Abstract
Legumain is a lysosomal cysteine protease that has been implicated in an increasing amount of physiological and pathophysiological processes. However, the upstream mechanisms regulating the expression and function of legumain are not well understood. Here, we provide in vitro and in vivo data showing that vitamin D3 (VD3) enhances legumain expression and function. In turn, legumain alters VD3 bioavailability, possibly through proteolytic cleavage of vitamin D binding protein (VDBP). Active VD3 (1,25(OH)2D3) increased legumain expression, activity, and secretion in osteogenic cultures of human bone marrow stromal cells. Upregulation of legumain was also observed in vivo, evidenced by increased legumain mRNA in the liver and spleen, as well as increased legumain activity in kidneys from wild-type mice treated with 25(OH)D3 (50 µg/kg, subcutaneously) for 8 days compared to a control. In addition, the serum level of legumain was also increased. We further showed that active legumain cleaved purified VDBP (55 kDa) in vitro, forming a 45 kDa fragment. In vivo, no VDBP cleavage was found in kidneys or liver from legumain-deficient mice (Lgmn−/−), whereas VDBP was cleaved in wild-type control mice (Lgmn+/+). Finally, legumain deficiency resulted in increased plasma levels of 25(OH)D3 and total VD3 and altered expression of key renal enzymes involved in VD3 metabolism (CYP24A1 and CYP27B1). In conclusion, a regulatory interplay between VD3 and legumain is suggested.
Keywords: asparaginyl endopeptidase, legumain, metabolism, proteolysis, vitamin D
1. Introduction
Legumain is a cysteine endopeptidase with strict specificity for hydrolysis of peptide bonds C-terminally of asparagine residues [1], hence the synonym asparaginyl endopeptidase (AEP). Although mainly lysosomal, legumain has also been shown to be secreted and detectable in plasma. In addition, legumain is postulated to have autocrine/paracrine functions (reviewed in [2]). Legumain is highly expressed in the kidneys, liver, and spleen [3] and is described as being involved in the pathogenesis of several disorders, such as cardiovascular diseases (reviewed in [2]). We have previously shown that legumain expression is altered in the bone microenvironment of patients with osteoporosis [4]. An increasing number of proteins have been identified as substrates of legumain (reviewed in [2]). However, despite the increased knowledge of legumain substrates, mechanisms of action, and roles in the pathogenesis of different malignant and non-malignant diseases, the upstream mechanisms regulating legumain expression are not well understood.
Vitamin D3 (VD3) is a hormone involved in different biological processes such as calcium homeostasis, immune regulation, as well as cell growth and differentiation [5]. VD3 is primarily synthesized in the skin upon exposure to sunlight but can also be obtained from dietary sources. VD3 is then transported to the liver, where it undergoes hydroxylation by the vitamin D 25-hydroxylase (CYP2R1), resulting in the formation of 25-hydroxyvitamin D3 (25(OH)D3; calcidiol), which is the most abundant circulating form of VD3. In the kidneys, 25(OH)D3 undergoes further hydroxylation by 1α-hydroxylase (CYP27B1), generating the active form of VD3, i.e., 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3; calcitriol) [6,7]. In addition, both 25(OH)D3 and 1,25(OH)2D3 can be catabolized through 24-hydroxylation by CYP24A1 to form the inactive 24,25(OH)2D3 and 1,24,25(OH)3D3 metabolites, respectively [8]. Furthermore, extra-renal expression of both CYP27B1 [9,10,11] and CYP24A1 [12,13,14] have been demonstrated, indicating a paracrine role of VD3 metabolites in some tissues (reviewed in [15]). The active 1,25(OH)2D3 metabolite exerts its biological effects through interaction with the nuclear vitamin D receptor (VDR) [16], which, upon ligand binding, heterodimerizes with the retinoid X receptor and translocates to the nucleus to alter transcription of target genes through binding to specific DNA sequences known as vitamin D-responsive elements (VDRE) [17,18].
VD3 is a fat-soluble molecule and is hence transported in the bloodstream bound to carrier proteins, mainly vitamin D binding protein (VDBP). VDBP is synthesized in the liver and secreted to the circulation, where it binds and transports the majority of VD3 metabolites [19,20]. VDBP is partially filtered in the glomerulus and subsequently reabsorbed in the proximal tubuli through megalin/cubilin-mediated internalization [21]. After reabsorption, VDBP is proteolytically cleaved in the endolysosomal compartments, and VD3 is released for hydroxylation. Legumain has been shown to cleave VDBP in incubates with purified bovine legumain [22], although this has not been corroborated in vivo.
Both VD3 and legumain regulate bone homeostasis; thus, we aimed to investigate potential interactions between VD3 and legumain. Using in vitro and in vivo studies, we showed that VD3 is an upstream regulator of legumain expression and that the presence or absence of legumain alters the processing of VDBP, as well as metabolism and the circulating levels of VD3.
2. Materials and Methods
2.1. Chemicals and Reagents
Bovine serum albumin, CHAPS (3-((3-cholamidopropyl) dimethylammonium)-1-propanesulfonate), citric acid, DAPI, dexamethasone, 1,25(OH)2D3, 25(OH)D3 (for in vitro experiments), VDBP (Gc-globulin) from human plasma (Catalog # G8764), DTT (dithiothreitol), β-glycerophosphate, L-ascorbic acid, Na2HPO4, Na2EDTA, TRI Reagent®, Tween® 20, and p-nitrophenyl phosphate were purchased from Sigma-Aldrich, St. Louis, MO, USA. Ethanol (96%—rectified) was purchased from Antibac, Asker, Norway. For in vivo treatment with 25(OH)D3, D3-Vicotrat® (Hilden, Germany) was used. The Z-Ala-Ala-Asn-AMC peptide substrate was purchased from Bachem, Bubendorf, Switzerland. Chameleon® Duo Protein Ladder, donkey anti-goat IR Dye 680LT and 800CW, donkey anti-mouse IR dye 800CW, donkey anti-rabbit IR dye 680LT and 800CW, and Odyssey® Blocking Buffer were obtained from LI-COR, Cambridge, UK. High-Capacity cDNA Reverse Transcription Kit (Catalog # 4368814), Minimal Essential Media (MEM), and Power SYBR™ Green PCR Master Mix (Catalog # 4367659) were obtained from Thermo Fischer Scientific, Waltham, MA, USA. RNeasy® Plus Mini Kit and Buffer RLT Plus were purchased from QIAGEN, Hilden, Germany. Trans-Blot® Turbo™ Mini-size nitrocellulose membrane and Trans-Blot® Turbo™ Transfer System were purchased from Bio-Rad, Copenhagen, Denmark. RNeasy® Plus Mini Kit was purchased from QIAGEN, Hilden, Germany. GentleMACS™ M Tubes were purchased from Miltenyi Biotec, Bergisch Gladbach, Germany. EconoSpin® spin columns were purchased from Epoch Life Science, Missouri City, TX, USA. Primers for mouse legumain, VDBP, CYP27B1, CYP24A1, RPLP0, GAPDH, and β-actin were purchased from Ebersberg, Germany. Bovine mature active legumain (36 kDa) was acquired as previously described [23].
