Abstract
The microsatellite instability (MSI) phenotype is related to a deficiency of the DNA mismatch repair (dMMR) system and is observed in 5% of metastatic colorectal cancers (mCRCs). MSI/dMMR phenotype testing should be routine for all CRCs regardless of stage. Two complementary techniques with a high concordance (90–97%) allow us to determine the MSI/dMMR status of a tumor: immunohistochemistry and polymerase chain reaction. Since 2020 and the results of the phase III KEYNOTE 177 trial, pembrolizumab [anti-programmed cell death protein 1 (PD1)] is the new standard of care in first-line MSI/dMMR mCRC. To date, no combination of chemtotherapy ± targeted therapy with immune checkpoint inhibitors (ICIs) has been validated in the management of MSI/dMMR mCRC, and it is not known whether this combination would be beneficial. It is also unclear whether dual therapy with two ICIs is more effective than monotherapy. Several phase III trials are ongoing to answer these questions. Despite a high response rate and long-term benefit of a first line by anti-PD1, 30–50% of patients with MSI/dMMR mCRC experience an early or secondary progression. There are currently no validated predictive biomarkers of anti-PD1 ± anti-cytotoxic T lymphocyte antigen-4 resistance in patients with MSI/dMMR mCRC. In case of early progression on ICIs, the first two questions to consider are the possibility of pseudoprogression and the correct diagnosis of MSI/dMMR status. To date, there are no data on the use of adjuvant ICIs for MSI/dMMR resected colon cancers. By contrast, data are accumulating regarding the efficacy of neoadjuvant ICIs, with at least two-thirds of patients in the different trials in pathological complete response, making it possible to envisage ‘Watch and wait’ strategies in future.
Keywords: checkpoint inhibitor, colorectal cancer, diagnostic, immunotherapy, MSI
Introduction
Microsatellite unstable (MSI) colorectal cancers (CRC) are due to DNA mismatch repair (MMR) deficiency and occurs in 5% in the metastatic setting. Deficient mismatch repair (dMMR)/MSI metastatic CRC (mCRC) seemed to have a worse prognosis than proficient mismatch repair/microsatellite stable (pMMR/MSS). This unfavorable prognosis in the metastatic setting is related to the high frequency of BRAFV600E mutation (20–30% of cases) and to a lower efficacy of standard chemotherapies.1,2 The character MSI/dMMR is observed in 10–20% of localized colic cancers (CCs). Unlike the metastatic stage, it is associated with a favorable prognosis for stage II and stage III pTxN1 CC, and with prognosis equivalent to that of MSS/pMMR stage III T4 or N2 CC. 3
Immunotherapy with immune checkpoint inhibitors (ICIs) has revolutionized the management of patients with MSI/dMMR mCRC at the first line of treatment. 4 However, many challenges remain in the management of these patients: mainly, the management of non-responding patients with ICIs, in dissociated response or late progression, but also for patients with ICIs regarding the duration of treatment or the place of surgery of residual masses. Immunotherapy also bursts in for localized cancers, justifying a universal screening of the MSI/dMMR status for all CRC regardless of stage. 5
Determination of microsatellite instability and mismatch repair deficiency in CRC
Current guidelines recommend determining MMR status in front of any CRC at any stage. MSI phenotype is characterized by length heterogeneity of noncoding repeated DNA sequences. It reflects a deficiency in the repair system of DNA mismatches encoded by the genes MLH1, MSH2, MSH6, and PMS2. Malfunction of these genes results in a hypermutability whose microsatellite instability is the reflection. Malfunction of the DNA repair system can be sporadic (methylation of the MLH1 gene promoter, bi-allelic somatic mutation at the tumor level of an MMR gene) or constitutional (germline mutation of MLH1, MSH2, MSH6, or PMS2).6,7 The presence of a BRAFV600E mutation is highly predictive of hypermethylation of the MLH1 promoter in case of loss of expression of MLH1/PMS2, and therefore of sporadic origin, even if rare cases of constitutional hypermethylation can be reported.
