Abstract
Neutrophils have both antimicrobial ability and pathogenic effect in the immune system, neutrophil extracellular traps (NETs) formation is one of the representative behaviors of their dual role. NETs formation was triggered by pathogen-related components and pathogen non-related proteins as cytokines to exert its effector functions. Recent studies indicate that the pathogenicity of NETs contributed to several skin diseases such as psoriasis, Stevens-Johnson syndrome, toxic epidermal necrolysis, and neutrophilic dermatosis. Especially in neutrophilic dermatosis, a heterogeneous group of inflammatory skin disorders characterized with sterile neutrophilic infiltrate on dermis, NETs formation was reported as the way of participation of neutrophils in the pathogenesis of these diseases. In this review, we describe the different processes of NETs formation, then summarized the most recent updates about the pathogenesis of neutrophilic dermatosis and the participation of NETs, including pyoderma gangrenosum and PAPA syndrome, Behçet syndrome, hidradenitis suppurativa, Sweet Syndrome, pustular dermatosis and other neutrophilic dermatosis. Furthermore, we discuss the link between NETs formation and the development of neutrophilic dermatosis.
Subject terms: Mechanisms of disease, Interleukins
Facts
Neutrophil extracellular traps have been reported in some skin diseases.
Aberrant neutrophil activation is crucial in the development of neutrophilic dermatoses.
Neutrophil extracellular traps play a crucial role in the pathogenesis of neutrophilic dermatoses.
Open Questions
How do neutrophils generate neutrophil extracellular traps?
What role does neutrophils play in the pathogenesis of neutrophilic dermatoses?
Are neutrophil extracellular traps considered a pivotal factor in the pathogenesis of neutrophilic dermatoses?
What is the regulatory mechanism underlying the generation of neutrophil extracellular traps in neutrophilic dermatoses?
Introduction
Neutrophils are the main innate immune effectors in human defense system. In physiologic conditions, neutrophils exit in peripheral blood as the first line of antimicrobial system [1]. After septic or aseptic injury, abundant neutrophils released from the bone marrow into the circulation and compromised tissue [2]. Lower neutrophil blood counts caused by impaired maturation of neutrophil granulocytes lead to recurrent and life-threatening infections beginning after birth [3], which indicate that neutrophils play a vital role in human homeostasis. In other ways, however, neutrophils can also initiate and exacerbate life-threatening diseases like Stevens-Johnson syndrome and toxic epidermal necrolysis [4], play the harmful role in human system. As a double-edged sword for immune system, neutrophils have antimicrobial ability and pathogenic effect after inappropriate activation.
Activated neutrophils work through several ways to exert their effector functions, including phagocytosis, degranulation, and neutrophil extracellular traps (NETs) formation [5]. The release of NETs occurs through a cell death process named NETosis [6]. It can be triggered by a variety of agents such as pathogens, cytokines, pathogen-associated molecular pattern molecules, damage-associated molecular patterns molecules, immune complexes [7]. In humans, two heterogenous groups of neutrophils have been reported: typical polymorphonuclear neutrophils (PMNs) and low-density granulocytes (LDGs) found in individuals suffering from autoimmunity [8]. As LDGs reported with higher staining for neutrophil elastase (NE) and lower staining for secretory leukocyte proteinase inhibitor (SLPI, an inhibitor for NE) in contrast to PMNs, LDGs are more prone to occur NETosis [9]. In addition to neutrophils, eosinophils, macrophages, mast cells, and basophils have all reported to release extracellular DNA to form different DNA trap types [10–14].
Upon septic or aseptic conditions, the dysregulation of NETs release can cause damage in multi-system in humans. NETs was reported in the pathophysiology of several conditions including infection, sepsis, cancer, thrombosis and connective tissue diseases [15]. With regard to dermatological diseases, the research on NETosis was involved in psoriasis, pustular dermatosis and neutrophilic dermatosis [16, 17]. Especially in neutrophilic dermatosis, a recent study has revealed the role of neutrophils in autoinflammation and tissue damage in these diseases. Therefore, we sought to update the effect of NETs in the pathogenesis of neutrophilic dermatosis.
Mechanisms of NETs formation
NETs formation is one of the major ways for neutrophils to exert their dual physiological functions described above. Appropriate NETs release is the first-line immune response to assist human body resist the invasion of external stimuli, while dysregulated NETs will lead to a variety of immune diseases [7]. The concept of NETs was first put forward by Takei et al. in 1996 and the systematic research of NETs was reported by Brinkmann et al. in 2004 [18, 19]. In general, the NET formation involves the extrude large amounts of intracellular contents accompanied with cell death and termed this process with NETosis (Fig. 1) [6]. According to the survival status of neutrophils, the process of NETs formation is divided into two types: lytic form of NETosis with the death of neutrophils and non-lytic form of NETosis with survival of neutrophils [19–21]. Although the non-lytic form of NETosis is considered should not be called as NETosis based on its definition [22].