2.2. Identification of Putative Vitamin D-Responsive Elements in the LGMN Promoter Region
The nucleotide sequence cut-off of the human legumain (LGMN) gene promoter (accession no.: NM_005606) was set to 1485 base pairs downstream and 15 base pairs upstream of the transcription start site. The nucleotide sequence was retrieved using the Sequence Retrieval Tool in the Eukaryotic Promoter Database (https://epd.epfl.ch (accessed on 20 September 2022)). Putative vitamin D-responsive elements (VDRE) were identified using the PROMO database [24,25] with the maximum matrix dissimilarity rate set to 15.
2.3. Cell Culturing
For cell culture experiments, human bone marrow-derived mesenchymal stromal cells stably overexpressing the catalytic subunit of human telomerase (hBMSC-TERT cell line, RRID:CVCL_Z017; further referred to as hBMSC) were used [4]. The cells were grown in a basal medium containing Minimal Essential Media (MEM) with L-glutamine, 10% (v/v) foetal bovine serum, 1% penicillin (100 U/mL), and streptomycin (100 µg/mL). The cells were seeded at a density of 20,000 cells/cm2, and at 80% confluence, the cells were differentiated using osteoblastic induction medium containing basal medium supplemented with 10 mM β-glycerophosphate, 50 µg/mL L-ascorbic acid, 10 nM dexamethasone [4], and 1,25-dihydroxyvitamin D3 (0–100 nM) or 25(OH)D3 (0–1000 nM) in ethanol solution (for controls, an equivalent volume of ethanol was used) for seven days. The induction medium was renewed every three or four days. Monoclonal legumain over-expressing HEK293 (M38L) and HEK293 (human embryonic kidney 293; RRID:CVCL_0045) cells were cultured as previously described [26]. In brief, the cells were seeded at a density of 5 × 106 cells/75 cm2 flask and cultured in Dulbecco′s Modified Eagle′s Medium with 10% (v/v) foetal bovine serum. G418 (800 μg/mL) was added to the culture medium of M38L cells.
2.4. Harvesting of Cell-conditioned Media and Lysates
Cell-conditioned media were collected and centrifuged at 800 rpm for 5 min at 4 °C, and the supernatants were frozen at −20 °C. Adherent cells were washed with PBS before harvesting in legumain lysis buffer (100 mM sodium citrate, 1 mM disodium-EDTA, 1% n-octyl-β-D-glucopyranoside, pH 5.8) for quantitation of legumain activity or Buffer RLT Plus for mRNA isolation. Cell lysates harvested in legumain lysis buffer were frozen (−70 °C) and thawed (30 °C) three times before centrifugation at 10,000× g for 5 min, and the supernatants were frozen at −20 °C or directly analyzed. Total protein concentrations in cell lysates were determined by measuring absorbance at 595 nm in a microplate reader (Wallac Victor® 3™ or Wallac Victor® Nivo™, Perkin Elmer, Boston, MA, USA) according to Bradford [27] and the manufacturer. Bovine serum albumin (0–400 µg/mL) was used to generate a standard curve for the calculation of total protein concentrations. All measurements were performed in triplicates.
2.5. Legumain-Deficient Mice
Legumain-deficient (Lgmn−/−) mice were produced using CRISPR/Cas9 gene targeting in C57BL/6J mouse embryos following established molecular and animal husbandry techniques [28]. A single guide RNA (sgRNA) was designed to target within exon 1 of Lgmn (target with protospacer-associated motif underlined GGATGGAGGCAAGCACTGGGTGG) and co-injected with polyadenylated Cas9 mRNA into C57BL/6J zygotes. Microinjected embryos were cultured overnight and introduced into pseudo-pregnant foster mothers. Pups were screened by PCR and Sanger sequencing of ear-punch DNA and a founder mouse was identified that carried a 10 bp frame-shift deletion in exon 1. The targeted allele was maintained by breeding on a C57BL/6J background.
2.6. Treatment of Mice with 25(OH)D3 and Tissue Harvesting
Twelve-week-old female legumain wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) mice were bred and housed under standard conditions (21 °C, 55% relative humidity) on a 12 h light/dark cycle. The mice were injected subcutaneously (s.c.) on day 0, 2, 4, and 7 with 50 µg/kg 25(OH)D3 (Vicotrat®) in 5% DMSO and 95% saline (n = 7) or an equal volume of vehicle (5% DMSO and 95% saline control, n = 7)). After the final injection, the mice were fasted overnight and anesthetized before blood was collected by retro-orbital bleeding and plasma was obtained after centrifugation and frozen at −80 °C. Subsequently, the mice were euthanized, and kidneys, liver, and spleen were collected, snap-frozen in liquid nitrogen, and stored at −80 °C. Mice experiments were carried out in accordance with permissions issued by the Danish Animal Experiments Inspectorate (2022-15-0201-01225). Tissue samples were homogenized in gentleMACS™ M Tubes (Miltenyi Biotec) using a gentleMACS™ Octo Dissociator (Miltenyi Biotec) in either TRI Reagent® (Sigma) or lysis buffer for subsequent mRNA isolation or protein analysis, respectively.
2.7. Legumain Activity Measurement
Cleavage of the peptide substrate Z-Ala-Ala-Asn-AMC was used to measure the proteolytic activity of legumain, as previously described [29]. In brief, 20 µL of cell lysates or tissue homogenates, 100 µL assay buffer (39.5 mM citric acid, 121 mM Na2HPO4, 1 mM Na2EDTA, pH 5.8, 1 mM DTT, and 0.1% CHAPS) and 50 µL peptide substrate solution (final concentration 10 µM) were added in black 96-well microtiter plates (Corning Life Science, Lowell, MA, USA). Kinetic measurements based on the increase in fluorescence (360EX/460EM) for 10 or 60 min were performed at 30 °C in a microplate reader (Wallac Victor® 3™ or Wallac Victor® Nivo™ (Perkin Elmer)).
2.8. Immunoblotting and Enzyme-Linked Immunosorbent Assay (ELISA)
Gel electrophoresis and immunoblotting were performed by loading 15–20 µg of total protein using NuPAGE 4–12% gels (Life Technologies, Carlsbad, CA, USA) and NuPAGE MOPS SDS running buffer prior to transfer to a nitrocellulose membrane (Trans-Blot® Turbo™ Mini-size nitrocellulose) in the Trans-Blot® Turbo™ Transfer System for 30 min. The membranes were blocked for 1 h at room temperature with Odyssey® Blocking Buffer and probed with polyclonal goat anti-human legumain (1:200, R&D Systems, Minneapolis, MN, USA, Catalog # AF2199, RRID: AB_416565), polyclonal rabbit anti-human/mouse VDBP (1:500, Bio-Techne, Minneapolis, MN, USA, Catalog # NBP1-88027, RRID: AB_11023579), monoclonal mouse anti-human VDBP (1:500, R&D Systems, Catalog # MAB3778, RRID: AB_2232276), monoclonal mouse anti-human GAPDH antibody (1:10,000, Santa Cruz Biotechnology Inc., Dallas, TX, USA, Catalog # sc-47724, RRID: AB_627678), or monoclonal mouse anti-human GAPDH (1:10,000, R&D Systems, Catalog # MAB5718, RRID: AB_10892505) antibody in Tris-buffered saline containing 0.1% Tween 20 (T-TBS) overnight at 4 °C. Membranes were subsequently washed 3–4 times in T-TBS buffer and incubated with donkey anti-mouse 800CW (1:10,000), donkey anti-goat, or donkey anti-rabbit IR Dye 680LT (1:10,000) or 800CW (1:10,000) for 1 h at room temperature. After another washing procedure, membranes were briefly dried and analyzed using Odyssey-CLx Imaging System (LI-COR).
