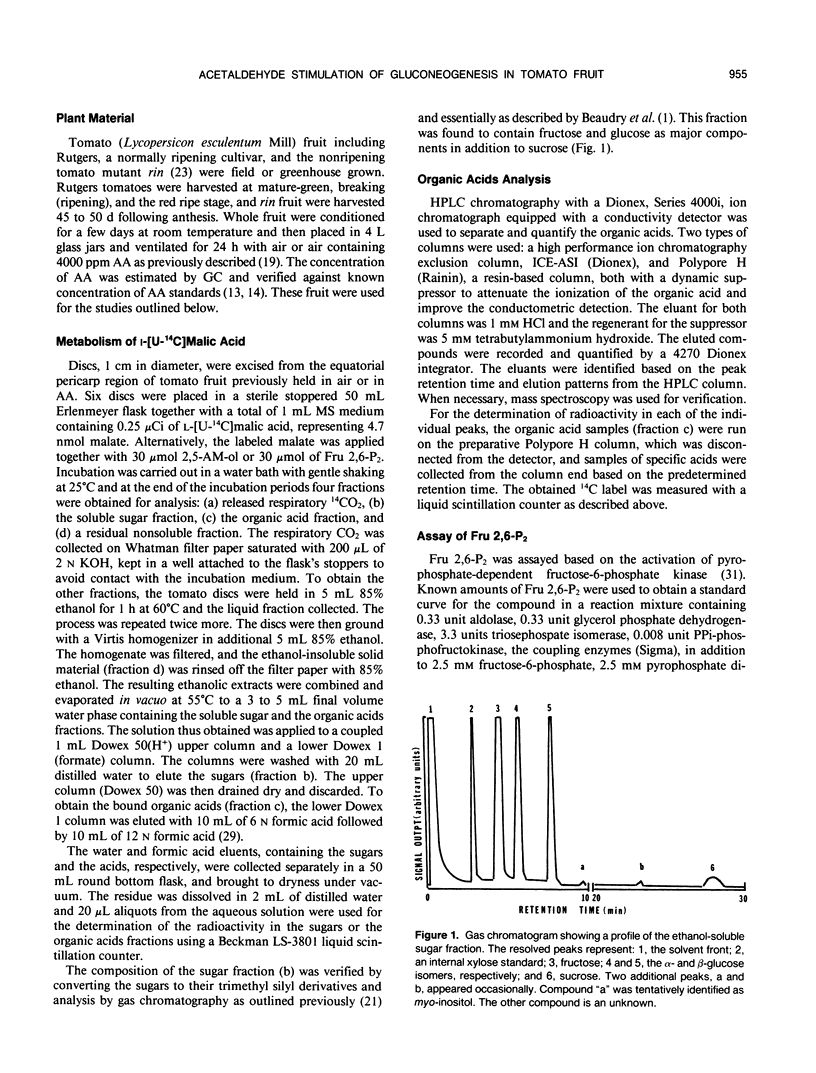
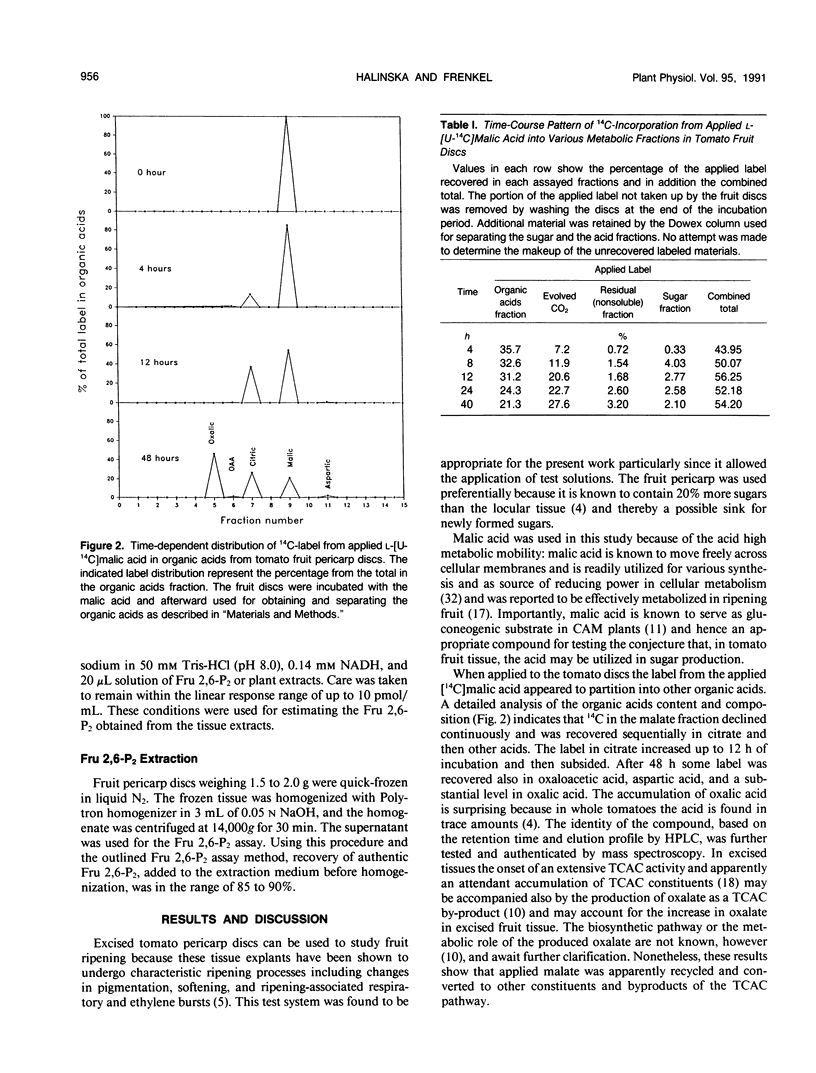
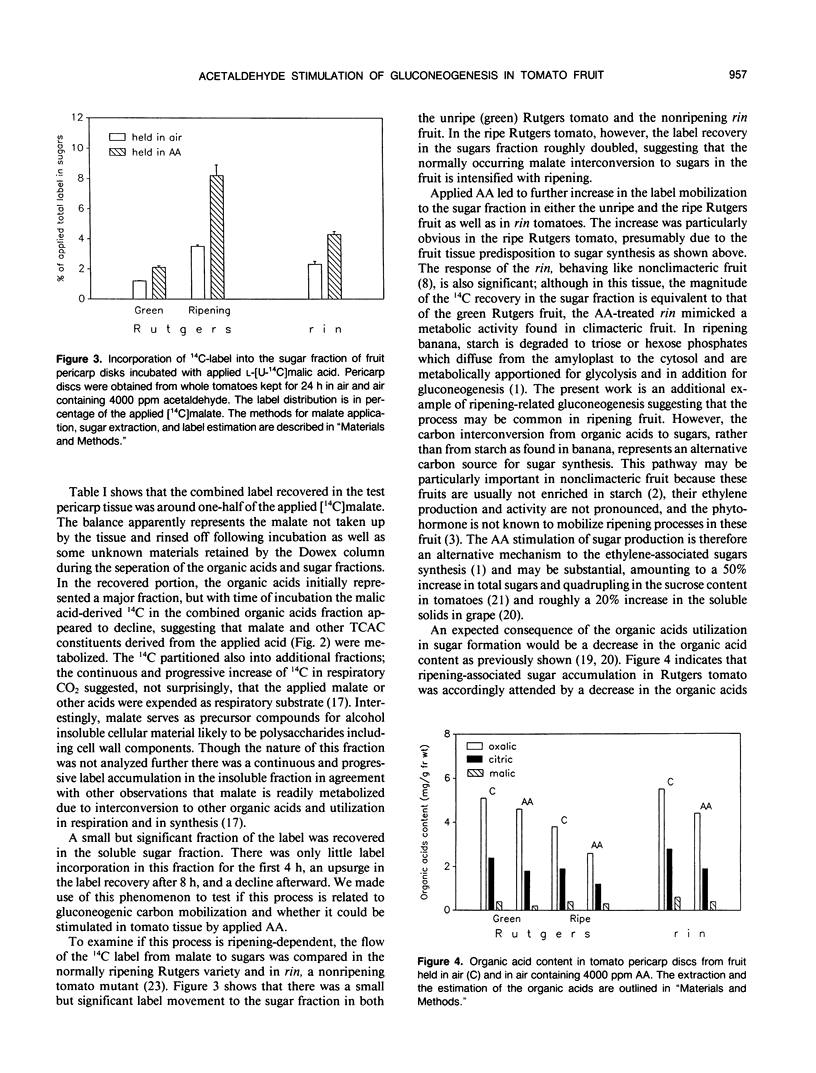
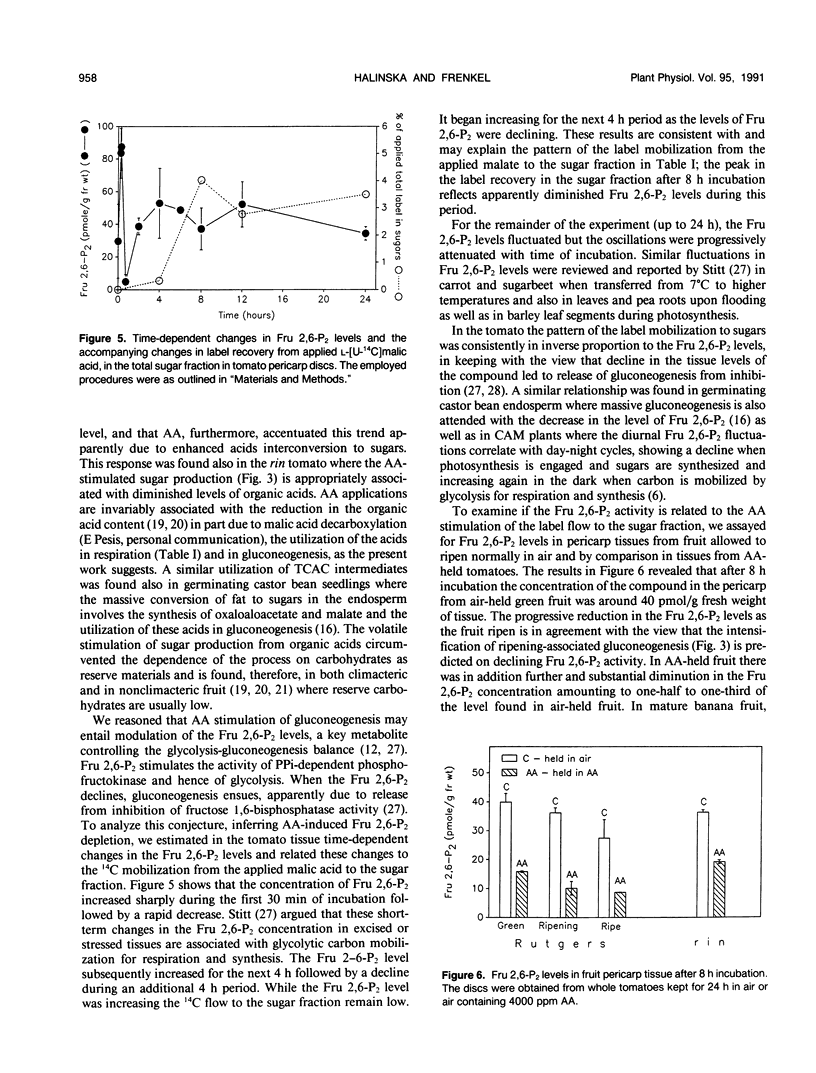
Abstract
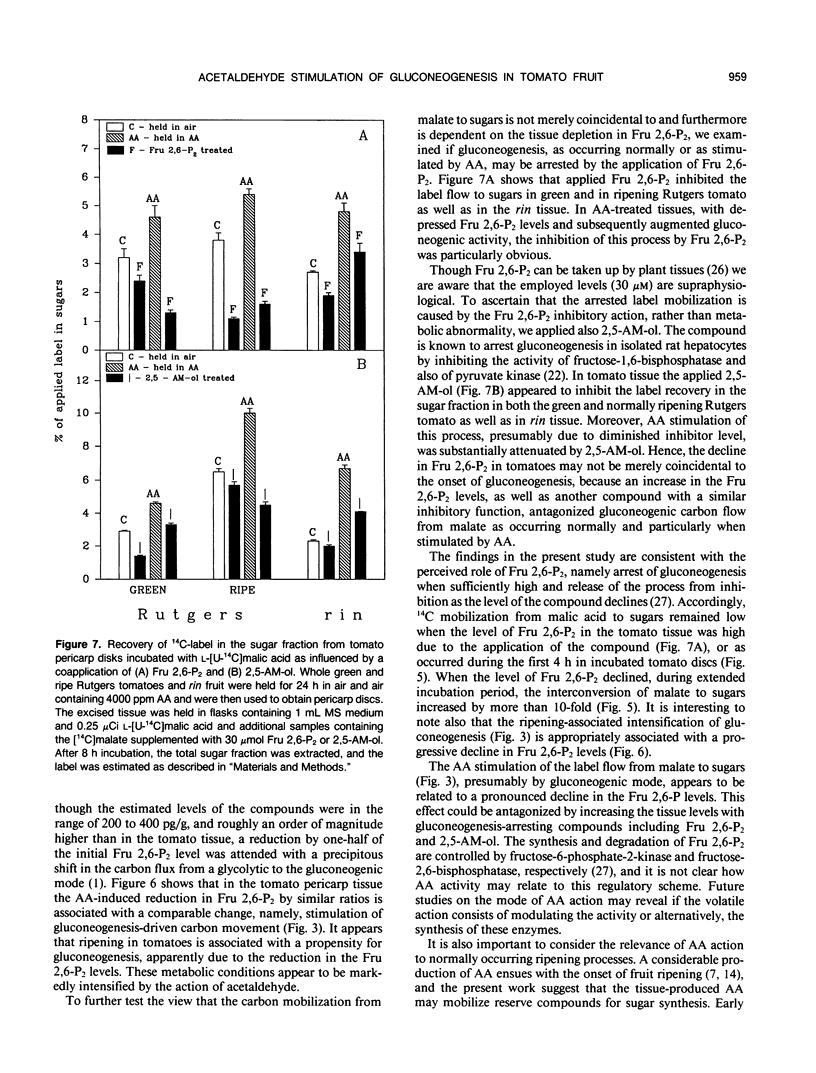
Applied acetaldehyde is known to lead to sugar accumulation in fruit including tomatoes (Lycopersicon esculentum) (O Paz, HW Janes, BA Prevost, C Frenkel [1982] J Food Sci 47: 270-274) presumably due to stimulation of gluconeogenesis. This conjecture was examined using tomato fruit pericarp discs as a test system and applied i-[U-14C]malic acid as the source for gluconeogenic carbon mobilization. The label from malate was recovered in respiratory CO2, in other organic acids, in ethanol insoluble material, and an appreciable amount in the ethanol soluble sugar fraction. In Rutgers tomatoes, the label recovery in the sugar fraction and an attendant label reduction in the organic acids fraction intensified with fruit ripening. In both Rutgers and in the nonripening tomato rin, these processes were markedly stimulated by 4000 ppm acetaldehyde. The onset of label apportioning from malic acids to sugars coincided with decreased levels of