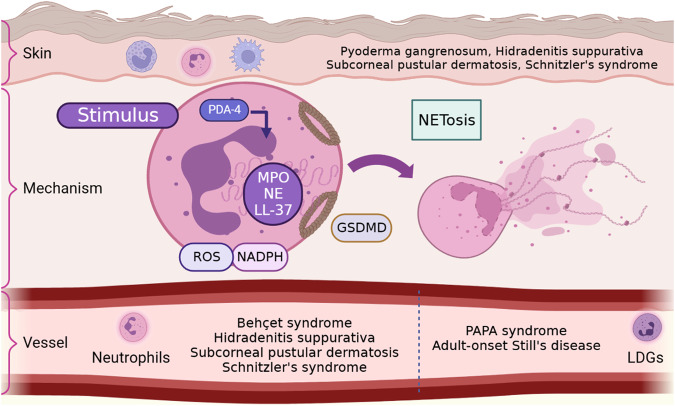
Fig. 1. The formation of neutrophil extracellular traps (Created with Biorender.com).
After being stimulated by external factors, neutrophils activate NADPH to release ROS. The activation of NADPH and ROS leads to the secretion of PAD4, which acts on eosinophilic granules and induces the release of proteins such as NE and MPO. These granular proteins will cause chromatin decondensation, and the secreted PAD4 will lead to histone citrullination. After the neutrophil plasma membrane ruptures, the decondensed chromatin combines with the granular proteins and is released to form a network-like DNA structure extracellularly. GSDMD is involved in the formation of eosinophilic granules and membrane pores. There are two types of neutrophils that can produce NETs, PMNs and LDGs. The production of NETs by neutrophils is involved in the occurrence of various skin diseases, such as pyoderma gangrenosum, PAPA syndrome, Behcet’s syndrome, pyogenic hidradenitis, adult Still’s disease, subcorneal pustular dermatosis, and Schnitzler’s syndrome. NADPH: nicotinamide adenine dinucleotide phosphate, ROS: reactive oxygen species, PAD: peptidylarginine deiminase, NE: neutrophil elastase, MPO: myeloperoxidase, DNA: deoxyribonucleic acid, PMNs: polymorphonuclear neutrophils, LDGs: low-density granulocytes, NETs: neutrophil extracellular traps, GSDMD: gasdermin D.
NETs formation can be initiated by various factors, including microorganisms such as bacteria, fungi and their products like cytokines, chemokines and oxidized mitochondrial DNA [23–26]. In the initial of cell-death-dependent NETosis, neutrophils were stimulated with phorbol 12-myristate 13-acetate (PMA), which activates protein kinase C (PKC) and lead to the generation of reactive oxygen species (ROS) with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [27]. Upon activation of NADPH oxidase and ROS, the secretion of PAD4 is facilitated, acting upon eosinophilic granules, thereby promoting the release of proteins such as NE and MPO. Activated myeloperoxidase (MPO) and NE from azurophilic granules by ROS dislocated into the nucleus, leading to the disconnection of histones with chromatin, resulting in chromatin decondensation [19, 27]. In addition, immune complexes such as nicotine can trigger NETosis independently of NADPH oxidase [28], but still accompanied with cell death, named NADPH oxidase-independent, mitochondrial ROS-dependent NETosis [26]. Besides, NETs formation generated with non-lytic form when exposure to bacteria such as S. aureus [29]. The chromatin material released from live cells using an intact plasma membrane through intracellular vesicles [30].
In addition to the processes mentioned above, calcium channels on the cell membrane also participate in NETs production. Studies have shown that the opening of calcium channels on the cell membrane is necessary for raising intracellular calcium levels and triggering NETosis after neutrophil activation. It has also been demonstrated that reducing the concentration of extracellular free calcium can effectively inhibit NETs production, which further confirms the participation of calcium channels [31–34]. Blocking receptors such as Toll-like receptors (TLR) 2, cluster of differentiation (CD) 18, and G protein-coupled receptors (GPCRs) can also inhibit the generation of NETs in response to corresponding stimuli, indicating the involvement of TLR2, CD18, and GPCRs in NETs production [20, 33, 35]. Additionally, Janus kinase (JAK) 2 is also involved in the process of NETs formation [36, 37].
Gasdermin D (GSDMD) is also involved in NETs formation. Chen et al. found in 2018 that GSDMD can induce NETosis in neutrophils, leading to cell death, which is a novel form of GSDMD-mediated cell death [38]. Subsequently, Silva et al. found that blocking GSDMD can reduce NETs and alleviate symptoms in a septic mouse model [39]. In later studies, Silva et al. also found that GSDMD-mediated NETs formation is involved in tissue damage caused by COVID-19 [40]. The mechanism by which GSDMD mediates NETs formation may be that the pores formed by GSDMD promote the release of NE and MPO from azurophilic granules, while NE can reactivate GSDMD formation in a loop, and GSDMD also participates in the formation of membrane pores on neutrophils, releasing chromatin and bound granule proteins through the pores.