Total human or mouse legumain ELISA kit was used to determine concentrations of legumain in cell-conditioned media or mice plasma, respectively, according to the manufacturer’s protocol (R&D Systems, Catalog # DY4769, RRID: AB_294369 and MyBioSource, Catalog # MBS9718081, RRID: AB_2943631, respectively). Plasma VDBP concentrations were measured using a mouse VDBP ELISA kit (R&D Systems, Catalog # DY4188-05, RRID: AB_2943630).
2.9. Quantitative PCR
Total RNA was extracted from cell lysates harvested in Buffer RLT Pluss using an RNeasy® Plus Kit according to the manufacturer’s protocol or from tissue homogenates by chloroform phase separation and subsequent EconoSpin column purification (Epoch Life Science). RNA was quantified using Nanodrop™ (Thermo Scientific, Waltham, MA, USA) and stored at −80 °C until analysis. Complementary DNA (cDNA) was synthesized from 2 µg mRNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and ProFlex™ 3 × 32-well thermal cycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C until analysis. Primers (Supplementary Table S1) were designed by the Primer Express software version 1 (Applied Biosystems, Thermo Fisher Scientific). Gene expressions were examined by real-time quantitative PCR (qPCR) using Power SYBR™ Green PCR Master Mix and the Applied Biosystems StepOnePlus™ Instrument with the accompanying software StepOne™ Version 2.3 (Applied Biosystems, Thermo Fisher Scientific). Gene expression was normalised against the geometric means of the CT values of housekeeping controls (RPLP0, GAPDH, β-actin, 18s) [30].
2.10. Measurement of Total VD3 Metabolites in Mouse Plasma
Vitamin D metabolite concentrations in mouse plasma were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS), as previously described [31]. The assays were calibrated using the National Institute of Science and Technology (NIST) standard reference material SRM972a. Total 25(OH)D3 was calculated from the sum of the measurements of VD3 and VD2 forms. Inter-assay coefficient of variation (CV) was <10.0% across the assay working range of 0.1 to 200.0 nmol/L.
1,25(OH)2D3 and 1,25(OH)2D2 were analyzed using a Waters Acquity Xevo TQXS LC-MS/MS system (Waters, Wilmslow, UK) [32]. Prior to analysis, plasma samples underwent immunoaffinity pretreatment to enrich the sample load, followed by derivatisation with Cookson-type dienophilic agents DAP-TAD (4-4-dimethylaminophenyl-1,2,4-triazoline-3,5-dione). The assays were calibrated using certified pure internal standards (Cerilliant, LGC). Inter-assay coefficient of variation (CV) was < 9.8% across the assay working range of 20 to 800.0 pmol/L.
All vitamin D metabolite assays met the requirements specified by vitamin D external quality assessment (DEQAS) scheme (http://www.deqas.org/; accessed on 30 January 2023). The 25OHD3 and 25OHD2 assays showed <6% accuracy bias against the Center for Disease Control and Prevention (CDC) reference measurement (RMP) target values on the DEQAS scheme.
2.11. Statistical Analysis
The data are represented as mean ± SEM. Student t-test, Kruskal–Wallis, Mann–Whitney, simple linear regression, and one-way or two-way ANOVA were performed when appropriate. Statistical significance was considered at p ˂ 0.05. All calculations were performed with GraphPad Prism (Version 9.0; GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. 1,25(OH)2D3 Regulates Legumain Expression in Pre-Osteoblastic Cells
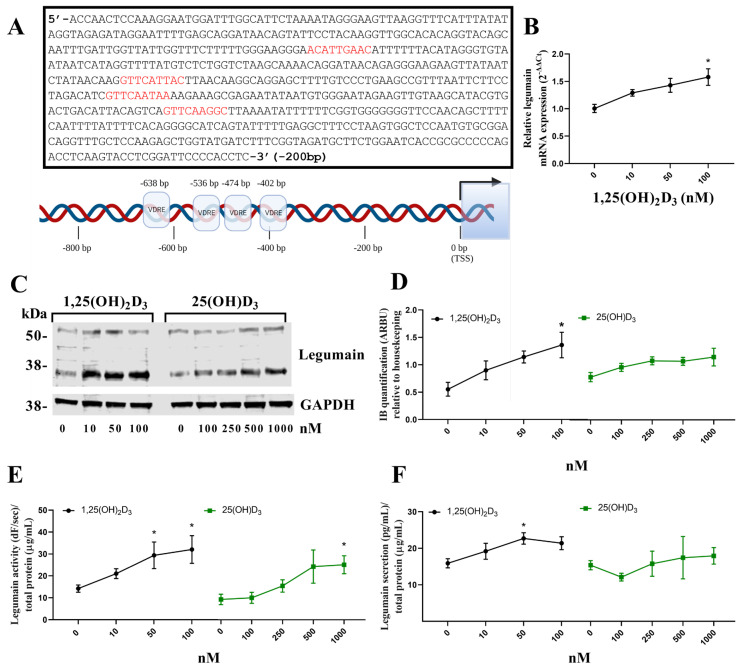
Given the role of VD3 in regulating the expression of several bone-related factors, we first aimed to investigate if VD3 could regulate the expression of the legumain encoding gene (LGMN). Analysis of the human LGMN gene promoter region using in silico analysis by the PROMO database revealed the presence of four potential vitamin D-responsive elements (VDRE) at the following nucleotide positions relative to the transcription start site: nucleotide −638 (dissimilarity (ds) = 4.62%), −536 (ds = 8.08%), −474 (ds = 8.93%), and −402 (ds = 6.93%) (Figure 1A). This suggested a possible regulation of LGMN expression by VD3. To test this hypothesis in a cell-based model, the effect of VD3 on legumain mRNA expression was investigated in osteogenic hBMSC cultures in the presence or absence of 1,25(OH)2D3 (10, 50 or 100 nM). We found a dose-dependent increase in legumain mRNA expression, reaching significance at 100 nM 1,25(OH)2D3 (Figure 1B).
Figure 1.