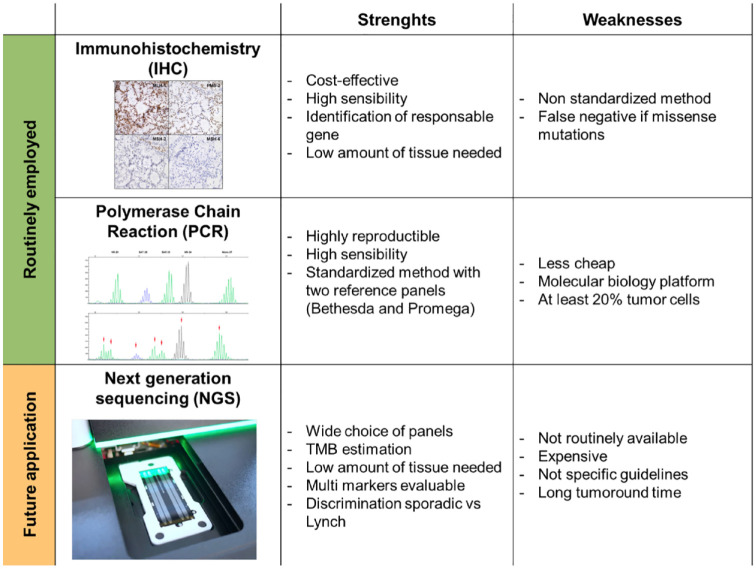
Two complementary methods are currently recommended to determine the MSI/dMMR status: screening for loss of MMR protein expression using immunohistochemistry (IHC) and testing for MSI using polymerase chain reaction (PCR) (Table 1). These two methods are implemented on tumor material from biopsies or surgical parts. The analysis of the MSI/dMMR status by IHC is an indirect method that allows us to highlight the loss of expression of MMR proteins (MLH1, MSH2, MSH6, and PMS2) at the tumor level. MMR proteins work in heterodimers, MLH1 with PMS2, and MSH2 with MSH6. MLH1 and MSH2 proteins are the obligatory partners of their respective heterodimers. However, the absence of PMS2 or MSH6 is not systematically associated with a loss of MLH1 or MSH2. Isolated losses of MSH6 or PMS2 are rarer and should lead to systematic research for microsatellite status by PCR.8,9 Multiple studies are underway to develop deep-learning algorithms to diagnose the phenotype dMMR directly on hematoxylin and eosin colored slides and scanned. 10
Table 1.
ORR, PFS, and OS under ICIs in MSI/dMMR mCRC.
| Trial | Design | Line | Experimental arm | N ( ICIs arm) | ORR (%) | PFS (%) at 1 year | OS (%) at 1 year | PFS (%) at 3 years | OS (%) at 3 years | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy | ||||||||||
| KEYNOTE-1774 | Phase III | 1 | Pembrolizumab versus CT ± bevacizumab or cetuximab | 153 | 45 | 55 | 78 | 42 | 61 | 44.5 |
| KEYNOTE-16411 | Phase II | ⩾3 | Pembrolizumab (cohort A) | 61 | 33 | 34 | 72 | – | – | 31 |
| ⩾2 | Pembrolizumab (cohort B) | 63 | 33 | 41 | 76 | – | – | 24 | ||
| CHECKMATE-14212 | Phase II | ⩾2 | Nivolumab | 74 | 31 | 48 | 73.8 | – | – | 21 |
| SAMCO 13 | Randomized phase II | ⩾2 | Avelumab versus CT ± bevacizumab or cetuximab | 61 | 29.5 | 31 | – | – | – | 33.3 |
| Dual therapy | ||||||||||
| CHECKMATE-14212 | Phase II | 1 | Nivolumab + ipilimumab | 45 | 69 | – | 85 | – | – | 29 |
| ⩾2 | Nivolumab + ipilimumab | 119 | 65 | 76.4 | 84 | 60 | 71.4 | 50.9 | ||
| NIPICOL 14 | Phase II | ⩾3 | Nivolumab + ipilimumab | 57 | 59.6 | 75.4 | 84.1 | 70 | 73.1 | 34.5 |
CT, chemotherapy; dMMR, deficient mismatch repair; ICIs, immune checkpoint inhibitors; mCRC, metastatic colorectal cancer; MSI, microsatellite unstable; N, number of patients; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Analysis of the MSI phenotype by PCR is based on the measure of amplification of specific noncoding repeats and requires sufficient tumor cellularity of the histological specimen to extract tumor DNA. Currently, one of the reference panels is the Promega panel which allows us to conclude to a phenotype MSI without comparison to the non-tumoral tissue of the patient when at least three markers are unstable. 15 When two markers are unstable, the PCR pattern must be compared with the patient’s healthy tissue to conclude with an MSI phenotype. IHC and PCR have high concordance (90–97%). 16 European Society of Medical Oncology’s (ESMO) guidelines are to use IHC as a first-line therapy to determine MMR status. 17
MSI/dMMR status testing by next-generation sequencing was recently approved by the Food and Drug Administration (FDA) for patients with mCRC prior to ICI therapy. 18 In contrast to PCR, which studies a small number of microsatellite sequences, this technology allows the study of several hundred microsatellite sequences via gene panel analysis (MSK-IMPACT) 19 or coding sequences (MSISensor). 18 Although validated by the FDA in pre-therapeutic test to detect MSI/dMMR status prior ICIs treatment in mCRC, the MSISensor panel fails to detect MSI phenotype in 9–32% depending on cohort. 20 A new analysis algorithm named MSICare allows us to obtain a better sensitivity with 100% specificity for the detection of the MSI phenotype. 20 It is therefore necessary to remain cautious on these new techniques, which, although advantageous compared with the pentaplex PCR (a same test allowing here to obtain not only the microsatellite status but also the RAS/RAF mutations), must be validated on a larger scale (Figure 1).