fructose-2,6-biphosphate, the gluconeogenesis inhibitor. In acetaldehyde-treated tissues, with enhanced label mobilization, this decline reached one-half to one third of the initial fructose-2,6-biphosphate levels. Application of 30 micromolar fructose-2,6-biphosphate or 2,5-anhydro-d-mannitol in turn led to a precipitous reduction in the label flow to sugars presumably due to inhibition of fructose-1,6-biphosphatase by the compounds. We conclude that malic and perhaps other organic acids are carbon sources for gluconeogenesis occurring normally in ripening tomatoes. The process is stimulated by acetaldehyde apparently by attenuating the fructose-2,6-biphosphate levels. The mode of the acetaldehyde regulation of fructose-2,6-biphosphate metabolism awaits clarification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudry R. M., Severson R. F., Black C. C., Kays S. J. Banana ripening: implications of changes in glycolytic intermediate concentrations, glycolytic and gluconeogenic carbon flux, and fructose 2,6-bisphosphate concentration. Plant Physiol. 1989 Dec;91(4):1436–1444. doi: 10.1104/pp.91.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. N., Hobson G. E. The constituents of tomato fruit--the influence of environment, nutrition, and genotype. Crit Rev Food Sci Nutr. 1981;15(3):205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- Fahrendorf T., Holtum J. A., Mukherjee U., Latzko E. Fructose 2,6-bisphosphate, carbohydrate partitioning, and crassulacean Acid metabolism. Plant Physiol. 1987 May;84(1):182–187. doi: 10.1104/pp.84.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner R. C., Sink K. C. Ethylene Production and Respiratory Behavior of the rin Tomato Mutant. Plant Physiol. 1973 Jul;52(1):38–42. doi: 10.1104/pp.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster K. L., Leopold A. C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988 Nov;88(3):829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N. J., Beevers H. Synthesis and degradation of fructose 2,6-bisphosphate in endosperm of castor bean seedlings. Plant Physiol. 1985 Feb;77(2):358–364. doi: 10.1104/pp.77.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme P. T., Wernette-Hammond M. E., Kneer N. M., Lardy H. A. Regulation of carbohydrate metabolism by 2,5-anhydro-D-mannitol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4301–4305. doi: 10.1073/pnas.80.14.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychter A., Janes H. W., Chin C. K., Frenkel C. Effect of ethanol, acetaldehyde, acetic Acid, and ethylene on changes in respiration and respiratory metabolites in potato tubers. Plant Physiol. 1979 Jul;64(1):108–111. doi: 10.1104/pp.64.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Macdonald F. D., Buchanan B. B. Studies on the entry of fructose-2,6-bisphosphate into chloroplasts. Plant Physiol. 1989 Apr;89(4):1270–1274. doi: 10.1104/pp.89.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : V. Modulation of the Spinach Leaf Cytosolic Fructose 1,6-Bisphosphatase Activity in Vitro by Substrate, Products, pH, Magnesium, Fructose 2,6-Bisphosphate, Adenosine Monophosphate, and Dihydroxyacetone Phosphate. Plant Physiol. 1985 Nov;79(3):590–598. doi: 10.1104/pp.79.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. A micromethod for the purification and quantification of organic acids of the tricarboxylic acid cycle in plant tissues. Anal Biochem. 1979 May;95(1):311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]