Vitamin D3 increases legumain expression, activity, and secretion in pre-osteoblastic cells. (A) The nucleotide sequence of the LGMN gene promoter region with annotations of potential vitamin D-responsive elements (VDRE; red) relative to the transcription start site (TSS). (B–F) Human BMSC-TERT cells (20,000 cells/cm2) were incubated with 1,25(OH)2D3 (B–F; 10, 50 or 100 nM), 25(OH)D3 (C–F; 100, 250, 500 or 1000 nM) or an equal volume of ethanol (control, 0 nM) in osteoblast induction medium for seven days before harvesting. (B) Legumain mRNA expression relative to housekeeping control (GAPDH) (2−ΔΔCT; n = 3). (C) One representative immunoblot of legumain (proform 56 kDa, mature form 36 kDa) and GAPDH (housekeeping) in cell lysates (n = 3). (D) Quantification of the 36 kDa mature legumain immunoband (IB) intensity as arbitrary units (ARBU) relative to GAPDH in immunoblots represented in C (n = 3). (E) Legumain activity (dF/s) in cell lysates adjusted for the total protein concentration (µg/mL) (n = 6–9). (F) Secreted legumain (pg/mL) in conditioned media measured by ELISA and adjusted for the total protein concentration in the corresponding cell lysates (n = 3–5). (B,D–F) Data represent mean ± SEM. (B,D) Kruskal–Wallis test. (E,F) One-way ANOVA. * p < 0.05 vs. 0 nM 1,25(OH)2D3 or 25(OH)D3. Numbers (n) represent individual biological replicates.
To further investigate the effect of VD3 on legumain expression and proteolytic activity, osteogenic hBMSC were cultured with or without 1,25(OH)2D3 (10, 50, or 100 nM) or 25(OH)D3 (100, 250, 500, or 1000 nM) for 7 days. Immunoblot analysis showed a dose-dependent tendency of increased levels of 36 kDa mature legumain in the presence of 1,25(OH)2D3, reaching significance at 100 nM 1,25(OH)2D3 (Figure 1C,D). However, the expression was not significantly affected by 25(OH)D3. The effect of VD3 on legumain function was investigated by quantifying the proteolytic activity of legumain in the lysates. Increased legumain activity was observed in cells treated with 50 or 100 nM 1,25(OH)2D3 and with 1000 nM 25(OH)D3 (Figure 1E). As legumain can also be secreted and mediate autocrine/paracrine functions, we investigated whether VD3 could alter legumain secretion. ELISA measurements of legumain in the conditioned media showed increased legumain secretion by pre-osteoblastic cells in the presence of 50 nM 1,25(OH)2D3 (Figure 1F).
3.2. 25(OH)D3 Administration Increases Legumain Expression and Activity In Vivo
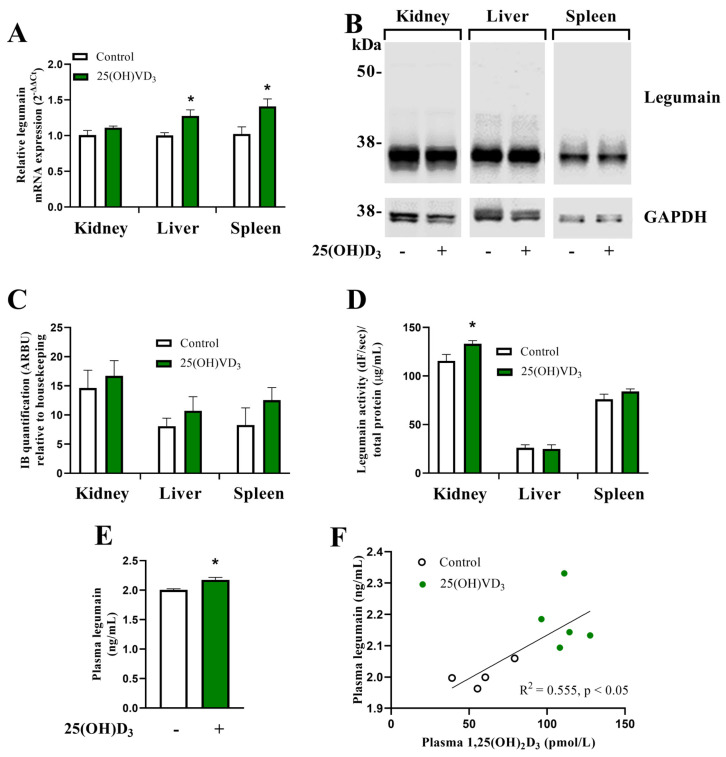
To investigate whether VD3 also regulated the levels of legumain in vivo and whether legumain expression is important for vitamin D metabolism through VDBP processing (see below), high dose 25(OH)D3 (50 μg/kg) or vehicle was subcutaneously (sc) administrated to wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) C57BL6/J mice for 8 days. Legumain deficiency in the kidneys, liver and spleen of Lgmn−/− mice was verified by immunoblotting and qPCR (Figure S1). In the wild-type mice, qRT-PCR analysis showed increased expression of legumain mRNA in the liver and spleen from the 25(OH)D3-treated compared to control mice (Figure 2A). In addition, immunoblot analysis showed a tendency towards increased level of 36 kDa mature legumain in the kidneys, liver, and spleen of 25(OH)D3-treated mice, although not statistically significant (Figure 2B,C). No prolegumain (56 kDa) was observed in these organs. Furthermore, an increased level of legumain proteolytic activity was detected in the kidneys of the 25(OH)D3-treated mice compared to control mice (Figure 2D). Importantly, ELISA measurement of legumain in the plasma revealed increased circulating legumain levels in mice treated with 25(OH)D3 versus control (Figure 2E). Plasma levels of VD3 metabolites in 25(OH)D3 and vehicle-treated mice were also measured and showed a positive correlation between the level of 1,25(OH)2D3 and circulating legumain (Figure 2F).
Figure 2.
Treatment with 25(OH)D3 increases legumain levels and activity in wild-type mice. Wild-type mice (Lgmn+/+) were treated with 50 µg/kg 25(OH)D3 (n = 7) or an equal volume vehicle (n = 7, control) subcutaneously every two to three days (four times in total). Tissues were harvested 24 h after the final injection (day 8). (A) Legumain mRNA expression relative to the geometric mean of CT values of four housekeeping controls in kidney, liver, and spleen (2−ΔΔCT; n = 5). (B) One representative immunoblot of legumain and GAPDH in kidney, liver, and spleen (n = 3). (C) Quantification of the 36 kDa mature legumain immunoband (IB) intensity as arbitrary units (ARBU) relative to GAPDH (housekeeping) in kidney, liver, and spleen from immunoblots represented in (C) (n = 3). (D) Legumain activity (dF/s) in kidney, liver, and spleen adjusted for total protein concentration (μg/mL, n = 5). (E) Legumain plasma concentration (ng/mL) measured by ELISA (n = 5). (F) Correlation between legumain (ng/mL and 1,25(OH)2D3 (pmol/L) concentrations in plasma (n = 5). (A,C,E) Two-tailed unpaired Student’s t-test. (D) Mann–Whitney test. Data represent mean ± SEM. * p < 0.05. (F) Simple linear regression. Numbers (n) represent individual biological replicates.