Neutrophilic dermatosis
Pyoderma gangrenosum and pyogenic arthritis, PG and acne (PAPA) syndrome
Pyoderma gangrenosum (PG) is a typical neutrophilic dermatosis characterized by painful skin ulcers with undermined borders and peripheral erythema [41]. The frequency of second immune-related systemic diseases in PG patients was 33% to 56.8% [42, 43], including inflammatory bowel disease, inflammatory arthritis and hematological malignancies. The most recognized inducer of disease is trauma, which causes the dysregulation of inflammatory cytokines and immune responses. The release of cytokines like IL-36 leading to the recruitment of neutrophils, cause the upregulated expression of neutrophil-attracting cytokines like IL-8, finally result in tissue damage [44].
One guess about how neutrophils produce its marked effect is through NETs formation. NETs formation was first reported in the PG skin lesion samples when compared to Schnitzler’s syndrome as a positive control [45]. This manifestation was further confirmed in recent studies [46, 47], one of them concluded 17 PG cutaneous lesions, with 62.9% neutrophils showing NETs, higher than different entities of neutrophilic dermatoses including Sweet’s syndrome and subcorneal pustular dermatosis [46].
PAPA syndrome is one of syndromic PG, which belongs to the autosomal dominant disorder. The study of PAPA syndrome revealed the elevated circulating LDGs and these LDGs displayed enhanced NETs formation and impaired NETs degradation. The research also indicated the effect of IL-1 signaling in exacerbated neutrophil responses in PG patients [48].
Behçet syndrome
Behçet syndrome is a systemic vasculitis that affects both small and large veins and arteries, involving the multi-organs including skin, mucosa, eyes, etc. [49]. The pathophysiology of Behçet syndrome is complicated, with a strong contribution of genetic factors that almost half of patients carry the allele HLA-B*51 [50, 51]. As a result, some researchers preferred Behçet syndrome to the group of MHC-I-opathy disease with T lymphocyte-mediated immune dysfunction [52–54]. Besides, Behçet syndrome is also an autoinflammatory syndromes with neutrophil hyperactivation [16]. It is very important to reveal the mechanism of infiltrated neutrophils in circulation and other organs.
Circulating Neutrophils from patients with Behçet syndrome were more prone to release spontaneous or stimulated NETosis compared healthy control, with higher level of soluble CD40 ligand, peptidylarginine deiminase 4 and interleukin-17 [55–57]. Markers of NETs levels including cell-free DNA and myeloperoxidase-DNA were elevated in Behçet syndrome patients and DNAse treatment significantly decreased thrombin generation in Behçet syndrome plasm, indicated that inhibition of NETs may represent as a potential therapeutic target for Behçet syndrome [58]. Besides, macrophages stimulated with Behçet syndrome-derived NETs produced higher levels of IL-8 and TNF-α compared to heathy controls, and promoted IFN-γ CD4 T cells differentiation [59]. Conversely, morning saliva from patients with Behçet syndrome prone to oral ulcers failed to induce NETosis, it may demonstrate the different disordered homeostasis in the oral cavity [60].
Hidradenitis suppurativa
Hidradenitis suppurativa is an inflammatory disorder that manifested with painful nodules, abscesses and sinus tracts [61]. The disease usually occurs in adulthood and last for a long time, and affect the patients both in physical and mental health because of its severe pain and bad smell [62, 63]. The initial part of disease onset is immune cells activation induced by microbial components and danger-associated molecular patterns (DAMPs). These immune responses lead to the release of cytokines including IL-1β and tumor necrosis factor (TNF) [64], then induce the production of chemokines such as CXCL8, CXCL11, CCL2 and CCL20 in keratinocytes and CXCL1 and CXCL6 in fibroblasts [65, 66]. Dysregulated cytokines and chemokines contribute together to the infiltration of neutrophils, T cells, B cells, monocytes and developed the disease [67].
The prominent presence of NETs was found in the hidradenitis suppurativa lesions and coexisted with plasmacytoid dendritic cells, in association with a type I interferon (IFN) gene signature. Also, the NETs were correlated with disease severity. Circulating neutrophils also manifested enhanced spontaneous NETs formation compared to healthy control neutrophils [68]. Besides, the increased formation of NETs was associated with tunnels according to the immunohistochemistry of hidradenitis suppurativa biopsy specimens [69]. To find the association between microbial components and NETs, the result of the RNA gene amplicon sequencing showed that Finegoldia magna was overabundant in skin samples and derived local inflammation to promote the formation of NETs [70, 71].
