Abstract
In Klebsiella pneumoniae, NifA-dependent transcription of nitrogen fixation (nif) genes is inhibited by a flavoprotein, NifL, in the presence of molecular oxygen and/or combined nitrogen. We recently demonstrated that the general nitrogen regulator NtrC is required to relieve NifL inhibition under nitrogen (N)-limiting conditions. We provide evidence that the sole basis for the NtrC requirement is its role as an activator of transcription for glnK, which encodes a PII-like allosteric effector. Relief of NifL inhibition is a unique physiological function for GlnK in that the structurally related GlnB protein of enteric bacteria—apparently a paralogue of GlnK—cannot substitute. Unexpectedly, although covalent modification of GlnK by uridylylation normally occurs under N-limiting conditions, several lines of evidence indicate that uridylylation is not required for relief of NifL inhibition. When GlnK was synthesized constitutively from non-NtrC-dependent promoters, it was able to relieve NifL inhibition in the absence of uridylyltransferase, the product of the glnD gene, and under N excess conditions. Moreover, an altered form of GlnK, GlnKY51N, which cannot be uridylylated due to the absence of the requisite tyrosine, was still able to relieve NifL inhibition.
In proteobacteria, the NifA protein activates the transcription of genes whose products are required for biological nitrogen fixation (nif genes; reviewed in references 18, 25, and 39). Both the expression and the activity of NifA can be regulated in response to the cellular oxygen (O2) and/or nitrogen (N) status, but the mechanisms for regulation differ with the organism. (O2 is a signal because it destroys the function of metal cofactors essential for nitrogen fixation.) In members of the γ subdivision of the proteobacteria, e.g., Klebsiella pneumoniae and Azotobacter vinelandii, NifA activity is inhibited by a second regulatory protein, NifL, in the presence of O2 and/or combined N (32, 40). Immunological studies imply that NifL inhibits NifA activity by means of a stoichiometric interaction with NifA (30). Prerequisite to such an interaction, NifL and NifA are synthesized from the chromosome in comparable amounts because their synthesis is translationally coupled (26).
The NifL protein of A. vinelandii is known to be a flavoprotein with flavin adenine dinucleotide (FAD) as a prosthetic group (31), and the same is true for the NifL protein of K. pneumoniae (47). In vitro, reduction of the FAD cofactor of NifL relieves NifL inhibition of NifA activity in a purified transcription system (31). Hence, in vitro NifL appears to act as a redox switch that allows NifA activity under O2-limiting conditions. In vivo, however, NifL inhibition can be relieved only under both O2- and N-limiting conditions (32, 40). This leads to the hypothesis that perhaps the FAD cofactor of NifL can be reduced in vivo only when both of the above limitations pertain. Because environmental iron (Fe) is required for relief of NifL inhibition but is not present in NifL itself, we have postulated that an unidentified Fe-containing protein may be the physiological reductant for NifL (47, 48). In addition, we have demonstrated that transcriptional activation by the general nitrogen regulatory protein NtrC is required for relief of NifL inhibition (29) and that regulation of the K. pneumoniae NifL protein in response to cellular nitrogen or oxygen availability can be studied in the related enteric bacterium Escherichia coli (29).
NtrC is required for transcription of the glnK gene (51, 52), which encodes a PII-like protein allosteric effector that is known to be involved in the regulation of nitrogen metabolism (5, 52). Therefore, we tested the hypothesis that GlnK is directly required to relieve NifL inhibition. We present evidence that GlnK is the only protein under NtrC control that is needed for relief of NifL inhibition, that the related GlnB protein cannot substitute and hence is a paralogue of GlnK, and that covalent modification of GlnK by uridylylation is not required for relief of NifL inhibition. The latter finding was surprising because GlnK is normally highly uridylylated under N-limiting conditions.
MATERIALS AND METHODS
Strains and strain constructions.
The bacterial strains and plasmids used in this work are listed in Table 1. Plasmid transformation was done as described previously (29). Ampicillin, spectinomycin, chloramphenicol, kanamycin, and tetracycline were generally used at 100, 50, 25, 25, and 10 μg/ml, respectively. For selection of pZC320 (a mini-F-based plasmid), the concentration of antibiotics was reduced to half of the normal value.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or characteristic(s) | Reference or description |
|---|---|---|
| Strains | ||
| NCM1529 | araD139 Δ(argF-lacU)169 flhD5301 gyrA219 non-9 rpsL150 ptsF25 relA1 deoC1 trpDC700:: putPA1303::[Kanr-Φ(nifH′-′lacZ)] (wild type) | 29 |
| NCM1528a | NCM1529/pNH3 | 29 |
| NCM1527 | NCM1529/pJES851 | 29 |
| NCM1686 | glnD99::Tn10 [Kanr-Φ(nifH′-′lacZ)] | 29 |
| NCM1687b | NCM1686/pNH3 | 29 |
| NCM1851c | ntrC10::Tn5 [Kanr-Φ(nifH′-′lacZ)]/pNH3 | 29 |
| NCM1971 | glnK::Spcr [Kanr-Φ(nifH′-′lacZ)] | See Materials and Methods |
| NCM1974d | NCM1971/pNH3 | See Materials and Methods |
| NCM1975 | NCM1971/pJES851 | See Materials and Methods |
| NCM1799 | [araD139] Δ(argF-lacU)205 flhD5301 fruA25 rpsL150 (strR) rha-10 deoC1 Δ[(ntrC?-ntrB-glnA)229] trpDC700::putPA1303::[Kanr-Φ(nifH′-′lacZ)]/pNH3 | 29 |
| Plasmids | ||
| p149B6 | 6-kb BamHI fragment of E. coli mdl-glnK-amtB-tesB′ region in pBluescriptII KS | 2 |
| pDK601 | E. coli ′yfhA-glnB region in pUC18 | 53 |
| pNH3 | K. pneumoniae nifLA controlled by tac promoter | 30 |
| pJES851 | K. pneumoniae nifA controlled by tac promoter | 48 |
| pUT-Sm/Spc | Spectinomycin resistance cassette (Ω-Spc) | 15 |
| pCVD442 | Suicide vector | 21 |
| pZC320 | Mini-F vector | 49 |
| pJES1006 | ′mdl-glnK-amtB′ in pUC19 | See Materials and Methods |
| pJES1007 | ′mdl-glnK::Spcr-amtB′ in pUC19 | See Materials and Methods |
| pJES1073 | ′mdl-glnK::Spcr-amtB′ in pCVD442 | See Materials and Methods |
| pJES1086 | glnK at EcoRI site of pACYC184 (Pcat-glnK+) | See Materials and Methods |
| pJES1104 | glnK at SalI site of pACYC184 (Ptet-glnK+) | See Materials and Methods |
| pJES1106 | glnK5 at SalI site of pACYC184 [Ptet-glnK5 (GlnKY51N)] | See Materials and Methods |
| pJES1111 | glnB in pACYC184 | See Materials and Methods |
| pJES1118 | ′mdl-glnK-cat in pUC19 (PglnK-glnK+) | See Materials and Methods |
| pJES1119 | ′mdl-glnK5-cat in pUC19 [PglnK-glnK5 (GlnKY51N)] | See Materials and Methods |
| pJES1160 | glnB in pZC320 | See Materials and Methods |
| pJES1161 | ′mdl-glnK-cat in pZC320 (PglnK-glnK+) | See Materials and Methods |
| pJES1162 | ′mdl-glnK5-cat in pZC320 [Ptet-glnK5 (GlnKY51N)] | See Materials and Methods |
| pJES1163 | cat in pZC320 | See Materials and Methods |
Strains NCM2086 and NCM2089 carry plasmids pJES1104 and pJES1106, respectively, in NCM1528.