3.3. Legumain Cleaves VDBP In Vitro and In Vivo
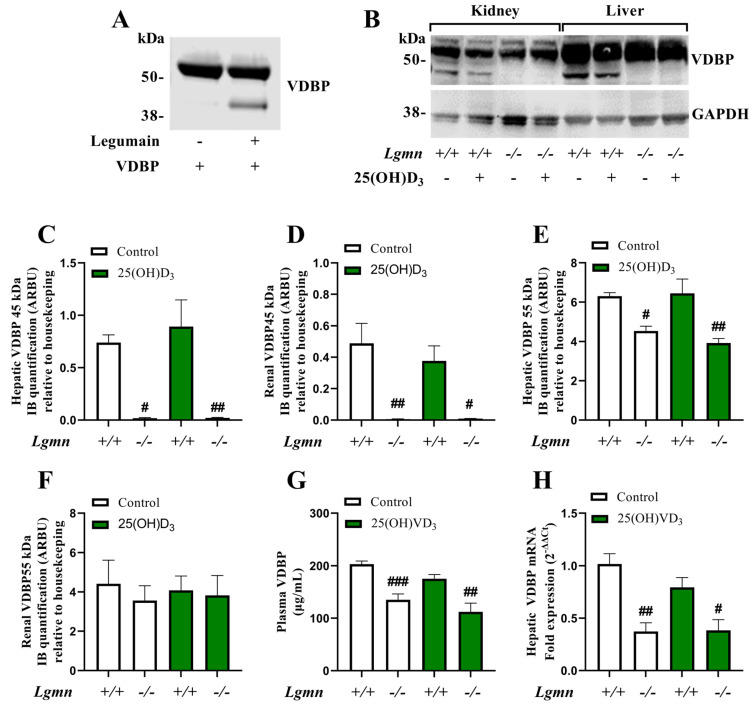
VDBP has previously been reported as a legumain substrate [22]; thus, we aimed to investigate the possible role of legumain in the regulation of VD3 metabolism. First, we examined VDBP processing by legumain using incubation of purified VDBP from human plasma with or without purified active bovine legumain, followed by immunoblot analysis. Cleavage of full-length VDBP (55 kDa) by active legumain generated a VDBP cleavage product of approximately 45 kDa, which was not observed in the absence of legumain (Figure 3A). In addition, purified VDBP was incubated with or without lysate from legumain over-expressing HEK293 (M38L) cells [26], and a similar cleavage product (~45 kDa) was detected (Figure S2).
Figure 3.
Legumain is required for VDBP processing and regulation. (A) Purified VDBP from human plasma (1.9 μM) was incubated in legumain assay buffer (pH 5.8) at 37 °C with or without purified active bovine legumain (2 μM) for 5 h before gel electrophoresis and immunoblotting of VDBP (n = 1). (B–H) Wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) mice were treated with 50 µg/kg 25(OH)D3 (n = 6–7) or an equal volume vehicle (n = 7, control) subcutaneously every two to three days (four times in total). Tissues were harvested 24 h after the final injection (day 8). (B) One representative immunoblot of VDBP and GAPDH (housekeeping) in kidney and liver (n = 4). (C–F) Quantification of VDBP immunoband (IB) intensity as arbitrary units (ARBU) relative to GAPDH in immunoblots represented in (B) (n = 4). (C) Hepatic VDBP 45 kDa immunoband. (D) Renal VDBP 45 kDa immunoband. (E) Hepatic VDBP 55 kDa immunoband. (F) Renal VDBP 55 kDa immunoband. (G) Plasma VDBP concentration (μg/mL) was measured by ELISA (n = 6–7). (H) Hepatic VDBP mRNA expression relative to the geometric mean of CT values of four housekeeping controls (2−ΔΔCT, n = 5). (C–H) Data represent mean ± SEM. Two-way ANOVA. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. different genotype, same treatment. Numbers (n) represent individual biological replicates.
To further investigate the role of legumain in VDBP processing in vivo, the abovementioned wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) mice were treated (sc) with 25(OH)D3 or vehicle for 8 days. Immunoblot analysis of VDBP in homogenates from the liver and kidney of Lgmn−/− mice did not show the generation of the 45 kDa VDBP cleavage product compared to the wild-type control (Lgmn+/+) mice (Figure 3B–D). Interestingly, significantly decreased expression of full-length VDBP was detected in the liver from Lgmn−/− compared to control mice, as observed by immunoblotting (Figure 3B,E), whereas legumain deficiency did not alter the levels of full-length VDBP in the kidneys (Figure 3B,F). No effect of 25(OH)D3 treatment on VDBP levels or its processing was observed in kidneys or liver from either Lgmn+/+ or Lgmn−/− mice (Figure 3B–F). The level of VDBP in plasma was analyzed using ELISA and showed decreased circulating VDBP levels in Lgmn−/− compared to Lgmn+/+ mice (Figure 3G). In addition, qRT-PCR analysis revealed a significantly decreased level of VDBP mRNA expression in the liver from Lgmn−/− mice (Figure 3H). We also observed a tendency towards decreased levels of VDBP in plasma and VDBP mRNA expression in the liver from 25(OH)D3-treated wild-type mice (Figure 3G and Figure 3H, respectively).
3.4. Legumain Deficiency Alters Vitamin D Metabolism In Vivo
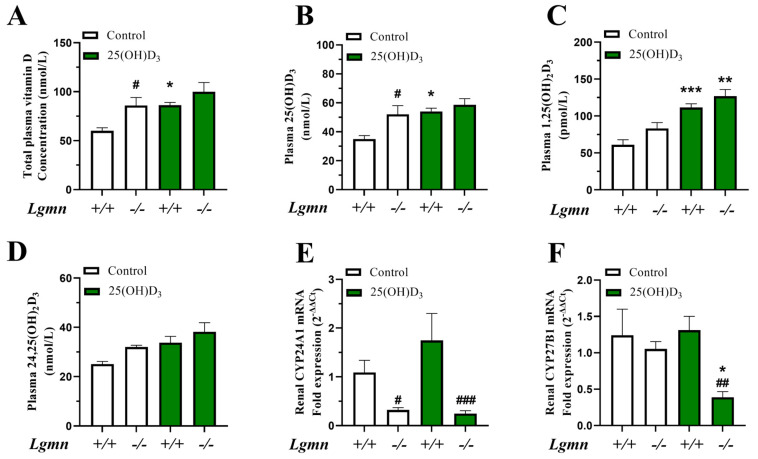
To examine VD3 metabolism in Lgmn+/+ versus Lgmn−/− mice, LC-MS/MS technology was employed to determine the circulating levels of VD3 metabolites. Interestingly, increased basal plasma levels of total VD3 and 25(OH)D3, as well as a tendency towards increased 1,25(OH)2D3 and 24,25(OH)2D3 levels, were found in Lgmn−/− compared to Lgmn+/+ control mice (Figure 4A–D). As expected, 25(OH)D3 treatment increased the plasma levels of all VD3 metabolites in Lgmn+/+ mice. In addition, 25(OH)D3 treatment significantly increased the plasma level of 1,25(OH)2D3 in Lgmn−/− mice (Figure 4C).
Figure 4.