Figure 1.
Strengths and weaknesses of methods for determining MSI/dMMR status.
dMMR, deficient mismatch repair; MSI, microsatellite unstable.
Which management in 2022 for a patient with a MSI/dMMR outside of therapeutic trials
Since 2020 and the results of the phase III KEYNOTE 177 trial, pembrolizumab [anti-programmed cell death protein 1 (PD1)] is the new standard of care in first-line MSI/dMMR mCRC. 4
This trial demonstrated the superiority of pembrolizumab versus doublet chemotherapy plus targeted therapy [anti-epidermal growth factor receptor (EGFR) or anti-vascular endothelial growth factor (VEGF)] in first-line MSI/dMMR mCRC. A total of 307 patients were randomized. The co-primary end points were progression-free survival (PFS) (centralized review) and overall survival (OS). Pembrolizumab was administered as a 30-min infusion, every 3 weeks, for 2 years or until progression or unacceptable toxicity. Median PFS was significantly improved in the pembrolizumab arm: 16.5 months versus 8.2 months. At 24 months of randomization, 48.3% of patients treated with pembrolizumab had not progressed and were able to stop treatment compared to 18.6% of those treated with chemotherapy. The objective response rate was 45.1% in the immunotherapy arm as compared to 33.1% in the chemotherapy arm. Quality of life was maintained or improved by pembrolizumab and was significantly better than that observed in patients treated with chemotherapy. 21 At 5 years of follow-up, the median OS was not reached in the pembrolizumab arm and was 36.7 months in the chemotherapy arm. Improvement in OS was not statistically significant, probably related to the fact that 60% of patients in the control group had received cross-over immunotherapy in subsequent lines of treatment. 22 Grade 3–4 toxicities occurred in 21.6% of patients treated with pembrolizumab versus 66.4% in the chemotherapy arm. This study resulted in a European marketing authorization in January 2021 for pembrolizumab in first-line MSI/dMMR mCRC.
For patients who did not receive ICIs on the first line, nivolumab (anti-PD1) and ipilimumab [anti-cytotoxic T lymphocyte antigen-4 (CTLA4)] obtained a European marketing authorization in June 2021 based on the CheckMate-142 phase II trial.12,23 The randomized phase II trial SAMCO-PRODIGE 54 presented at ESMO 2022 demonstrated efficacy with a favorable tolerance profile of avelumab (anti-PDL1) monotherapy in the same indication versus doublet chemotherapy plus targeted therapy (anti-EGFR or anti-VEGF). The primary end point was PFS. Median PFS was significantly improved in the avelumab arm: 12 months versus 7 months. 13 OS, quality of life, and biomarkers are currently being analyzed.
Ongoing randomized trials for MSI/dMMR mCRC
Currently, no combination of chemotherapy ± targeted therapy is validated with ICIs in the management of MSI/dMMR mCRC and it is not known whether this combination would be beneficial. This is the question that the phase III COMMIT trial (NCT02997228) currently being enrolled will attempt to answer. It is an initial three-arm trial (the mFOLFOX6 + bevacizumab arm was subsequently closed) comparing mFOLFOX6 + bevacizumab + atezolizumab with atezolizumab monotherapy (Table 2).