Strains NCM2093, NCM2094, NCM2080, and NCM2082 carry plasmids pJES1104, pJES1106, pJES1161, and pJES1162, respectively, in NCM1687.
Strains NCM2088 and NCM2091 carry plasmids pJES1104 and pJES1106, respectively, in NCM1851.
Strains NCM1982, NCM2087, NCM2090, NCM2048, NCM2068, NCM2069, NCM2081, NCM2070, and NCM1990 carry plasmids pJES1086, pJES1104, pJES1106, pJES1111, pJES1160, pJES1161, pJES1162, pJES1163, and pACYC184, respectively, in NCM1974.
Construction of a glnK::Spcr null mutation.
Strain NCM1971 was obtained by insertion of a spectinomycin resistance cassette (Ω-Spc) into the glnK gene of strain NCM1529 (29), as achieved in the following steps. (i) A 1.3-kb NdeI-EcoNI fragment from plasmid p149B6 (2) (also known as [aka] pJES999), which carries the mdl-glnK-amtB-tesB′ region of E. coli, was subcloned into the SmaI site of pUC19 to yield pJES1006. (ii) A 2-kb SmaI fragment carrying the spectinomycin resistance cassette from pUT-Sm/Spc (15) (aka pJES857) was inserted into pJES1006, which had been cleaved with BstEII and made blunt ended by the Klenow fragment of DNA polymerase I, to yield pJES1007. (iii) A 3.3-kb SacI-SalI fragment carrying glnK::Spcr was ligated into the suicide vector pCVD442 (21) (aka pJES1034), which had been digested with SacI and SalI, and the resulting plasmid, pJES1073, which contains the sacB gene of Bacillus subtilis, was then transferred by electroporation into E. coli SM10λpir. (iv) Plasmid pJES1073 was transformed into NCM1529, and ampicillin-resistant transformants were chosen for further analysis. Because pJES1073 cannot replicate in NCM1529 (there is no π protein available), ampicillin-resistant transformants should be the ones in which pJES1073 has been integrated into the chromosome. (v) After overnight growth in LB medium (0.5% NaCl, 1% tryptone, 0.5% yeast extract) without ampicillin, transformants were plated on 7% sucrose–LB medium and grown at 30°C. Among the sucrose-resistant colonies should be those that have achieved allelic exchange at glnK (Aps glnK::Spcr). The presence of the glnK::Spcr insertion mutation was confirmed by Southern blot analysis (data not shown).
Construction of plasmids.
To construct a plasmid carrying Pcat-glnK+, the small EcoRI-BsgI (blunt ended by the Klenow fragment) fragment of pJES1006, which carries the intact glnK region (including the ribosomal binding site for glnK) and the first 50 bp of amtB, was ligated to the large EcoRI-ScalI fragment of pACYC184 to yield plasmid pJES1086.
Because pJES1086 encodes tetracycline resistance as its only drug resistance, it cannot be selectively maintained in glnD99::Tn10 mutant strains. Therefore, we constructed plasmid pJES1104, in which the 0.4-kb XhoI-SalI glnK fragment from pKOP2 (3), which carries the intact glnK coding region from the first codon (ATG) and a strong ribosomal binding site of atpE (46), was inserted into the SalI site of pACYC184 and thereby expressed from the tet promoter. Plasmid pJES1104 confers chloramphenicol resistance. The orientation of the insertion was checked by digestion with appropriate restriction enzymes. Plasmid pJES1106 was constructed similarly to pJES1104, except that it carries glnK5 (GlnKY51N) (5) from pKOPY51N (3).
To express glnK and the glnK5 allele from the native glnK promoter in a very low-copy-number vector, we made use of the vector pZC320 (aka pJES1120), which is a mini-F vector (49). To do so, we first inserted a chloramphenicol resistance gene into pJES1006 (PglnK-glnK+) by replacing the small BstEII-BsgI (blunt ended by the Klenow fragment) fragment of pJES1006 with the large BstEII-BstZ17I fragment of pJES1104 (or pJES1106) to yield pJES1118 (or pJES1119); the large Ecl136II-HindIII fragment of pJES1118 (or pJES1119) was then ligated to the large ScalI-HindIII fragment of pZC320 to yield pJES1161 (or pJES1162). Thus, plasmid pJES1161 (or pJES1162) carries the 3′ portion of the mdl gene and the glnK promoter and coding regions (or PglnK-glnK5 [GlnKY51N]). To construct a control plasmid, pJES1163, the EcoRV-HindIII fragment of pJES1118, which carries only the chloramphenicol resistance gene, was inserted into the large ScalI-HindIII fragment of pZC320.