Pustular dermatosis
Pustular dermatoses are a group of skin diseases characterized by aseptic pustules of varying sizes, with the pathological feature of massive neutrophil infiltration in the pustules [16]. Pustular skin diseases include generalized pustular psoriasis (GPP), impetigo herpetiformis (IH), acute generalized exanthematous pustulosis (AGEP), and subcorneal pustular dermatosis (SPD) [72–75]. The onset of these diseases is related to neutrophil infiltration, but the specific form of neutrophil activation and regulation mechanism are still unclear.
Research has demonstrated that compared with HC, there is a significant increase in the proportion of neutrophils producing NETs in the skin lesions of patients with SPD. This suggests a potential involvement of NETs in the pathogenesis of SPD [46]. IL-36 and its receptor antagonist (IL-36Ra) are crucial in maintaining physiological homeostasis [76–78]. Loss-of-function mutations in IL36RN, which encodes IL-36Ra, lead to a dominantly inherited autoimmune inflammatory disease termed Deficiency of Interleukin-36 Receptor Antagonist (DITRA) [79]. Using imiquimod cream to construct a psoriasis mouse model in IL-36Ra knockout mice, researchers found that the epidermis significantly proliferates, the number of neutrophils increases, and NETs expression significantly increases, indicating the involvement of IL-36 in NETs formation and the pathogenesis of psoriasis [80]. MPO, similar to IL-36, also plays a crucial role in neutrophil NETs formation and other physiological functions [81, 82]. Haskamp et al. discovered that mutations in MPO genes in 31 GPP patients affected neutrophils and monocytes, which results in reducing the induction of NETs formation in MPO-deficient neutrophils. Furthermore, CD47 expression is increased in MPO-deficient neutrophils. CD47 is an inhibitory protein for monocyte-mediated phagocytosis. The elevated CD47 expression can inhibit the phagocytosis of neutrophils by monocytes, thereby prolonging the survival time of neutrophils [83].
Other neutrophilic dermatoses
Other neutrophilic dermatoses also showed the formation of NETs in skin lesions and circulation, including Schnitzler’s syndrome and adult-onset Still’s disease (AOSD).
Schnitzler’s syndrome is a rare autoinflammatory disorder characterized by neutrophil-dominated inflammation, which is induced by IL-1β [84]. Immunofluorescence co-staining showed widespread and substantial NETs formation in lesion skin of Schnitzler’s syndrome patients compared to the control skin. Blood neutrophils from patients showed significantly elevated NETosis rates compared to control neutrophils following stimulation with PMA [45].
AOSD is a rare systemic autoimmune inflammatory disease with an unknown etiology [85]. The disease is characterized by recurrent high fever, arthritis and joint pain, transient skin rash, leukocytosis, and hyperferritinemia [86–88]. The pathogenesis of AOSD remains uncertain, and multiple factors, such as infection, genetics, and immune dysfunction, may contribute to the onset of the disease. Macrophages and neutrophils, in addition to the cytokines released after their activation, play a crucial role in the pathogenesis of AOSD. Various external stimuli, such as DAMPs, can activate the inflammasome and ultimately cause dysregulated cytokine secretion by increasing the expression levels of IL-1β and IL-18, leading to cytokine storms and disease onset [89–92]. Additionally, the inflammasome pathway also participates in the pathogenesis of AOSD and its complications, macrophage activation syndrome, by mediating cytokine secretion through GSDMD [93]. Studies have found that AOSD patients have significantly increased levels of cell-free DNA, NET-DNA complexes, and α-defensin in circulation, as well as NE-positive and MPO-positive neutrophils in AOSD skin lesions compared to HC. Furthermore, these indicators are positively correlated with disease severity, and AOSD patients have a significantly enhanced ability of neutrophils to spontaneously produce NETs [89, 94, 95]. The number of LDGs and the expression levels of NETs are significantly higher in active AOSD patients, and the serum levels of NETs are positively correlated with the number of joint swelling and monocyte count [96]. Research has also found that type I interferons can trigger NETs enrichment in mitochondrial DNA of AOSD patients, highlighting IFN as a potential target for AOSD treatment [97].