Legumain deficiency alters plasma levels of vitamin D metabolites and induces changes in renal expression of vitamin D-metabolizing enzymes. Wild-type (Lgmn+/+) and legumain-deficient (Lgmn−/−) mice were treated with 50 µg/kg 25(OH)D3 (n = 6–7) or an equal volume vehicle (n = 7, control) subcutaneously every two to three days (four times in total). Tissues were harvested 24 h after the final injection (day 8). (A–D) Vitamin D3 metabolites in plasma were analyzed by LC-MS/MS. (A) Total plasma concentration of vitamin D3 metabolites (25(OH)D3, 1,25(OH)2D3, and 24,25(OH)2D3) (nmol/L, n = 4–5). (B) Plasma 25(OH)D3 concentration (nmol/L, n = 5). (C) Plasma 1,25(OH)2D3 concentration (pmol/L, n = 4–5). (D) Plasma 24,25(OH)2D3 concentration (nmol/L, n = 5). (E,F) Renal CYP24A1 (E) and CYP27B1 (F) mRNA expressions relative to the geometric mean of CT values of four housekeeping controls (2−ΔΔCT; n = 5). Data represent mean ± SEM. (A–D). Two-way ANOVA. (E,F) Two-way ANOVA on ΔCT values. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. same genotype, different treatment. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. different genotype, same treatment. Numbers (n) represent individual biological replicates.
To further investigate the effect of legumain deficiency on VD3 metabolism, renal mRNA expressions of the two key metabolic enzymes CYP24A1 (24-hydroxylase) and CYP27B1 (1α-hydroxylase) were analyzed. Significantly decreased CYP24A1 mRNA was detected in kidneys obtained from Lgmn−/− compared to Lgmn+/+ mice (Figure 4E). However, hepatic CYP24A1 mRNA expression increased significantly in Lgmn−/− mice in response to 25(OH)D3 treatment, an effect that was not seen in Lgmn+/+ mice (Figure S3). 25(OH)D3 treatment did not have any effect on renal expression of CYP24A1 mRNA (Figure 4E). In addition, no difference in renal expression of CYP27B1 mRNA was observed in either Lgmn−/− or Lgmn+/+ mice. However, after 25(OH)D3 administration, a significantly decreased renal level of CYP27B1 mRNA was detected in Lgmn−/− compared to Lgmn+/+ mice (Figure 4F).
4. Discussion
In the present study, VD3 was identified as an inducer of legumain expression and proteolytic activity in pre-osteoblasts and mouse tissues. In addition, the cleavage of VDBP by legumain was, for the first time, demonstrated in vivo. Interestingly, legumain deficiency resulted in transcriptional downregulation of hepatic VDBP synthesis, resulting in reduced levels of circulating VDBP. Furthermore, legumain deficiency also altered VD3 metabolism due to changes in the renal expression of key metabolic enzymes (CYP27B1 and CYP24A1), resulting in altered basal levels of VD3 metabolites, as well as in response to 25(OH)D3 treatment.
Initially, in silico studies indicated the presence of vitamin D-responsive elements (VDRE) in the promoter region of the legumain encoding gene (LGMN). Therefore, we hypothesized that VD3 could be a regulator of legumain expression. Our cell-based studies using osteogenic cultures of human BMSC showed increased mRNA expression, proteolytic activity, and secretion of legumain by pre-osteoblasts in the presence of 1,25(OH)2D3. Although the promoter of the LGMN gene contains VDRE, it is most likely that the enhancing effect of VD3 on legumain expression is mediated through an indirect mechanism, as a direct transcriptional regulation of legumain expression by VD3 would likely result in a more pronounced effect.
We also observed increased legumain activity in the presence of the VD3 metabolite 25(OH)D3. This was likely due to the conversion of 25(OH)D3 to 1,25(OH)2D3 by the pre-osteoblasts, as CYP27B1 is expressed and functional in these cells [33,34,35]. In addition, administration of 25(OH)D3 to wild-type mice increased the expression and activity of legumain in various tissues and, importantly, increased the circulating levels of legumain in the plasma. These data provide strong evidence that VD3 is an upstream regulator of legumain expression. It has previously been shown that there is minimal overlap in genes regulated by VD3 between different cell types or species [36]. Interestingly, we observed that legumain was regulated in a similar manner in human pre-osteoblastic cells and mice. However, whether the functional and physiological consequences of VD3-induced production of legumain are conserved in mice and humans is currently not known.
Identification of VD3 as an upstream regulator of legumain expression provides new insights into the role of VD3 in modulating cellular processes beyond its well-known roles in, i.e., regulation of calcium homeostasis. The ability of VD3 to regulate legumain expression suggests a possible involvement of VD3 in legumain-mediated physiological and pathological processes. In this regard, and since legumain has an inhibitory role in osteoblast maturation [4], it is possible that legumain plays a role in the inhibition of osteoblast differentiation and reduction of bone mass associated with a high dose of VD3 administration [37,38,39].
The present study provides evidence that VDBP is processed by legumain both in vitro and in vivo, corroborating a previous study presenting VDBP as a legumain substrate [22]. We observed no VDBP processing in mouse kidneys or liver upon legumain deficiency, which could possibly lead to an increased level of VDBP in the circulation. However, interestingly, significantly lower plasma levels of VDBP were observed in Lgmn−/− compared to Lgmn+/+ mice. Renal dysfunction manifested as decreased glomerular filtration rate, increased plasma creatinine, and fibrosis, and premature senescence has been demonstrated in legumain-deficient mice [40,41]. Whether the decrease in circulating VDBP levels in Lgmn−/− mice is caused or exacerbated by proteinuria is not known. However, the present data show a significant decrease in mRNA and protein expressions of VDBP in the liver upon legumain deficiency, which suggests a negative regulatory feedback loop that ensures decreased hepatic production of VDBP to counteract the systemic lack of VDBP processing by legumain. In addition, the observed increase in total VD3 metabolite concentration in conjunction with decreased VDBP levels upon legumain deficiency indicates that proteinuria is not the cause of the reduced plasma VDBP level as the absolute majority of VD3 metabolites are bound to VDBP and would be excreted along with the carrier protein [42,43]. In a normal state, the plasma VDBP level is in a substantial surplus with regard to the VD3 metabolite levels, and the binding capacity of VDBP far exceeds the level of available VD3 metabolites [44,45,46]. In addition, VD3 metabolites are also bound to albumin, although to a lesser extent. Therefore, the observed increase in total VD3 metabolite concentration seen in Lgmn−/− mice is not a contradiction to the decrease in plasma VDBP.
The mice used for in vivo experiments were kept on a regular diet (chow) with sufficient amounts of dietary VD3. Therefore, in order to provoke detectable changes in the levels of circulating VD3 metabolites, high doses of parenteral 25(OH)D3 were administered. However, the total exposure was within the range of what has previously been used in comparable experiments [47,48], and the detected levels of VD3 metabolites were well below what has been considered toxic [48]. Results in the present study showed increased plasma levels of total VD3 and 25(OH)D3 in Lgmn−/− compared to Lgmn+/+ mice, which could be explained by reduced tissue distribution of VD3 or reduced clearance due to decreased levels of VDBP upon legumain deficiency. In addition, our data indicated a tendency towards increased plasma levels of 1,25(OH)2D3 in Lgmn−/− mice, which could reflect increased total VD3 and 25(OH)D3 plasma levels upon legumain deficiency. However, the lack of major changes in the plasma levels of 1,25(OH)2D3 upon legumain deficiency indicates the presence of legumain-independent mechanisms that could play a role in the release of VD3 from VDBP in the kidneys, which is required for hydroxylation to the active 1,25(OH)2D3.