To construct PglnB-glnB+, a 1.5-kb SalI-BglII fragment of pDK601 (53) was ligated into the SalI and BamHI sites of pACYC184 to yield plasmid pJES1111, which carries the 3′ part of yfhA and the intact glnB gene of E. coli. Plasmid pJES1160 carries PglnB-glnB+ in the pZC320 vector; it was constructed by ligating the large HindIII-BstZ17I fragment of pJES1111 to the HindIII and PmlI sites of pZC320.
Growth conditions and β-galactosidase assay.
We recently constructed E. coli strains that carry a single copy of a K. pneumoniae Φ(nifH′-′lacZ) (hereafter designated simply nifH′-′lacZ) translational fusion at the trp locus and a plasmid that carries either Ptac-nifLA (pNH3) or Ptac-nifA (pJES851) of K. pneumoniae (29). NifA-mediated expression from the nifH promoter was monitored by measuring the differential rate of β-galactosidase synthesis during exponential growth. Inhibitory effects of NifL on NifA were assessed by virtue of a decrease in nifH expression. Transcription of nifA or nifLA was induced from the tac promoter with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG) unless specified otherwise. Cells were grown in modified K medium at 30°C as described previously (29). Samples of the growing cultures were taken every 2 to 3 h to determine the optical density at 600 nm (OD600) and β-galactosidase activity [units/milliliter = 1,000 (OD420 − 1.75 × OD550)/(Δt × v)] (41). The differential rates (35) of β-galactosidase synthesis reported here were obtained by determining the slopes of plots of β-galactosidase activity versus the OD of the culture (units/milliliter/OD600).
Western blotting.
Western blotting was performed as reported previously (29). At an OD600 of 0.8 to 1.0, cells were harvested from 1 ml of culture and concentrated 20-fold into sodium dodecyl sulfate (SDS) gel-loading buffer (44). (The differential rate of β-galactosidase synthesis was also measured in these cultures.) The NifL and NifA proteins were separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) using a Tris-glycine system (44) and were detected with rabbit polyclonal antisera against the proteins from K. pneumoniae. To compare the amounts of these proteins in different strains or under different conditions of induction, the sample that gave the higher intensity of staining was diluted serially by a factor of 2 until its staining intensity was less than that of the other. The GlnK protein was separated by SDS–16.5% PAGE in a Tris-Tricine system (45) or by SDS–20% PAGE in a 2× Tris-glycine system (12). It was detected with either rabbit polyclonal antiserum against the E. coli GlnK protein (42) or the E. coli GlnB protein (see figure legends). Purified NifL, NifA, and GlnK and prestained protein molecular size markers (Broad Range; New England Biolabs) were used as standards.
RESULTS
GlnK is required to relieve NifL inhibition of NifA activity.
To determine whether GlnK is required for relief of NifL inhibition, we constructed a glnK null mutation in E. coli by inserting a spectinomycin cassette into the very beginning of the glnK gene (glnK-amtB operon) and measured the differential rate of expression of a nifH′-′lacZ fusion in the resulting strain (see Materials and Methods). In agreement with our previous results, the inhibition of NifA activity by NifL was relieved only under anaerobic and N-limiting (derepressing) conditions (29) (Tables 2 and 3, parent strain NCM1528). However, in the glnK null mutant (NCM1974), NifL inhibition apparently persisted under derepressing conditions: expression from the nifH promoter was more than 100-fold lower than in the parent strain (NCM1528). In the absence of NifL, NifA was able to activate transcription from the nifH promoter in the glnK mutant strain (NCM1975), indicating that effects of GlnK were indeed transduced through NifL.
TABLE 2.
Effects of a glnK null allele on activity of the K. pneumoniae NifL protein
| Straina | Relevant genotype | Expression of nifH′-′lacZb (U/ml/OD600)
|
|
|---|---|---|---|
| With IPTGc | Without IPTG | ||
| NCM1528 | Wild type/Ptac-nifLA | 5,780 | 2,060 |
| NCM1974 | glnK/Ptac-nifLA | <10 | <10 |
| NCM1975 | glnK/Ptac-nifA | 6,310 | NDd |
| NCM1990 | glnK/Ptac-nifLA/pACYC184 | <10 | ND |
| NCM1982 | glnK/Ptac-nifLA Pcat-glnK+ | ND | 1,180 |
| NCM2069 | glnK/Ptac-nifLA PglnK-glnK+ | 5,160 | 1,950 |
All strains carry a single chromosomal copy of a nifH′-′lacZ fusion at the trp locus.
Specific activity is the slope of a plot of β-galactosidase activity versus the OD600 of the culture. Cultures were grown in K medium under anaerobic conditions with 2 mM glutamine as the sole nitrogen source.
10 μM.
ND, not determined.
TABLE 3.
Effects of GlnK and GlnKY51N on NifL activity in different backgrounds
| Straina | Relevant genotype | Expression of nifH′-′lacZb (U/ml/OD600)
|
|
|---|---|---|---|
| −N | +N | ||
| NCM1528 | Wild type | 3,580 | <10 |
| NCM1974 | glnK | <10 | <10 |
| NCM1851 | ntrC | <10 | <10 |
| NCM1687 | glnD | 20 | <10 |
| NCM2086 | Wild type/Ptet-glnK+ | 3,090 | 1,060 |
| NCM2087 | glnK/Ptet-glnK+ | 3,860 | 1,240 |
| NCM2088 | ntrC/Ptet-glnK+ | 3,510 | 1,850 |
| NCM2093 | glnD/Ptet-glnK+ | 3,940 | 1,340 |
| NCM2089 | Wild type/Ptet-glnK5 (GlnKY51N) | 1,450 | 1,330 |
| NCM2090 | glnK/Ptet-glnK5 (GlnKY51N) | 2,200 | 910 |
| NCM2091 | ntrC/Ptet-glnK5 (GlnKY51N) | 2,990 | 1,450 |
| NCM2094 | glnD/Ptet-glnK5 (GlnKY51N) | 3,390 | 2,670 |
All strains carry a single chromosomal copy of a nifH′-′lacZ fusion at the trp locus and plasmid pNH3 (Ptac-nifLA).