Conclusions
Neutrophilic dermatosis are a heterogeneous group of cutaneous disorders characterized by the histologic finding of a predominantly sterile neutrophilic infiltrate within the various layers of the skin, in the epidermal layer like pustular dermatoses, in the dermal layer like Sweet’s syndrome, in the hypodermal layer like pyoderma gangrenosum and in the overlapping layer like PAPA syndrome [98, 99]. All of which share a common pathological feature of non-infectious neutrophilic infiltration. While the clinical manifestations, laboratory indicators, and mechanisms of onset vary among this group of diseases, activated neutrophils play an important role in the pathogenesis of each. Research has found that the production of NETs by neutrophils may serve as a major form of neutrophil activation in the pathogenesis of NDs. This is demonstrated by the elevated expression levels of NETs in skin lesions and serum of NDs patients, enhanced ability of neutrophils from NDs patients to spontaneously produce NETs, and increased expression levels of upstream regulators of NETs production such as IL-36 and IFN. However, the regulatory mechanism of neutrophil production of NETs in these diseases lacks further investigation and exploration. In light of these findings, it is imperative to deepen our understanding of the mechanisms underlying neutrophil activation in NDs. Such an understanding may pave the way for the development of novel therapies that can effectively target the dysregulated immune response and promote better outcomes for patients affected by this group of disorders.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82173400 to JQ, 82273548 to HF).
Author contributions
All authors approved the version to be published and agreed to be accountable for all aspects of this review. SL and SY: literature retrieval and original draft writing. YW and YL: figure making. JQ and HF: article check and revision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sheng Li, Shuni Ying, Yuqian Wang.
Contributor Information
Jianjun Qiao, Email: qiaojianjun@zju.edu.cn.
Hong Fang, Email: fanghongzy@zju.edu.cn.
References
- 1.Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, et al. Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood. 2016;127:3431–8. doi: 10.1182/blood-2016-03-700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54:1377–91. doi: 10.1016/j.immuni.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Prim. 2017;3:17032. doi: 10.1038/nrdp.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinoshita M, Ogawa Y, Hama N, Ujiie I, Hasegawa A, Nakajima S, et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci Transl Med. 2021;13:eaax2398. [DOI] [PMC free article] [PubMed]
- 5.Lawrence SM, Corriden R, Nizet V. How neutrophils meet their end. Trends Immunol. 2020;41:531–44. doi: 10.1016/j.it.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–47. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 8.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–63. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skrzeczynska-Moncznik J, Zabieglo K, Osiecka O, Morytko A, Brzoza P, Drozdz L, et al. Differences in staining for neutrophil elastase and its controlling inhibitor SLPI reveal heterogeneity among neutrophils in psoriasis. J Invest Dermatol. 2020;140:1371–8.e3. doi: 10.1016/j.jid.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 11.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–80. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 13.Morshed M, Hlushchuk R, Simon D, Walls AF, Obata-Ninomiya K, Karasuyama H, et al. NADPH oxidase-independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314–23. doi: 10.4049/jimmunol.1303418. [DOI] [PubMed] [Google Scholar]
- 14.Daniel C, Leppkes M, Munoz LE, Schley G, Schett G, Herrmann M. Extracellular DNA traps in inflammation, injury and healing. Nat Rev Nephrol. 2019;15:559–75. doi: 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- 15.Wigerblad G, Kaplan MJ. NETs spread ever wider in rheumatic diseases. Nat Rev Rheumatol. 2020;16:73–74. doi: 10.1038/s41584-019-0352-1. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Muto Y, Kinoshita M, Shimada S, Kawamura T. Neutrophil extracellular traps in skin diseases. Biomedicines. 2021;9:1888. [DOI] [PMC free article] [PubMed]
- 17.Shao S, Xue K, Wang G. Neutrophils in neutrophilic dermatoses: emerging roles and promising targeted therapies. J Allergy Clin Immunol. 2022;149:1203–5. [DOI] [PubMed]
- 18.Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59:229–40. doi: 10.1002/jlb.59.2.229. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–93. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017;6:e24437. [DOI] [PMC free article] [PubMed]
