senesced membranes and for membranes aged in vitro suggests that the phospholipid composition of membranes is continuously altered during senescence by acyl chain desaturation and possibly retailoring so as to generate molecular species that are more prone to catabolism. The results collectively indicate that provision of particular molecular species of phospholipids with increased susceptibility to degradation contributes to enhanced phospholipid catabolism in the senescing carnation petal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bomalaski J. S., Clark M. A. The effect of sn-2 fatty acid substitution on phospholipase C enzyme activities. Biochem J. 1987 Jun 15;244(3):497–502. doi: 10.1042/bj2440497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H., Shinitzky M. Senescence and the Fluidity of Rose Petal Membranes : RELATIONSHIP TO PHOSPHOLIPID METABOLISM. Plant Physiol. 1982 Feb;69(2):296–299. doi: 10.1104/pp.69.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Corona M. L., Peters S. G., Narr B. J., Thompson R. L. Infections related to central venous catheters. Mayo Clin Proc. 1990 Jul;65(7):979–986. doi: 10.1016/s0025-6196(12)65159-3. [DOI] [PubMed] [Google Scholar]
- Dickens B. F., Thompson G. A., Jr Phospholipid molecular species alterations in microsomal membranes as an initial key step during cellular acclimation to low temperature. Biochemistry. 1982 Jul 20;21(15):3604–3611. doi: 10.1021/bi00258a012. [DOI] [PubMed] [Google Scholar]
- Fobel M., Lynch D. V., Thompson J. E. Membrane deterioration in senescing carnation flowers : coordinated effects of phospholipid degradation and the action of membranous lipoxygenase. Plant Physiol. 1987 Sep;85(1):204–211. doi: 10.1104/pp.85.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Zevallos M., Farkas T. Manipulation of plasma membrane physical state affects desaturase activity in rat lymphocytes. Arch Biochem Biophys. 1989 Jun;271(2):546–552. doi: 10.1016/0003-9861(89)90306-8. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. Delta 6- and delta 12-desaturase activities and phosphatidic acid formation in microsomal preparations from the developing cotyledons of common borage (Borago officinalis). Biochem J. 1988 Jun 15;252(3):641–647. doi: 10.1042/bj2520641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M. Phospholipase D. Adv Lipid Res. 1978;16:267–326. doi: 10.1016/b978-0-12-024916-9.50011-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol phosphodiesterase in higher plants. Biochem J. 1980 Oct 15;192(1):279–283. doi: 10.1042/bj1920279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Koizumi K. Effect of different physical states of phospholipid substrates on partially purified platelet phospholipase A2 activity. Biochim Biophys Acta. 1979 Oct 5;556(3):423–433. doi: 10.1016/0005-2736(79)90130-5. [DOI] [PubMed] [Google Scholar]
- Kim D. K., Kudo I., Inoue K. Detection in human platelets of phospholipase A2 activity which preferentially hydrolyzes an arachidonoyl residue. J Biochem. 1988 Oct;104(4):492–494. doi: 10.1093/oxfordjournals.jbchem.a122496. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McArthur J. A., Marsho T. V., Newman D. W. Lipid Transformations in Plastids of Bean Leaves and Pepper Fruits. Plant Physiol. 1964 Jul;39(4):551–554. doi: 10.1104/pp.39.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Lipid crystallization in senescent membranes from cotyledons. Plant Physiol. 1977 May;59(5):803–807. doi: 10.1104/pp.59.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myher J. J., Kuksis A. Resolution of diacylglycerol moieties of natural glycerophospholipids by gas-liquid chromatography on polar capillary columns. Can J Biochem. 1982 Jun;60(6):638–650. doi: 10.1139/o82-079. [DOI] [PubMed] [Google Scholar]
- Ramesha C. S., Thompson G. A., Jr Cold stress induces in situ phospholipid molecular species changes in cell surface membranes. Biochim Biophys Acta. 1983 Jun 10;731(2):251–260. doi: 10.1016/0005-2736(83)90016-0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene Action and Loss of Membrane Integrity during Petal Senescence in Tradescantia. Plant Physiol. 1980 Jun;65(6):1067–1072. doi: 10.1104/pp.65.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P., Khan M. U., Mitchell K., Johnson G. The Effect of Temperature on the Level and Biosynthesis of Unsaturated Fatty Acids in Diacylglycerols of Brassica napus Leaves. Plant Physiol. 1988 Aug;87(4):904–910. doi: 10.1104/pp.87.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]