We observed a significantly decreased level of CYP24A1 mRNA expression in kidneys from Lgmn−/− mice. However, it is intriguing that the plasma level of 24,25(OH)2D3 did not decrease in these mice. As 24,25(OH)2D3 is generated by CYP24A1-mediated hydroxylation of 25(OH)D3, the lack of change in the plasma levels of 24,25(OH)2D3 in Lgmn−/− mice could be due to the increased plasma levels of 25(OH)D3 upon legumain deficiency, together with CYP24A1-mediated hydroxylation of 25(OH)D3 in extra-renal vitamin D-targeted tissues. This notion is supported by studies indicating that extra-renal CYP enzymes are involved in the regulation of VD3 metabolism [49,50,51] and the increase in hepatic CYP24A1 mRNA expression in 25(OH)D3-treated Lgmn−/− mice. It has recently been shown that extra-renal CYP24A1 ameliorates severe hypercalcemia in mice with kidney-specific CYP24A1 ablation [52].
CYP27B1 is the key enzyme involved in the hydroxylation of 25(OH)D3 and the production of active 1,25(OH)2D3. Expression of CYP27B1 mRNA in kidneys of Lgmn−/− mice was significantly decreased upon 25(OH)D3 administration. Taking into account the increased plasma levels of total VD3 and 25(OH)D3 in Lgmn−/− mice, together with significantly decreased expression of the VD3 catabolizing enzyme CYP24A1 upon legumain deficiency, decreased renal expression of CYP27B1 mRNA in Lgmn−/− mice could be a feedback mechanism to avoid high levels of 1,25(OH)2D3 production and its associated side effects such as hypercalcemia [53]. This is in line with a previous study indicating decreased renal expression of CYP27B1 in mice that are unable to catabolize VD3 due to CYP24A1 deficiency [53].
5. Conclusions
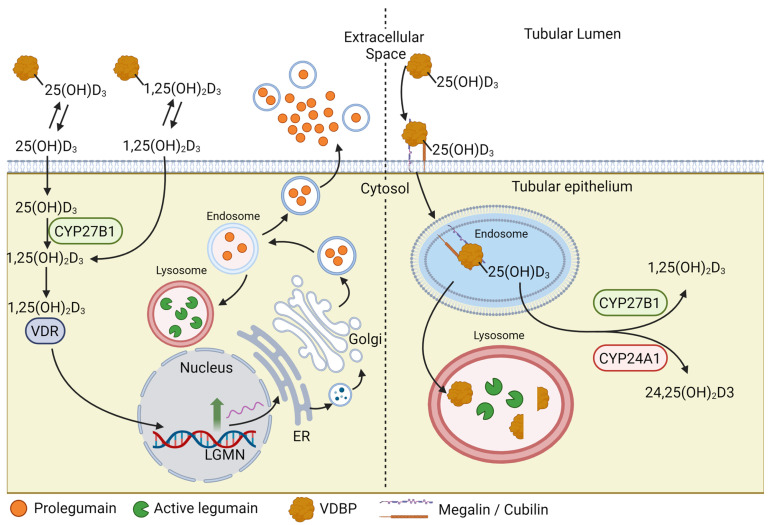
Overall, the present work revealed the role of VD3 as an upstream enhancer of legumain expression both in vitro and in vivo and that legumain plays a role in the regulation of VD3 metabolism. This suggests a potential feedback loop where legumain activity can modulate the bioavailability of VD3 and its metabolites and possibly its downstream physiological processes (Figure 5). These findings provide insight into the intricate relationship between VD3 and legumain and can possibly open new avenues for research and investigation of novel therapeutic opportunities in various diseases in which VD3 and legumain play crucial roles.
Figure 5.
Graphical representation of the suggested interplay between vitamin D and legumain. Left panel: Vitamin D (VD3) promotes legumain expression and activity through transcriptional upregulation of the legumain gene (LGMN). The free fraction of circulating VD3 metabolites diffuse through plasma membranes. 25-hydroxyvitamin D (25(OH)D3) is hydroxylated by 1α-hydroxylase (CYP27B1), forming the active metabolite 1α,25-dihydroxyvitamin D (1,25(OH)2D3). 1,25(OH)2D3 binds to the nuclear vitamin D receptor (VDR) and promotes transcription of legumain (LGMN). Synthesized prolegumain is either sorted and activated in the endolysosomal system or released to the extracellular environment. Right panel: In the proximal tubular epithelium, 25(OH)D3 bound to vitamin D binding protein (VDBP) is internalized from the tubular lumen through a megalin/cubilin-mediated process. The vitamin D metabolite is released, enabling subsequent hydroxylation by 1α-hydroxylase (CYP27B1) or 24-hydroxylase (CYP24A1), and VDBP is cleaved by legumain in the endolysosomal system. VDBP cleavage by legumain might be important in controlling the systemic level of vitamin D metabolites. Created with BioRender.com (accessed on 11 December 2023).
Acknowledgments
Hilde Nilsen is highly acknowledged for the technical assistance and the OPEN Lab, Odense University Hospital (OUH), for performing VDBP ELISA (financially supported by the Research Council at OUH). R.S. is a member of the COST action CA20113 ProteoCure (A sound proteome for a sound body: targeting proteolysis for proteome remodeling).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cells13010036/s1.