- 22.Vorobjeva NV. Neutrophil extracellular traps: new aspects. Mosc Univ Biol Sci Bull. 2020;75:173–88. doi: 10.3103/S0096392520040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, et al. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem. 2004;279:44123–32. doi: 10.1074/jbc.M405883200. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo A, Libby P, Soehnlein O, Aramburu IV, Papayannopoulos V, Silvestre-Roig C. Neutrophil extracellular traps: from physiology to pathology. Cardiovasc Res. 2022;118:2737–53. doi: 10.1093/cvr/cvab329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE. 2012;7:e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–53. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belambri SA, Rolas L, Raad H, Hurtado-Nedelec M, Dang PM, El-Benna J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur J Clin Invest. 2018;48:e12951. doi: 10.1111/eci.12951. [DOI] [PubMed] [Google Scholar]
- 28.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100:1105–12. doi: 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–25. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 30.Desai J, Mulay SR, Nakazawa D, Anders HJ. Matters of life and death. How neutrophils die or survive along NET release and is “NETosis” = necroptosis? Cell Mol Life Sci. 2016;73:2211–9. doi: 10.1007/s00018-016-2195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dixit N, Simon SI. Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest. Front Immunol. 2012;3:188. doi: 10.3389/fimmu.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Immler R, Simon SI, Sperandio M. Calcium signalling and related ion channels in neutrophil recruitment and function. Eur J Clin Invest. 2018;48:e12964. doi: 10.1111/eci.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 2012;92:841–9. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015;67:3190–200. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- 36.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184:460–75.e21. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dou H, Kotini A, Liu W, Fidler T, Endo-Umeda K, Sun X, et al. Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation. 2021;144:1940–54. doi: 10.1161/CIRCULATIONAHA.121.056414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol. 2018;3:eaar6676. [DOI] [PubMed]
- 39.Silva CMS, Wanderley CWS, Veras FP, Sonego F, Nascimento DC, Goncalves AV, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138:2702–13. doi: 10.1182/blood.2021011525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva CMS, Wanderley CWS, Veras FP, Goncalves AV, Lima MHF, Toller-Kawahisa JE, et al. Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology. Crit Care. 2022;26:206. doi: 10.1186/s13054-022-04062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araujo M, Ligeiro D, Costa L, Marques F, Trindade H, Correia JM, et al. A case of fulminant Type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy. 2017;9:531–5. doi: 10.2217/imt-2017-0020. [DOI] [PubMed] [Google Scholar]
- 42.Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132:2166–70. doi: 10.1038/jid.2012.130. [DOI] [PubMed] [Google Scholar]
- 43.Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19:479–87. doi: 10.1007/s40257-018-0356-7. [DOI] [PubMed] [Google Scholar]
- 44.Marzano AV, Fanoni D, Antiga E, Quaglino P, Caproni M, Crosti C, et al. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin Exp Immunol. 2014;178:48–56. doi: 10.1111/cei.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnekoh H, Scheffel J, Wu J, Hoffmann S, Maurer M, Krause K. Skin and systemic inflammation in schnitzler’s syndrome are associated with neutrophil extracellular trap formation. Front Immunol. 2019;10:546. doi: 10.3389/fimmu.2019.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eid E, Safi R, El Hasbani G, Aftimos V, Abbas O, Kibbi AG, et al. Characterizing the presence of neutrophil extracellular traps in neutrophilic dermatoses. Exp Dermatol. 2021;30:988–94. doi: 10.1111/exd.14360. [DOI] [PubMed] [Google Scholar]
- 47.Croia C, Dini V, Loggini B, Manni E, Romanelli M, Migliorini P. Evaluation of neutrophil extracellular trap deregulated formation in pyoderma gangrenosum. Exp Dermatol. 2021;30:1340–4. doi: 10.1111/exd.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mistry P, Carmona-Rivera C, Ombrello AK, Hoffmann P, Seto NL, Jones A, et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann Rheum Dis. 2018;77:1825–33. doi: 10.1136/annrheumdis-2018-213746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yazici Y, Hatemi G, Bodaghi B, Cheon JH, Suzuki N, Ambrose N, et al. Behcet syndrome. Nat Rev Dis Prim. 2021;7:67. doi: 10.1038/s41572-021-00301-1. [DOI] [PubMed] [Google Scholar]