Table 2.
Ongoing randomized trials involving ICIs for MSI/dMMR mCRC.
| Trial | Design | Line | Status | Experimental arm | Control arm | N | Primary end point | Clin.gov number |
|---|---|---|---|---|---|---|---|---|
| COMMIT | Phase III | 1 | Ongoing (USA) | mFOLFOX6 + bevacizumab + atezolizumab | Atezolizumab | 231 | PFS | NCT02997228 |
| CHECKMATE 8HW | Phase III | 1,2 | Ongoing | Nivolumab + ipilimumab | CT ± targeted therapy | 831 | PFS | NCT04008030 |
| Nivolumab | ||||||||
| NIPISAFE | Randomized phase II | 1, 2 | Ongoing | Nivolumab + ipilimumab 24 months | 12 weeks induction nivolumab + ipilimumab then 24 months maintenance nivolumab | 96 | Safety and PFS | NCT04730544 |
| SEAMARK | Randomized phase II ƚ | 1 | Ongoing | Encorafenib + cetuximab + pembrolizumab | Pembrolizumab | 104 | PFS | NCT05217446 |
For BRAFV600E-mutated dMMR/MSI mCRC.
CT, chemotherapy; dMMR, deficient mismatch repair; ICIs, immune checkpoint inhibitors; mCRC, metastatic colorectal cancer; MSI, microsatellite unstable; N, number of patients to be included; PFS, progression-free survival.
It is also unclear whether dual therapy with two ICIs is more effective than monotherapy. To answer this question, the phase III CA209-8HW trial (NCT04008030) is underway, randomizing in three arms nivolumab versus nivolumab + ipilimumab versus chemotherapy ± targeted therapy.
Since the publication of the CheckMate-142 trial and the NIPICOL trial, 14 the maintenance dose of nivolumab has evolved from 3 mg/kg to fixed doses of 240 mg every 2 weeks or 480 mg every 4 weeks. The health authorities have approved this dosing regimen. However, dose equivalence studies have involved other tumor locations and the question of using these doses in MSI/dMMR mCRC arises. The ongoing NIPISAFE trial designed to compare two regimens of nivolumab + ipilimumab in MSI/dMMR mCRC to identify the regimen with the highest level of clinical activity and lowest toxicity.
Currently, there are no first-line treatment options indicated specifically for patients with both MSI/dMMR and BRAFV600E-mutant mCRC. To assess the safety and efficacy of combining pembrolizumab with encorafenib + cetuximab, the SEAMARK trial (NCT05217446) will evaluate this combination versus pembrolizumab alone in patients with previously untreated BRAFV600E-mutant MSI/dMMR mCRC
Managing a patient on ICIs
Clinical and molecular predictive factors
Despite a high response rate and long-term benefit of a first line by anti-PD1, 30–50% of patients with MSI/dMMR mCRC experience an early or secondary progression. There are currently no validated predictive biomarkers of anti-PD1 ± anti-CTLA4 resistance in patients with MSI/dMMR mCRC. DNA or RNA biomarkers such as RAS/RAF status, hereditary or sporadic DNA mismatch repair system deficiency, and tumor mutational burden (TMB) have not been identified as predictive of resistance to ICIs to date.11,23,24 Two studies have suggested an impact of TMB25,26 but with a low level of evidence (small sample sizes, possible misdiagnosis of MSI/dMMR status). 27 KRAS-mutated tumors appeared to benefit less from immunotherapy in KEYNOTE-177 trial, but this subgroup analysis is not observed in other trials published in the literature.23,28 An analysis of 466 ICI-treated MSI/dMMR mCRC patients drawn from international cohorts (France, Italy, Spain, and the United States) show RAS/BRAFV600E mutations are not associated with survival while Lynch syndrome patients demonstrated improved PFS. The data suggest that Lynch syndrome is protective against PFS events. 29 Further translational studies are expected to identify factors for ICIs resistance in patients with MSI/dMMR mCRC.