See footnote b to Table 2. Cultures were grown in K medium under anaerobic conditions with 2 mM glutamine (−N) or with 2 mM glutamine plus 4 mM NH4Cl (+N).
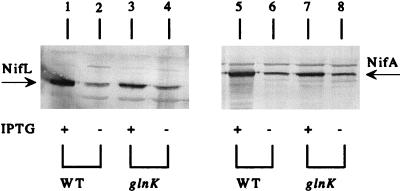
To demonstrate that failure of the glnK mutant strain to express nifH under derepressing conditions could not be accounted for by a decrease in the amount of NifA, we compared the amounts of the NifL and NifA proteins in the glnK mutant strain to those in a congenic wild-type strain by immunological means (see Materials and Methods). Under derepressing conditions and at our usual level of induction with 10 μM IPTG, the glnK mutant strain had about one-fourth as much NifL and NifA as the wild-type strain (Fig. 1, compare lanes 3 and 7 to lanes 1 and 5 and the results obtained with diluted samples [see Materials and Methods; data not shown]). However, it is unlikely that this 4-fold decrease in the amounts of the NifL and NifA proteins resulted in a greater-than-100-fold decrease in the level of nifH expression. When the amounts of NifL and NifA in the glnK mutant strain were adjusted to be greater than or equal to those in the wild-type strain by adding IPTG to the culture of the glnK mutant strain but not the wild-type strain (Fig. 1, lanes 3 and 7 versus lanes 2 and 6) or omitting IPTG in both cases (Fig. 1, lanes 4 and 8 versus lanes 2 and 6), the level of nifH expression in the glnK mutant strain remained >100-fold lower than that in the wild-type strain (Table 2, compare NCM1974 with or without IPTG induction to NCM1528 without IPTG induction). These results were very similar to those obtained previously by comparing an ntrC mutant strain to a wild-type strain (29).
FIG. 1.
Amounts of NifL and NifA in wild-type (wt) and glnK mutant strains of E. coli. Amounts of proteins in crude cell extracts were determined by Western blotting with polyclonal antisera against NifL (lanes 1 to 4) or NifA (lanes 5 to 8). All strains carried plasmid pNH3 (Ptac-nifLA). Cultures were grown in K medium under derepressing conditions, and expression of NifL and NifA was induced with 10 μM IPTG (+) or was not induced (−). Lanes: 1, 2, 5, and 6, strain NCM1528 (wild type); 3, 4, 7, and 8, strain NCM1974 (glnK mutant).
The glnK insertion mutation is expected to have a polar effect on amtB (ammonium-methylammonium transport B) because amtB lies downstream of glnK and is apparently cotranscribed with it (52). To determine whether AmtB plays any role in nif regulation, we constructed an amtB null mutation (51). Relief of NifL inhibition in the amtB mutant strain under derepressing conditions was similar to that in the wild-type strain (data not shown). In addition, when a glnK+ allele was provided in trans to the glnK disruption (Pcat-glnK+, pJES1086; Ptet-glnK+, pJES1104; PglnK-glnK+, pJES1161), the GlnK protein alone was sufficient to relieve NifL inhibition (Table 2, compare strains NCM1982 [glnK mutant strain with pJES1086] and NCM2069 [glnK mutant strain with pJES1161] with strain NCM1990 [glnK mutant strain with pACYC184 vector], Table 3, compare strain NCM2087 [glnK mutant strain with pJES1104] with strain NCM1974 [glnK mutant strain without pJES1104]). Results from the two sorts of control experiments—effects of disrupting amtB and of complementing glnK alleles with glnK+—indicated that the AmtB protein is not involved in nif regulation. Rather, GlnK is essential for relief of NifL inhibition under derepressing conditions.
GlnK is the missing link between NtrC and NifL.
We previously demonstrated that transcriptional activation by NtrC is required for relief of NifL inhibition under derepressing conditions (29) (Table 3, NCM1851 versus NCM1528). To determine whether this requirement could be accounted for by the role of NtrC in activating the transcription of glnK (51, 52), we expressed glnK from promoter Ptet (pJES1104) or Pcat (pJES1086) in an ntrC null mutant strain. Under these circumstances, NtrC was no longer needed to relieve NifL inhibition (Table 3, compare nifH expression in strains NCM1851 and NCM2088; data not shown). Similarly, the constitutive expression of glnK (pJES1086) suppressed effects of the ntrB mutation in NCM1872 (29) and those of the glnA ntrB ntrC deletion mutation in NCM1799 (Table 1) to allow relief of NifL inhibition (data not shown). Because the parental strain of NCM1799 is different from that of the other strains used in this study, the requirement for GlnK to relieve NifL inhibition does not appear to be strain specific. Hence, the NtrC requirement for relief of NifL inhibition can be accounted for entirely by the requirement for synthesis of GlnK.
Uridylylation of GlnK is not required for relief of NifL inhibition.
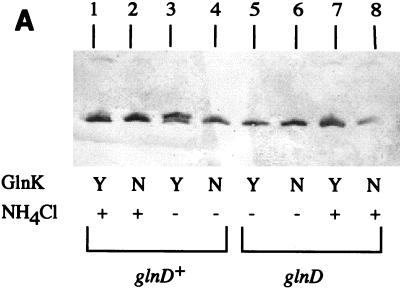
In agreement with the results of van Heeswijk et al. (52), we confirmed that the GlnK protein is highly uridylylated under N-limiting conditions in a glnD+ background (Fig. 2A, lane 3 versus lane 1) but not in a glnD mutant background (Fig. 2A, lanes 5 and 7). The GlnD protein, which has both uridylyltransferase and uridylyl-removing activities, uridylylates GlnK under N-limiting conditions but less so in the presence of ammonium (N excess conditions). Surprisingly, however, the constitutive expression of glnK resulted in relief of NifL inhibition in the presence of ammonium, as well as under N-limiting conditions (Table 3, β-galactosidase activities under N excess conditions). These results implied that uridylylation of GlnK might not be required for relief of NifL inhibition.