- 50.Yazici H, Seyahi E, Hatemi G, Yazici Y. Behcet syndrome: a contemporary view. Nat Rev Rheumatol. 2018;14:107–19. doi: 10.1038/nrrheum.2017.208. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz-Fernandez L, Sawalha AH. Genetics of Behcet’s disease: functional genetic analysis and estimating disease heritability. Front Med (Lausanne) 2021;8:625710. doi: 10.3389/fmed.2021.625710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behcet’s disease: New insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17:567–75. doi: 10.1016/j.autrev.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Giza M, Koftori D, Chen L, Bowness P. Is Behcet’s disease a ‘class 1-opathy’? The role of HLA-B*51 in the pathogenesis of Behcet’s disease. Clin Exp Immunol. 2018;191:11–18. doi: 10.1111/cei.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu J, Kaneko F, Suzuki N. Skewed helper T-cell responses to IL-12 family cytokines produced by antigen-presenting cells and the genetic background in behcet’s disease. Genet Res Int. 2013;2013:363859. doi: 10.1155/2013/363859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perazzio SF, Soeiro-Pereira PV, Dos Santos VC, de Brito MV, Salu B, Oliva MLV, et al. Soluble CD40L is associated with increased oxidative burst and neutrophil extracellular trap release in Behcet’s disease. Arthritis Res Ther. 2017;19:235. doi: 10.1186/s13075-017-1443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safi R, Kallas R, Bardawil T, Mehanna CJ, Abbas O, Hamam R, et al. Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet’s disease. J Dermatol Sci. 2018;92:143–50. doi: 10.1016/j.jdermsci.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Vural S, Kerl K, Ertop Dogan P, Vollmer S, Puchta U, He M, et al. Lesional activation of Tc 17 cells in Behcet disease and psoriasis supports HLA class I-mediated autoimmune responses. Br J Dermatol. 2021;185:1209–20. doi: 10.1111/bjd.20643. [DOI] [PubMed] [Google Scholar]
- 58.Le Joncour A, Martos R, Loyau S, Lelay N, Dossier A, Cazes A, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2019;78:1274–82. doi: 10.1136/annrheumdis-2018-214335. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Yu X, Liu J, Wang Z, Li C, Shi J, et al. Neutrophil extracellular traps promote aberrant macrophages activation in Behcet’s disease. Front Immunol. 2020;11:590622. doi: 10.3389/fimmu.2020.590622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohanty T, Sjogren J, Kahn F, Abu-Humaidan AH, Fisker N, Assing K, et al. A novel mechanism for NETosis provides antimicrobial defense at the oral mucosa. Blood. 2015;126:2128–37. doi: 10.1182/blood-2015-04-641142. [DOI] [PubMed] [Google Scholar]
- 61.Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Prim. 2020;6:18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 62.Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917–24. doi: 10.1111/bjd.16101. [DOI] [PubMed] [Google Scholar]
- 63.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol. 2010;62:706–8, 708.e1. doi: 10.1016/j.jaad.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 64.van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292–8. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 65.Witte-Handel E, Wolk K, Tsaousi A, Irmer ML, Mossner R, Shomroni O, et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139:1294–305. doi: 10.1016/j.jid.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 66.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87:523–36. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 67.List EK, Pascual JC, Zarchi K, Nurnberg BM, Jemec GBE. Mast cells in hidradenitis suppurativa: a clinicopathological study. Arch Dermatol Res. 2019;311:331–5. doi: 10.1007/s00403-019-01910-3. [DOI] [PubMed] [Google Scholar]
- 68.Byrd AS, Carmona-Rivera C, O’Neil LJ, Carlucci PM, Cisar C, Rosenberg AZ, et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 2019;11:eaav5908. [DOI] [PMC free article] [PubMed]
- 69.Navrazhina K, Frew JW, Gilleaudeau P, Sullivan-Whalen M, Garcet S, Krueger JG. Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa. J Allergy Clin Immunol. 2021;147:2213–24. doi: 10.1016/j.jaci.2020.12.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarthy S, Barrett M, Kirthi S, Pellanda P, Vlckova K, Tobin AM, et al. Altered skin and gut microbiome in hidradenitis suppurativa. J Invest Dermatol. 2022;142:459–68.e15. doi: 10.1016/j.jid.2021.05.036. [DOI] [PubMed] [Google Scholar]
- 71.Jiang SW, Whitley MJ, Mariottoni P, Jaleel T, MacLeod AS. Hidradenitis suppurativa: host-microbe and immune pathogenesis underlie important future directions. JID Innov. 2021;1:100001. doi: 10.1016/j.xjidi.2021.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watts PJ, Khachemoune A. Subcorneal pustular dermatosis: a review of 30 years of progress. Am J Clin Dermatol. 2016;17:653–71. doi: 10.1007/s40257-016-0202-8. [DOI] [PubMed] [Google Scholar]
- 73.Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843–8. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 74.Posso-De Los Rios CJ, Pope E. New insights into pustular dermatoses in pediatric patients. J Am Acad Dermatol. 2014;70:767–73. doi: 10.1016/j.jaad.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Marrakchi S, Puig L. Pathophysiology of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23:13–19. doi: 10.1007/s40257-021-00655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15:612–32. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 77.Elias M, Zhao S, Le HT, Wang J, Neurath MF, Neufert C, et al. IL-36 in chronic inflammation and fibrosis—bridging the gap? J Clin Invest. 2021;131:e144336. [DOI] [PMC free article] [PubMed]
- 78.Bassoy EY, Towne JE, Gabay C. Regulation and function of interleukin-36 cytokines. Immunol Rev. 2018;281:169–78. doi: 10.1111/imr.12610. [DOI] [PubMed] [Google Scholar]