Note the results of an international cohort study showing, on the one hand, an unfavorable prognosis of patients with an impaired performance status treated with ICIs for MSI/dMMR mCRC, and, on the other hand, the pejorative impact of the presence of ascites in this population (it should be noted that peritoneal carcinosis is a preferential metastatic site for MSI/dMMR mCRC).30–32
Pseudoprogressions
Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) criteria may underestimate response to ICIs because of pseudoprogression. Pseudoprogression was observed in only 5% of the 57 patients in the NIPICOL phase II trial 14 and was reported in 10% of the 123 patients with MSI/dMMR mRCRC treated with ICIs, accounting for approximately 50% of the cases of early progression. 33 Evaluation of response to ICIs should be performed according to immune RECIST criteria (iRECIST) for the first 3 months. The evolution of clinical status and the kinetics of tumor markers CEA and CA-19.9 may be useful to distinguish pseudo-progression from early progression on ICIs.
Beyond early treatment: prognosis and duration of treatment
In the 4-year follow-up update of the CheckMate-142 trial, an exploratory analysis was performed on OS based on best radiological response (BOR) according to RECIST 1.1 criteria after 6 months of nivolumab + ipilimumab (4 cycles nivolumab + ipilimumab followed by nivolumab monotherapy). The risk of late progression is low in the population with objective response at 6 months with 93% survivors at 48 months, late progression being mainly observed in patients with radiological stability at 6 months with 66% survivors at 48 months. This analysis shows a significant correlation between BOR at 6 months and OS. 12
The optimal duration of treatment with ICIs is unknown. Some trials have evaluated ICIs without a maximum duration of treatment, others such as the phase III KEYNOTE 177 trial demonstrated the superiority of 2 years of pembrolizumab versus chemotherapy ± targeted therapy. The results of the phase II NIPICOL trial showed a PFS at 24 months of 92.9% after treatment with nivolumab + ipilimumab for 1 year, with only four cases of progression beyond 1 year among the 42 patients alive and progression free at 12 months. These results raise questions about the optimal duration of treatment. However, it is interesting to note that patients who stopped ICIs early for toxicity have similar results.12,23
Dissociated tumor responses and management of residual lesions
Dissociated tumor responses under ICIs suggest that some organs may be sanctuary sites due to the tumoral microenvironment. A study published in 2002 on a series of five patients highlighted the possible sanctuary character of the adrenal glands. 28 The management of patients with a dissociated tumor response must be discussed in a multidisciplinary board to consider the possibility of surgical or interventional radiology strategies. The management of minimal residual disease in patients with a prolonged partial response to ICIs should also be discussed in a multidisciplinary board to assess the benefit–risk balance of resection. A retrospective analysis published in 2021 of 121 patients treated with ICIs for MSI/dMMR mCRC demonstrated that 93% (13/14) of the residual tumor disease resected were in complete pathological response. 34
What to do in case of progression with ICIs
In case of early progression on ICIs, the first two questions to consider are (i) the possibility of pseudoprogression (see above) and (ii) the correct diagnosis of MSI/dMMR status, which accounts for a non-negligible proportion of early progressions. 35 There are no validated data in the current literature to recommend a second line with another ICI after progression on first-line ICIs for MSI/dMMR mCRC.
The ongoing phase II NIPIRESCUE trial (NCT05310643) is investigating the efficacy of nivolumab + ipilimumab as a second-line treatment after progression to anti-PD1 monotherapy. Apart from inclusion in a clinical trial, current guidelines recommend referral to first-line chemotherapy adapted to the patient’s profile and the molecular profile of the tumor. In contrast to other cancer sites, no studies have documented increased efficacy of conventional chemotherapy after ICI treatment in MSI/dMMR mCRC. 36
For patients with localized CRC MSI/dMMR
To date, there are no data on the use of adjuvant ICIs for MSI/dMMR resected colon cancers (CCs). Currently, only one phase II trial is evaluating ICIs in this indication, the ATOMIC trial, which is evaluating 6 months of FOLFOX ± atezolizumab for stage III MSI/dMMR CC.