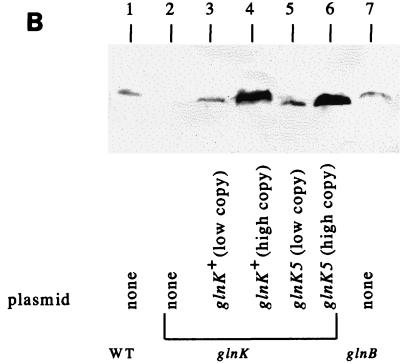
FIG. 2.
Uridylylation (A) and different levels of accumulation (B) of GlnK (Y) and GlnKY51N (N). (A) Cultures were grown in K medium under anaerobic conditions with (+) or without (−) NH4Cl (see Materials and Methods). Proteins were separated by SDS-PAGE in the Tris-Tricine system (see Materials and Methods) and detected with anti-GlnB serum. Lanes: 1 to 4, glnD+ background (NCM1851); 5 to 8, glnD mutant background (NCM1687). The strains used for lanes 1, 3, 5, and 7 contained pJES1104 (Ptet-glnK in vector pACYC184); the strains used for lanes 2, 4, 6, and 8 contained plasmid pJES1106 (Ptet-glnK5; GlnKY51N in vector pACYC184). When cell extracts were treated with snake venom phosphodiesterase to remove uridylyl groups prior to electrophoresis, only the lower band was detected (data not shown). Treatment with alkaline phosphatase had no effect. (B) Cultures were grown in K medium under derepressing conditions (see Materials and Methods). Proteins were separated by SDS-PAGE in the 2× Tris-glycine system (see Materials and Methods) and detected with anti-GlnK serum. Lanes: 1, strain NCM1528 (wild type); 2, strain NCM1974 (glnK mutant); 3, strain NCM2069 (glnK/pJES1161 [PglnK-glnK+ in mini-F vector]); 4, strain NCM2087 (glnK/pJES1104 [Ptet-glnK+ in vector pACYC184]); 5, strain NCM2081 (glnK/pJES1162 [PglnK-glnK5; GlnKY51N in mini-F vector]); 6, strain NCM2088 (glnK/pJES1106 [Ptet-glnK5; GlnKY51N in vector pACYC184]). In this panel, bands were not resolved well enough for assessment of uridylylation.
We used two additional approaches to investigate the role of uridylylation of the GlnK protein with respect to nif regulation. First, we examined the effects of a glnD::Tn10 allele that is known to reduce uridylyltransferase activity greatly (8, 9). Second, we examined effects of changing the uridylylated tyrosine in GlnK to another residue. Although we had shown previously that glnD mutations prevent relief of NifL inhibition (29) (Table 3, NCM1687), it was not clear whether their effects extend beyond control of the activity of NtrC. To assess whether GlnD is directly required to uridylylate GlnK, we transferred plasmid pJES1104 (Ptet-glnK+) into the glnD::Tn10 strain. Immunological (Western blot) analysis indicated that GlnK was in its unmodified form in this background (Fig. 2A, lanes 5 and 7). As shown in Table 3, NifL inhibition was relieved and nif expression was increased to levels characteristic of a wild-type background under both N-limiting and N excess conditions (Table 3, NCM2093 and NCM1528). The fact that constitutive expression of glnK+ could suppress the effects of a glnD mutation on nif expression provided further evidence that uridylylation of GlnK is not required for relief of NifL inhibition.
A second, independent test of the need for uridylylation of GlnK to relieve NifL inhibition investigated the effects of a mutant form of GlnK in which the presumed site of uridylylation, Y51 (1, 11, 13, 23, 36, 50), was altered. Western blot analysis (Fig. 2A, lanes 2, 4, 6, and 8) and in vitro assays (3, 42) confirmed that GlnKY51N was not uridylylated. In agreement with results obtained under N excess conditions and in a glnD mutant background, GlnKY51N allowed relief of NifL inhibition not only in a glnK mutant background but also in ntrC and glnD mutant backgrounds (Table 3, NCM2089, NCM2090, NCM2091, and NCM2094). As was the case for GlnK itself (NCM2086, NCM2087, NCM2088, and NCM2093), the altered protein allowed relief of NifL inhibition under both N excess and N-limiting conditions.
To determine whether the efficacy of GlnKY51N in relieving NifL inhibition depend on its being overproduced from a multicopy vector (pACYC184; copy number, about 10 to 15 per cell [43]), we further studied the effects of this GlnK protein when it was expressed from its native promoter carried on a very low-copy-number vector (pZC320 [49], a mini-F plasmid; 0.2 to 1.4 copies per chromosome [27]). Plasmids pJES1161 and pJES1162, which encode GlnK and GlnKY51N, respectively, are compatible with pNH3 (pBR322 based; 15 to 20 copies per cell [43]), which encodes NifL and NifA, and direct analysis showed that the amounts of the mini-F derivatives (pJES1161 and pJES1162) were much lower than that of pNH3 when the two coexisted in one strain (NCM2069 or NCM2081) (data not shown). Moreover, Western blot analysis showed that the accumulation of GlnK or GlnKY51N expressed from the mini-F vector was similar to that obtained when GlnK was expressed from the chromosome in the wild-type background (NCM1528) and was less than that obtained when GlnK was expressed from the pACYC184 vector (Fig. 2B). As shown in Table 4, glnK (encoding wild-type GlnK) and glnK5 (encoding GlnKY51N) carried on the mini-F vector could complement a glnK null allele for relief of NifL inhibition under N-limiting conditions (NCM2069 and NCM2081, respectively), indicating that large amounts of the GlnK protein are not required for this purpose. As expected, neither pJES1161, which encodes GlnK, nor pJES1162, which encodes GlnKY51N, allowed relief of NifL inhibition under N excess conditions or in a glnD or ntrC background (Table 4, strains NCM2080 and NCM2082, respectively; data not shown) because glnK was transcribed from its native promoter. Taken together, the results in this section indicate that uridylylation of GlnK is not required for relief of NifL inhibition.
TABLE 4.