- 79.Salik D, Zoghaib S, Dangoisse C, Sass U, Kolivras A, Soblet J, et al. New variant in deficiency of interleukin-36 receptor antagonist syndrome (DITRA) Int J Dermatol. 2021;60:899–900. doi: 10.1111/ijd.15522. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe S, Iwata Y, Fukushima H, Saito K, Tanaka Y, Hasegawa Y, et al. Neutrophil extracellular traps are induced in a psoriasis model of interleukin-36 receptor antagonist-deficient mice. Sci Rep. 2020;10:20149. doi: 10.1038/s41598-020-76864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizo-Tellez SA, Sekheri M, Filep JG. Myeloperoxidase: regulation of neutrophil function and target for therapy. Antioxidants (Basel). 2022;11:2302. [DOI] [PMC free article] [PubMed]
- 82.Dos Santos Ramos A, Viana GCS, de Macedo Brigido M, Almeida JF. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets. Pharm Res. 2021;171:105779. doi: 10.1016/j.phrs.2021.105779. [DOI] [PubMed] [Google Scholar]
- 83.Haskamp S, Bruns H, Hahn M, Hoffmann M, Gregor A, Lohr S, et al. Myeloperoxidase Modulates Inflammation in Generalized Pustular Psoriasis and Additional Rare Pustular Skin Diseases. Am J Hum Genet. 2020;107:527–38. doi: 10.1016/j.ajhg.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon A, Asli B, Braun-Falco M, De Koning H, Fermand JP, Grattan C, et al. Schnitzler’s syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68:562–8. doi: 10.1111/all.12129. [DOI] [PubMed] [Google Scholar]
- 85.Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Seve P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13:708–22. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 86.Li S, Zheng S, Tang S, Pan Y, Zhang S, Fang H, et al. Autoinflammatory pathogenesis and targeted therapy for adult-onset Still’s disease. Clin Rev Allergy Immunol. 2020;58:71–81. doi: 10.1007/s12016-019-08747-8. [DOI] [PubMed] [Google Scholar]
- 87.Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 2018;14:603–18. doi: 10.1038/s41584-018-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Y, Meng J, Jia J, Wang M, Teng J, Zhu D, et al. Current and emerging biological therapy in adult-onset Still’s disease. Rheumatol (Oxf) 2021;60:3986–4000. doi: 10.1093/rheumatology/keab485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Q, Shi H, Zeng T, Liu H, Su Y, Cheng X, et al. Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still’s disease. Arthritis Res Ther. 2019;21:9. doi: 10.1186/s13075-018-1800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hsieh CW, Chen YM, Lin CC, Tang KT, Chen HH, Hung WT, et al. Elevated expression of the NLRP3 inflammasome and its correlation with disease activity in adult-onset still disease. J Rheumatol. 2017;44:1142–50. doi: 10.3899/jrheum.161354. [DOI] [PubMed] [Google Scholar]
- 91.Mitrovic S, Fautrel B. New markers for adult-onset Still’s disease. Jt Bone Spine. 2018;85:285–93. doi: 10.1016/j.jbspin.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 92.Rau M, Schiller M, Krienke S, Heyder P, Lorenz H, Blank N. Clinical manifestations but not cytokine profiles differentiate adult-onset Still’s disease and sepsis. J Rheumatol. 2010;37:2369–76. doi: 10.3899/jrheum.100247. [DOI] [PubMed] [Google Scholar]
- 93.Tang S, Yang C, Li S, Ding Y, Zhu D, Ying S, et al. Genetic and pharmacological targeting of GSDMD ameliorates systemic inflammation in macrophage activation syndrome. J Autoimmun. 2022;133:102929. doi: 10.1016/j.jaut.2022.102929. [DOI] [PubMed] [Google Scholar]
- 94.Ahn MH, Han JH, Chwae YJ, Jung JY, Suh CH, Kwon JE, et al. Neutrophil Extracellular Traps May Contribute to the Pathogenesis in Adult-onset Still Disease. J Rheumatol. 2019;46:1560–9. doi: 10.3899/jrheum.181058. [DOI] [PubMed] [Google Scholar]
- 95.Jia J, Wang M, Ma Y, Teng J, Shi H, Liu H, et al. Circulating neutrophil extracellular traps signature for identifying organ involvement and response to glucocorticoid in adult-onset still’s disease: a machine learning study. Front Immunol. 2020;11:563335. doi: 10.3389/fimmu.2020.563335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres-Ruiz J, Carrillo-Vazquez DA, Tapia-Rodriguez M, Garcia-Galicia JA, Alcocer-Varela J, Gomez-Martin D. The role of low density granulocytes and NETosis in the pathogenesis of adult-onset Still’s Disease. Clin Exp Rheumatol. 2019;37:74–82. [PubMed] [Google Scholar]
- 97.Ma Y, Wang M, Jia J, Meng J, Teng J, Zhu D, et al. Enhanced type I interferon signature induces neutrophil extracellular traps enriched in mitochondrial DNA in adult-onset Still’s disease. J Autoimmun. 2022;127:102793. doi: 10.1016/j.jaut.2022.102793. [DOI] [PubMed] [Google Scholar]
- 98.Shao S, Xue K, Wang G. Neutrophils in neutrophilic dermatoses: emerging roles and promising targeted therapies. J Allergy Clin Immunol. 2022;149:1203–5. doi: 10.1016/j.jaci.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 99.Marzano AV, Borghi A, Wallach D, Cugno M. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol. 2018;54:114–30. doi: 10.1007/s12016-017-8621-8. [DOI] [PubMed] [Google Scholar]