On the other hand, data are accumulating regarding the efficacy of neoadjuvant ICIs, with at least two-thirds of patients in the different trials in pathological complete response (pCR). In particular, the phase II NICHE 37 study has initiated this line of research. Among 21 patients with MSI/dMMR CC treated with one dose of ipilimumab and two doses of nivolumab 4 weeks before surgery, the major pathologic response rate (MPR; ⩽10% viable residual tumor) was 95% and the pCR rate was 60%. These data are confirmed by the results of NICHE-2 (integrating data from the NICHE 1 trial), presented at the ESMO 2022 Congress (N = 112; 95% MPR; 67% pCR), with no distant relapse (median follow-up of 13 months) and satisfactory safety data (95% of surgeries performed on time, only 4% of grade 3–4 immuno-mediated adverse events). 38 To note, the pCR rate is high in the different trials, whether patients are treated with anti-PD1 alone or combined with an anti-CTLA4, and whether the duration of treatment is short or long (Table 3), and is also similar to that observed for MSI/dMMR esogastric cancers. 39 The ongoing phase II IMOTHEP trial (NCT04795661) is evaluating pembrolizumab as a neoadjuvant treatment for resectable MSI/dMMR digestive and endometrial tumors. These data support the possibility of ‘watch and wait’ strategies, as evaluated in a phase II trial for MSI/dMMR rectal cancer. 40 In this trial with dostarlimab (anti-PD1) every 3 weeks for 6 months, patients with a complete clinical response at the end of treatment were monitored without additional treatment, radiochemotherapy or surgery. Of the first 12 patients enrolled, all were in complete clinical response, none had undergone surgery, and none had relapsed after a median follow-up of 12 months.
Table 3.
Trials evaluating neoadjuvant ICIs for MSI/dMMR CCR.
| Study | Population | Duration of neoadjuvant period | Exploring a surgical de-escalation | Experimental arm | Number of patients included | Pathological complete response rate (%) | CCR rate |
|---|---|---|---|---|---|---|---|
| NICHE 37 | Colon cancers MSI/dMMR | 4 weeks | No | Nivolumab + ipilimumab | 21 | 60 | – |
| NICHE-238 | Colon cancers MSI/dMMR | 4 weeks | No | Nivolumab + ipilimumab | 112 | 67 | – |
| PICC 41 | Colon cancers MSI/dMMR | 12 weeks | No | Noripalimab + celecoxib | 17 | 88 | – |
| Toripalimab | 17 | 65 | – | ||||
| MD Anderson 42 | Locally advanced CRC MSI/dMMR | 24 weeks | Yes | Pembrolizumab | 35 | 71 | 12/22 (55%) |
| MSKCC 40 | Rectal cancers MSI/dMMR | 24 weeks | Yes | Dostarlimab | 12 | – | 100% |
CCR, clinical complete response; CRC, colorectal cancer; dMMR, deficient mismatch repair; ICIs, immune checkpoint inhibitors; MSI, microsatellite unstable.
These initial data on the use of ICIs in the neoadjuvant setting and watch and wait strategies raise a number of questions. On the one hand, it is difficult to assess the prognosis of the disease preoperatively, without knowing the pTN stage of the tumor. It is important to mention that the performance of computed tomography to predict pT4 or pN+ CC is mediocre,43,44, especially for lymph node involvement. Data concerning the existence of signs of tumor regression at the lymph node level on surgical specimens in NICHE-2 (26% of tumors initially classified as cN1 and 62% as cN2) could partially answer this question, but have not yet been reported. For ‘watch and wait’ strategies, the modalities of surveillance and decision of non-surgical management (endoscopic follow-up, systematic biopsies, etc.) remain to be specified. In addition, long-term data on the risk of relapse and second cancers after ICIs treatment for patients with Lynch syndrome are currently lacking, particularly in terms of how to reconcile organ preservation strategies with surgery to prevent the risk of second cancers. In an MSKCC trial, 11-second post-ICIs cancers were observed among 155 patients (7%) with Lynch syndrome at 32 months of follow-up.
Perspectives
To date, there are three main challenges in the management of patients with MSI/dMMR mCRC. The first is the identification of predictive factors of response to anti-PD(L)1 monotherapy, whether biological, molecular, genetic, or clinical. Therefore, the research and documentation of the mechanism of acquisition of the MSI phenotype is a major issue to constitute homogeneous cohorts between a sporadic or constitutional mechanism.
The second is the identification of patients for which combination of anti-PD(L)1 + anti-CTLA4 ICIs is more appropriate than anti-PD1 monotherapy. Finally, the third challenge is the determination of the optimal duration of a treatment with ICIs.