Effects of low levels of GlnK and GlnB on NifL activity
| Straina | Relevant genotype | Expression of nifH′-′lacZb (U/ml/OD600)
|
|
|---|---|---|---|
| −N | +N | ||
| NCM1528 | Wild type | 5,780 | <10 |
| NCM1974 | glnK | <10 | <10 |
| NCM2070 | glnK/pJES1163 | <10 | <10 |
| NCM2069 | glnK/PglnK-glnK+ (mini-F vector) | 5,200 | <10 |
| NCM2081 | glnK/PglnK-glnK5 (GlnKY51N) (mini-F vector) | 3,770 | <10 |
| NCM2080 | glnD/PglnK-glnK+ (mini-F vector) | 60 | NDc |
| NCM2082 | glnD/PglnK-glnK5 (GlnKY51N) (mini-F vector) | 60 | ND |
| NCM2048 | glnK/PglnB-glnB+ (in pACYC184) | 30 | <10 |
| NCM2068 | glnK/PglnB-glnB+ (mini-F vector) | <10 | <10 |
All strains carry a single chromosomal copy of a nifH′-′lacZ fusion at the trp locus and plasmid pNH3 (Ptac-nifLA).
See footnote b to Table 3.
ND, not determined.
The functions of GlnK have diverged from those of GlnB.
Although the amino acid sequence of GlnK is 67% identical to that of GlnB (52), it is known that glnB null alleles have no effect on NifL inhibition of NifA activity in either K. pneumoniae (33, 34) or E. coli (29). In addition, a glnB+ allele did not suppress effects of a glnK null mutation on NifL inhibition, whether the glnB gene was carried by a multicopy vector or a very low-copy vector (Table 4, NCM2048 and NCM2068, respectively). Moreover, GlnB was not able to suppress effects of ntrC or glnD mutations on nif gene regulation (data not shown). These findings indicate that the functions of GlnK have diverged from those of GlnB.
DISCUSSION
GlnK is required to relieve inhibition of NifA activity by the K. pneumoniae NifL protein.
A major question concerning regulation of nif transcription is how signals of O2 and fixed-N availability are transmitted to the transcriptional machinery. Although it has long been known that N status regulates transcriptional activation by NifA through the NifL protein in the γ-proteobacteria (32, 40), it was not clear whether NifL senses signals of N availability directly or additional components are required. Our recent finding that transcriptional activation by the general nitrogen regulator NtrC is essential for relief of NifL inhibition of NifA activity under derepressing conditions led us to propose the existence of other components in the signal transduction pathway (29). In this study, we have demonstrated that, indeed, a previously unrecognized protein, GlnK, acts as the missing link between the nitrogen sensor NtrC and the coupled redox/nitrogen sensor NifL (or a complex of NifL and NifA) (Tables 2, 3, and 4). Whereas NifL is a negative regulatory factor, GlnK acts positively to counteract inhibitory effects of NifL specifically under derepressing (N-limiting) conditions. Epistasis tests performed with constitutively expressed GlnK indicated that GlnK is the only link between NtrC and NifL.
Despite the fact that GlnK is normally uridylylated under N-limiting conditions, we have several lines of evidence that uridylylation is not required for relief of NifL inhibition, even when GlnK is expressed at normal levels (Tables 3 and 4; Fig. 2A and B). First, constitutively expressed (overexpressed) GlnK can relieve NifL inhibition, even in the presence of ammonium, a circumstance under which GlnK is not highly uridylylated normally. Second, the product of the glnD gene, which catalyzes uridylylation of GlnK, is not required under these conditions. Finally, when expressed at normal levels from the native glnK promoter, GlnKY51N, in which the uridylylated tyrosine has been changed to a residue that cannot be modified, allows relief of NifL inhibition.
The surprising finding that uridylylation of GlnK is not required for relief of NifL inhibition leads to two interesting questions. First, why is GlnK interposed between NtrC and NifL when transcription of both GlnK and NifL is under NtrC control? Second, how is NifL inhibition restored when ammonium (combined N) is added back to the medium? With respect to the first question, one can speculate that more extreme conditions of N limitation—which should result in accumulation of more phosphorylated (active) NtrC—are required for transcription of the glnK operon than for that of the nifLA operon. (It is known that larger amounts of phosphorylated NtrC are required for transcription of nifLA than for that of glnA [6, 54].) This postulate can be tested in vivo in ammonium-limited chemostat cultures. With respect to the second question, it is known that NifA-dependent transcription of the nifHDK operon of K. pneumoniae ceases less than 20 min after ammonium is added back to the medium and that cessation is mediated by NifL (10, 14). Because pre-existing GlnK would be diluted less than twofold during this time period, it is not apparent how inhibition would be restored. It is possible that GlnK is proteolyzed or covalently modified by a mechanism other than uridylylation upon replenishment of ammonium.
Role of PII-like proteins in regulation of nif gene expression in other organisms.
The GlnK and GlnB proteins of enteric bacteria are both “PII-like” protein allosteric effectors that regulate nitrogen metabolism (1, 5, 52). Because their functions have diverged (Table 4)—e.g., only GlnK can relieve NifL inhibition—they are most usefully referred to as paralogues rather than homologues. Interestingly, A. vinelandii, another member of the γ-proteobacteria, has only one PII-like protein, which has been designated GlnK (38). In A. vinelandii, GlnK is essential—strains carrying disruptions of the glnK gene cannot segregate mutant chromosomes (38). Moreover, lesions in nfrX, which is homologous to the glnD gene of enteric bacteria, cause a Nif− phenotype. If the (single) GlnK protein of A. vinelandii is required for relief of NifL inhibition, it is possible that it must be uridylylated to perform this function. Uridylylation of a single PII-like protein may serve as an alternative to the presence of a second such protein.
Finally, there is evidence for pairs of PII-like paralogues in diazotrophs among the α- and β-proteobacteria (7, 17). In Azospirillum brasilense (α) and Herbaspirillum seropedicae (β), at least one of the two PII-like proteins is required for a Nif+ phenotype (7, 16, 17, 37). Whether uridylylation of the pertinent PII-like protein is involved in nif regulation in these cases is not known. Finally, in α- and β-proteobacteria, there is no NifL protein (24). Rather, in A. brasilense, the PII-like protein appears to interact directly with NifA (4). Like the differences between NifA proteins with regard to the presence of cysteine-rich inserts and, presumably, metal clusters involved in O2 sensing, differences in the details of N sensing by the NifA protein or the NifA and NifL proteins of different organisms provide evidence for the regulatory volatility thought to be responsible for much phenotypic diversity (19, 20, 22, 28).