The results of the NICHE, NICHE-2, and MSKCC trials open the way for neoadjuvant immunotherapy in the management of patients with MSI/dMMR CC and potentially lead to curative strategies without the need for surgery.
Acknowledgments
Not applicable.
Contributor Information
Baptiste Cervantes, Department of Medical Oncology, Saint-Antoine Hospital, Sorbonne University, Paris, France AP-HP.
Thierry André, Department of Medical Oncology, Saint-Antoine Hospital, Sorbonne University, Paris, France; AP-HP; SIRIC CURAMUS, INSERM, Unité Mixte de Recherche Scientifique 938, Centre de Recherche Saint-Antoine, Equipe Instabilité des Microsatellites et Cancer, Equipe Labellisée par la Ligue Nationale Contre le Cancer, Paris, France.
Romain Cohen, Department of Medical Oncology, Saint-Antoine Hospital, Sorbonne University, Paris, France; AP-HP, SIRIC CURAMUS, INSERM, Unité Mixte de Recherche Scientifique 938, Centre de Recherche Saint-Antoine, Equipe Instabilité des Microsatellites et Cancer, Equipe Labellisée par la Ligue Nationale Contre le Cancer, Saint-Antoine Hospital, 184 rue du Fg Saint-Antoine 75012 Paris, France.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: All authors.
Author contribution(s): Baptiste Cervantes: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Thierry André: Supervision; Validation.
Romain Cohen: Supervision; Validation.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
BC declares no conflict of interest.
RC has received honoraria as a speaker or in an advisory role from MSD Oncology, Bristol Myers Squibb and Pierre Fabre and travel fees from MSD Oncology, Bristol Myers Squibb, MYLAN Medical and Servier.
TA has received honoraria as a speaker or in an advisory role from AstraZeneca, Astellas, Bristol Myers Squibb, Gritstone Oncology, GamaMabs Pharma Sa, GlaxoSmithKline, Merck & Co. Inc., Nordic Oncology, Pierre Fabre, Roche/Ventana, Sanofi, Seagen, Servier and Transgène and travel fees from Bristol Myers Squibb, Merck & Co. Inc. and Servier.
Availability of data and materials: All authors were provided with full access to all study material and BC takes responsibility for the integrity of the results and the accuracy of the review process.
References
- 1. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014; 20: 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tougeron D, Sueur B, Zaanan A, et al. Prognosis and chemosensitivity of deficient MMR phenotype in patients with metastatic colorectal cancer: an AGEO retrospective multicenter study. Int J Cancer 2020; 147: 285–296. [DOI] [PubMed] [Google Scholar]
- 3. Cohen R, Taieb J, Fiskum J, et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol 2021; 39: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020; 383: 2207–2218. [DOI] [PubMed] [Google Scholar]
- 5. Taieb J, Svrcek M, Cohen R, et al. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer 2022; 175: 136–157. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998; 58: 3455–3460. [PubMed] [Google Scholar]
- 7. Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997; 57: 808–811. [PubMed] [Google Scholar]
- 8. Colas C, Coulet F, Svrcek M, et al. Lynch or not lynch? Is that always a question? Adv Cancer Res 2012; 113: 121–166. [DOI] [PubMed] [Google Scholar]
- 9. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 2008; 10: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamashita R, Long J, Longacre T, et al. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol 2021; 22: 132–141. [DOI] [PubMed] [Google Scholar]
- 11. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020; 38: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142☆. Ann Oncol 2022; 33: P1052–P1060. [DOI] [PubMed] [Google Scholar]
- 13. Taieb J, Bouche O, André T. Avelumab versus standard second-line treatment chemotherapy in metastatic colorectal cancer (mCRC) patients with microsatellite instability (MSI). Ann Oncol 2022; 33(Suppl. 7): S808-S869, https://oncologypro.esmo.org/meeting-resources/esmo-congress/avelumab-versus-standard-second-line-treatment-chemotherapy-in-metastatic-colorectal-cancer-mcrc-patients-with-microsatellite-instability-msi [DOI] [PubMed] [Google Scholar]
- 14. Cohen R, Bennouna J, Meurisse A, et al. RECIST and iRECIST criteria for the evaluation of nivolumab plus ipilimumab in patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the GERCOR NIPICOL phase II study. J Immunother Cancer 2020; 8: e001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buhard O, Cattaneo F, Wong YF, et al. Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J Clin Oncol 2006; 24: 241–251. [DOI] [PubMed] [Google Scholar]
- 16. Svrcek M, Lascols O, Cohen R, et al. MSI/MMR-deficient tumor diagnosis: which standard for screening and for diagnosis? Diagnostic modalities for the colon and other sites: differences between tumors. Bull Cancer 2019; 106: 119–128. [DOI] [PubMed] [Google Scholar]
- 17. Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019; 30: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 18. Middha S, Zhang L, Nafa K, et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol 2017; 2017: PO.17.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol 2016; 34: 2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ratovomanana T, Cohen R, Svrcek M, et al. Performance of next generation sequencing for the detection of microsatellite instability in colorectal cancer with deficient DNA mismatch repair. Gastroenterology 2021; 161: P814–P826.E7. [DOI] [PubMed] [Google Scholar]
- 21. Andre T, Amonkar M, Norquist JM, et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol 2021; 22: 665–677. [DOI] [PubMed] [Google Scholar]
- 22. Diaz LA, Shiu KK, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 2022; 23: 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018; 36: 773–779. [DOI] [PubMed] [Google Scholar]
- 24. Colle R, Cachanado M, Rousseau A, et al. Parameters associated with outcomes in pretreated MSI/dMMR metastatic colorectal cancer (mCRC) treated with immune checkpoint inhibitors (ICI): subgroup analysis of a prospective cohort. J Clin Oncol 2021; 39: 3580. [Google Scholar]
- 25. Mandal R, Samstein RM, Lee KW, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science 2019; 364: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019; 30: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 27. Rousseau B, Foote MB, Maron SB, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med 2021; 384: 1168–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen R, Jonchère V, De La Fouchardière C, et al. Adrenal gland as a sanctuary site for immunotherapy in patients with microsatellite instability-high metastatic colorectal cancer. J Immunother Cancer 2021; 9: e001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colle R, Lonardi S, Cachanado M, et al. Impact of lynch syndrome, BRAF V600E, and RAS mutations on outcomes in MSI/dMMR metastatic colorectal cancer (mCRC) treated with immune checkpoint inhibitors (ICI): analysis of combined international cohorts. J Clin Oncol 2023; 41: 171. [Google Scholar]
- 30. Mazzoli G, Cohen R, Lonardi S, et al. Prognostic impact of performance status on the outcomes of immune checkpoint inhibition strategies in patients with dMMR/MSI-H metastatic colorectal cancer. Eur J Cancer 2022; 172: 171–181. [DOI] [PubMed] [Google Scholar]
- 31. Fucà G, Cohen R, Lonardi S, et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer 2022; 10: e004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011; 117: 4623–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colle R, Radzik A, Cohen R, et al. Pseudoprogression in patients treated with immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Eur J Cancer 2021; 144: 9–16. [DOI] [PubMed] [Google Scholar]
- 34. Ludford K, Cohen R, Svrcek M, et al. Pathological tumor response following immune checkpoint blockade for deficient mismatch repair advanced colorectal cancer. J Natl Cancer Inst 2021; 113: 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen R, Hain E, Buhard O, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2019; 5: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bui QL, Mas L, Hollebecque A, et al. Treatments after immune checkpoint inhibitors in patients with dMMR/MSI metastatic colorectal cancer. Cancers 2022; 14: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020; 26: 566–576. [DOI] [PubMed] [Google Scholar]
- 38. Chalabi M, Verschoor YL, van den Berg J, et al. Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study. Ann Oncol 2022; 33: S808–S869. [Google Scholar]
- 39. André T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol 2023; 41: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 2022; 386: 2363–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu H, Kang L, Zhang J, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2022; 7: 38–48. [DOI] [PubMed] [Google Scholar]
- 42. Ludford K, Raghav K, Murphy MAB, et al. 1758O neoadjuvant pembrolizumab in localized/locally advanced solid tumors with mismatch repair deficiency. Ann Oncol 2021; 32: S1210. [Google Scholar]
- 43. Rollvén E, Abraham-Nordling M, Holm T, et al. Assessment and diagnostic accuracy of lymph node status to predict stage III colon cancer using computed tomography. Cancer Imaging 2017; 17: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012; 13: 1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]