ACKNOWLEDGMENTS
We thank M. Atkinson, D. Biek, M. Dean, and K. Klose for providing plasmids pKOP2, pKOPY51N, pZC320, p1459B6, and pCVD442, respectively; W. van Heeswijk for providing polyclonal rabbit antiserum against GlnB; and L. Passaglia for helping to make polyclonal rabbit antiserum against GlnK. We thank L. Passaglia, V. Wendisch, and D. Yan for critical reading of the manuscript and S. Kato for help in its preparation.
This work was supported by National Science Foundation grant MCB-9405733 to S.K.
ADDENDUM
When expressed from the glnK promoter, the GlnKY51F protein, which carries a conservative amino acid substitution of phenylalanine for the tyrosine that is normally uridylylated, relieves NifL inhibition under derepressing conditions. Like GlnKY51N, GlnKY51F is not uridylylated, and hence, the result confirms the conclusion that uridylylation of GlnK is not required for relief of NifL inhibition.
REFERENCES
- 1.Adler S P, Purich D, Stadtman E. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975;250:6264–6272. [PubMed] [Google Scholar]
- 2.Allikmets R, Gerrard B, Court D, Dean M. Cloning and organization of the abc and mdl genes of Escherichia coli: relationship to eukaryotic multidrug resistance. Gene. 1993;136:231–236. doi: 10.1016/0378-1119(93)90470-n. [DOI] [PubMed] [Google Scholar]
- 3.Arkinson, M. R., and A. J. Ninfa. Unpublished data.
- 4.Arsene F, Kaminski P A, Elmerich C. Modulation of NifA activity by PII in Azospirillum brasilense: evidence for a regulatory role of the NifA N-terminal domain. J Bacteriol. 1996;178:4830–4838. doi: 10.1128/jb.178.16.4830-4838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson M R, Ninfa A J. Role of the GlnK signal transduction protein in the regulation of nitrogen assimilation in Escherichia coli. Mol Microbiol. 1998;29:431–448. doi: 10.1046/j.1365-2958.1998.00932.x. [DOI] [PubMed] [Google Scholar]
- 6.Austin S, Henderson N, Dixon R. Requirements for transcriptional activation in vitro of the nitrogen-regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol. 1987;1:92–100. doi: 10.1111/j.1365-2958.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 7.Benelli E M, Souza E M, Funayama S, Rigo L U, Pedrosa F O. Evidence for two possible glnB-type genes in Herbaspirillum seropedicae. J Bacteriol. 1997;179:4623–4626. doi: 10.1128/jb.179.14.4623-4626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom F R, Levin M S, Foor F, Tyler B. Regulation of glutamine synthetase formation in Escherichia coli: characterization of mutants lacking the uridylyltransferase. J Bacteriol. 1978;134:569–577. doi: 10.1128/jb.134.2.569-577.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bueno R, Pahel G, Magasanik B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J Bacteriol. 1985;164:816–822. doi: 10.1128/jb.164.2.816-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon M, Hill S, Kavanagh E, Cannon F. A molecular study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol Gen Genet. 1985;198:198–206. doi: 10.1007/BF00382996. [DOI] [PubMed] [Google Scholar]
- 11.Carr P D, Cheah E, Suffolk P M, Vasudevan S G, Dixon N E, Ollis D L. X-ray structure of the signal transduction protein P-II from Escherichia coli at 1.9Å. Acta Crystallogr Sect D Biol Crystallogr. 1996;D52:93–104. doi: 10.1107/S0907444995007293. [DOI] [PubMed] [Google Scholar]
- 12.Carr S A, Annan R S. Nonurea peptide separations with Tris buffer. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 10.2.12–10.2.14. [Google Scholar]
- 13.Cheah E, Carr P D, Suffolk P M, Vasudevan S G, Dixon N E, Ollis D L. Structure of the Escherichia coli signal transducing protein PII. Structure. 1994;2:981–990. doi: 10.1016/s0969-2126(94)00100-6. [DOI] [PubMed] [Google Scholar]
- 14.Collins J J, Roberts G P, Brill W J. Posttranscriptional control of Klebsiella pneumoniae nif mRNA stability by the nifL product. J Bacteriol. 1986;168:173–178. doi: 10.1128/jb.168.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zamaroczy M, Elmerich C. Regulatory roles of the structural homologues PII and Pz proteins in Azospirillum brasilense. In: Elmerich C, Kondorosi A, Newton W E, editors. Biological nitrogen fixation in the 21st century. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 111–114. [Google Scholar]
- 17.de Zamaroczy M, Paquelin A, Peltre G, Forchhammer K, Elmerich C. Coexistence of two structurally similar but functionally different PII proteins in Azospirillum brasilense. J Bacteriol. 1996;178:4143–4149. doi: 10.1128/jb.178.14.4143-4149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon R. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in gamma-proteobacteria. Arch Microbiol. 1998;169:371–380. doi: 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 19.Doebley J. Genetic dissection of the morphological evolution of maize. Aliso. 1995;14:297–304. [Google Scholar]
- 20.Doebley J, Stec A, Hubbard L. The evolution of apical dominance in maize [see comments] Nature. 1997;386:485–488. doi: 10.1038/386485a0. [DOI] [PubMed] [Google Scholar]
- 21.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorweiler J E, Doebley J. Developmental analysis of teosinte glume architecture 1: a key locus in the evolution of maize (Poaceae) Am J Bot. 1997;84:1313–1322. [PubMed] [Google Scholar]
- 23.Edwards K J, Suffolk P M, Carr P D, Wegman M, Cheah E, Ollis D L. Crystallization and preliminary X-ray diffraction studies of new crystal forms of Escherichia coli P-II complexed with various ligands. Acta Crystallogr Sect D Biol Crystallogr. 1996;D52:738–742. doi: 10.1107/S0907444996003241. [DOI] [PubMed] [Google Scholar]
- 24.Elmerich C, de Zamaroczy M, Arsene F, Pereg L, Paquelin A, Kaminski A. Regulation of NIF gene expression and nitrogen metabolism in Azospirillum. Soil Biol Biochem. 1997;29:847–852. [Google Scholar]
- 25.Fischer H M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govantes F, Molina-Lopez J A, Santero E. Mechanism of coordinated synthesis of the antagonistic regulatory proteins NifL and NifA of Klebsiella pneumoniae. J Bacteriol. 1996;178:6817–6823. doi: 10.1128/jb.178.23.6817-6823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyer M S, Reed R R, Steitz J A, Low K B. Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harbor Symp Quant Biol. 1981;45:135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Hanson M A, Gaut B S, Stec A O, Fuerstenberg S I, Goodman M M, Coe E H, Doebley J F. Evolution of anthocyanin biosynthesis in maize kernels: the role of regulatory and enzymatic loci. Genetics. 1996;143:1395–1407. doi: 10.1093/genetics/143.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L, Soupene E, Kustu S. NtrC is required for control of Klebsiella pneumoniae NifL activity. J Bacteriol. 1997;179:7446–7455. doi: 10.1128/jb.179.23.7446-7455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson N, Austin S, Dixon R A. Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae. Mol Gen Genet. 1989;216:484–491. [Google Scholar]
- 31.Hill S, Austin S, Eydmann T, Jones T, Dixon R. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc Natl Acad Sci USA. 1996;93:2143–2148. doi: 10.1073/pnas.93.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill S, Kennedy C, Kavanagh E, Goldberg R B, Hanau R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature. 1981;290:424–426. doi: 10.1038/290424a0. [DOI] [PubMed] [Google Scholar]
- 33.Holtel A, Merrick M. Identification of the Klebsiella pneumoniae glnB gene: nucleotide sequence of wild-type and mutant alleles. Mol Gen Genet. 1988;15:134–138. doi: 10.1007/BF00331314. [DOI] [PubMed] [Google Scholar]
- 34.Holtel A, Merrick M J. The Klebsiella pneumoniae PII protein (glnB gene product) is not absolutely required for nitrogen regulation and is not involved in NifL-mediated nif gene regulation. Mol Gen Genet. 1989;217:474–480. doi: 10.1007/BF02464920. [DOI] [PubMed] [Google Scholar]
- 35.Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- 36.Jaggi R, Ybarlucea W, Cheah E, Carr P D, Edwards K J, Ollis D L, Vasudevan S G. The role of the T-loop of the signal transducing protein PII from Escherichia coli. FEBS Lett. 1996;391:223–228. doi: 10.1016/0014-5793(96)00737-5. [DOI] [PubMed] [Google Scholar]
- 37.Liang Y Y, de Zamaroczy M, Arsene F, Paquelin A, Elmerich C. Regulation of nitrogen fixation in Azospirillum brasilense Sp7: involvement of nifA, glnA and glnB gene products. FEMS Microbiol Lett. 1992;79:113–119. doi: 10.1111/j.1574-6968.1992.tb14028.x. [DOI] [PubMed] [Google Scholar]
- 38.Meletzus D, Rudnick P, Doetsch N, Green A, Kennedy C. Characterization of the glnK-amtB operon of Azotobacter vinelandii. J Bacteriol. 1998;180:3260–3264. doi: 10.1128/jb.180.12.3260-3264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merrick M. Regulation of nitrogen fixation genes in free-living and symbiotic bacteria. In: Stacey G, Burris R, Evans H, editors. Biological nitrogen fixation. New York, N.Y: Chapman & Hall; 1992. pp. 835–876. [Google Scholar]
- 40.Merrick M, Hill S, Hennecke H, Hahn M, Dixon R, Kennedy C. Repressor properties of the nifL gene product in Klebsiella pneumoniae. Mol Gen Genet. 1982;185:75–81. [Google Scholar]
- 41.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 42.Passaglia, L., L. He, and S. Kustu. Unpublished data.
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. 1.4. [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 3. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 18.49–18.54. [Google Scholar]
- 45.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 46.Schauder B, Blöcker H, Frank R, McCarthy J E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52:279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz R A. NifL of Klebsiella pneumoniae carries an N-terminally bound FAD cofactor, which is not directly required for the inhibitory function of NifL. FEMS Microbiol Lett. 1997;157:313–318. doi: 10.1111/j.1574-6968.1997.tb12791.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmitz R A, He L, Kustu S. Iron is required to relieve inhibitory effects of NifL on transcriptional activation by NifA in Klebsiella pneumoniae. J Bacteriol. 1996;178:4679–4687. doi: 10.1128/jb.178.15.4679-4687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi J, Biek D P. A versatile low-copy-number cloning vector derived from plasmid F. Gene. 1995;164:55–58. doi: 10.1016/0378-1119(95)00419-7. [DOI] [PubMed] [Google Scholar]
- 50.Son H S, Rhee S G. Cascade control of Escherichia coli glutamine synthetase. Purification and properties of PII protein and nucleotide sequence of its structural gene. J Biol Chem. 1987;262:8690–8695. [PubMed] [Google Scholar]
- 51.Soupene E, He L, Yan D, Kustu S. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci USA. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Heeswijk W C, Hoving S, Molenaar D, Stegeman B, Kahn D, Westerhoff H V. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol Microbiol. 1996;21:133–146. doi: 10.1046/j.1365-2958.1996.6281349.x. [DOI] [PubMed] [Google Scholar]
- 53.van Heeswijk W C, Rabenberg M, Westerhoff H V, Kahn D. The genes of the glutamine synthetase adenylylation cascade are not regulated by nitrogen in Escherichia coli. Mol Microbiol. 1993;9:443–457. doi: 10.1111/j.1365-2958.1993.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 54.Wong P K, Popham D, Keener J, Kustu S. In vitro transcription of the nitrogen fixation regulatory operon nifLA of Klebsiella pneumoniae. J Bacteriol. 1987;169:2876–2880. doi: 10.1128/jb.169.6.2876-2880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]