Author Contributions
Conceptualization, A.J., H.T.J., K.M.F., M.K., N.N.L., R.S. and T.B.-O.; Methodology, A.J., D.H., G.A., H.T.J., J.C.Y.T., K.M.F., M.K., M.O., N.N.L., R.S. and T.B.-O.; Validation, A.J., H.T.J., J.C.Y.T., K.M.F., M.K., N.N.L. and R.S.; Formal Analysis, A.J., H.T.J., K.M.F., M.K., M.O., N.N.L. and R.S.; Investigation, G.A., J.C.Y.T., K.M.F., M.O., N.N.L. and T.B.-O.; Resources, A.J., D.H., H.T.J., M.K. and R.S.; Data Curation, K.M.F., N.N.L. and T.B.-O.; Writing—Original Draft Preparation, K.M.F.; Writing—Review and Editing, all co-authors; Visualization, K.M.F.; Supervision, A.J., M.K., M.O. N.N.L. and R.S.; Project Administration, A.J. and R.S.; Funding Acquisition, A.J., D.H., H.T.J., M.K. and R.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Animal experiments were performed with approval issued by the Danish Animal Experiments Inspectorate at the Ministry of Food, Agriculture and Fisheries of Denmark (approval number: 2022-15-0201-01225) and in accordance with local animal welfare laws and regulations. All technicians were FELASA-accredited.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Olav Thon Foundation and the University of Oslo, Norway; University of Copenhagen, Odense University Hospital, and University of Southern Denmark, Denmark; Garvan Institute of Medical Research and St. Vincent’s Clinical School, Sydney, Australia; Gerda og Aage Haenschs Fond, Direktør Michael Hermann Nielsens mindelegat, Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis’ Legat; and The Norwegian Pharmaceutical Society.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen J.-M., Dando P.M., Rawlings N.D., Brown M.A., Young N.E., Stevens R.A., Hewitt E., Watts C., Barrett A.J. Cloning, Isolation, and Characterization of Mammalian Legumain, an Asparaginyl Endopeptidase. J. Biol. Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- 2.Solberg R., Lunde N.N., Forbord K.M., Okla M., Kassem M., Jafari A. The Mammalian Cysteine Protease Legumain in Health and Disease. Int. J. Mol. Sci. 2022;23:15983. doi: 10.3390/ijms232415983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J.-M., Dando P.M., Stevens R.A.E., Fortunato M., Barrett A.J. Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 1998;335:111–117. doi: 10.1042/bj3350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafari A., Qanie D., Andersen T.L., Zhang Y., Chen L., Postert B., Parsons S., Ditzel N., Khosla S., Johansen H.T., et al. Legumain Regulates Differentiation Fate of Human Bone Marrow Stromal Cells and Is Altered in Postmenopausal Osteoporosis. Stem Cell Rep. 2017;8:373–386. doi: 10.1016/j.stemcr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLuca H.F. Vitamin D: The vitamin and the hormone. Fed. Proc. 1974;33:2211–2219. [PubMed] [Google Scholar]
- 7.Zehnder D., Bland R., Walker E.A., Bradwell A.R., Howie A.J., Hewison M., Stewart P.M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J. Am. Soc. Nephrol. 1999;10:2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]
- 8.Jones G., Prosser D.E., Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Bikle D.D., Nemanic M.K., Whitney J.O., Elias P.W. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 10.Turner R.T., Puzas J.E., Forte M.D., Lester G.E., Gray T.K., Howard G.A., Baylink D.J. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc. Natl. Acad. Sci. USA. 1980;77:5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollis B.W. 25-Hydroxyvitamin D3-1 alpha-hydroxylase in porcine hepatic tissue: Subcellular localization to both mitochondria and microsomes. Proc. Natl. Acad. Sci. USA. 1990;87:6009–6013. doi: 10.1073/pnas.87.16.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer M.B., Lee S.M., Carlson A.H., Benkusky N.A., Kaufmann M., Jones G., Pike J.W. A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues. J. Biol. Chem. 2019;294:14467–14481. doi: 10.1074/jbc.RA119.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechner D., Kállay E., Cross H.S. 1α,25-dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol. Cell. Endocrinol. 2007;263:55–64. doi: 10.1016/j.mce.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.El-Boshy M., BaSalamah M.A., Ahmad J., Idris S., Mahbub A., Abdelghany A.H., Almaimani R.A., Almasmoum H., Ghaith M.M., Elzubier M., et al. Vitamin D protects against oxidative stress, inflammation and hepatorenal damage induced by acute paracetamol toxicity in rat. Free Radic. Biol. Med. 2019;141:310–321. doi: 10.1016/j.freeradbiomed.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Morris H.A., Anderson P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010;31:129–138. [PMC free article] [PubMed] [Google Scholar]
- 16.Brumbaugh P.F., Haussler M.R. 1α,25-Dihydroxycholecalciferol Receptors in Intestine. J. Biol. Chem. 1974;249:1258–1262. doi: 10.1016/S0021-9258(19)42969-4. [DOI] [PubMed] [Google Scholar]
- 17.Liao J., Ozono K., Sone T., McDonnell D.P., Pike J.W. Vitamin D Receptor Interaction with Specific DNA Requires a Nuclear Protein and 1,25-Dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1990;87:9751–9755. doi: 10.1073/pnas.87.24.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sone T., Kerner S., Pike J.W. Vitamin D receptor interaction with specific DNA. Association as a 1,25-dihydroxyvitamin D3-modulated heterodimer. J. Biol. Chem. 1991;266:23296–23305. doi: 10.1016/S0021-9258(18)54496-3. [DOI] [PubMed] [Google Scholar]
- 19.Bikle D.D., Siiteri P.K., Ryzen E., Haddad J.G., Gee E. Serum protein binding of 1,25-dihydroxyvitamin D: A reevaluation by direct measurement of free metabolite levels. J. Clin. Endocrinol. Metab. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 20.Cooke N.E., McLeod J.F., Wang X.K., Ray K. Vitamin D binding protein: Genomic structure, functional domains, and mRNA expression in tissues. J. Steroid Biochem. Mol. Biol. 1991;40:787–793. doi: 10.1016/0960-0760(91)90304-N. [DOI] [PubMed] [Google Scholar]
- 21.Nykjaer A., Dragun D., Walther D., Vorum H., Jacobsen C., Herz J., Melsen F., Christensen E.I., Willnow T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/S0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 22.Yamane T., Takeuchi K., Yamamoto Y., Li Y.H., Fujiwara M., Nishi K., Takahashi S., Ohkubo I. Legumain from bovine kidney: Its purification, molecular cloning, immunohistochemical localization and degradation of annexin II and vitamin D-binding protein. Biochim. Biophys. Acta. 2002;1596:108–120. doi: 10.1016/S0167-4838(02)00209-1. [DOI] [PubMed] [Google Scholar]
- 23.Lunde N.N., Haugen M.H., Bodin Larsen K.B., Damgaard I., Pettersen S.J., Kasem R., Rut W., Drag M., Poreba M., Johansen H.T., et al. Glycosylation is important for legumain localization and processing to active forms but not for cystatin E/M inhibitory functions. Biochimie. 2017;139:27–37. doi: 10.1016/j.biochi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Messeguer X., Escudero R., Farré D., Núñez O., Martínez J., Albà M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 25.Farré D., Roset R., Huerta M., Adsuara J.E., Roselló L., Albà M.M., Messeguer X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3653. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith R., Johansen H.T., Nilsen H., Haugen M.H., Pettersen S.J., Mælandsmo G.M., Abrahamson M., Solberg R. Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie. 2012;94:2590–2599. doi: 10.1016/j.biochi.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Yang H., Wang H., Jaenisch R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc. 2014;9:1956–1968. doi: 10.1038/nprot.2014.134. [DOI] [PubMed] [Google Scholar]
- 29.Johansen H.T., Knight C.G., Barrett A.J. Colorimetric and fluorimetric microplate assays for legumain and a staining reaction for detection of the enzyme after electrophoresis. Anal. Biochem. 1999;273:278–283. doi: 10.1006/abio.1999.4221. [DOI] [PubMed] [Google Scholar]
- 30.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang J.C.Y., Nicholls H., Piec I., Washbourne C.J., Dutton J.J., Jackson S., Greeves J., Fraser W.D. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J. Nutr. Biochem. 2017;46:21–29. doi: 10.1016/j.jnutbio.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Tang J. Ph.D. Thesis. University of East Anglia; Norwich, UK: 2019. Methods for the Measurement of Vitamin D Metabolites and Studies on their Relationships in Health and Disease. [Google Scholar]
- 33.Van Driel M., Koedam M., Buurman C.J., Hewison M., Chiba H., Uitterlinden A.G., Pols H.A., van Leeuwen J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 34.Kidd P.M. Vitamins D and K as pleiotropic nutrients: Clinical importance to the skeletal and cardiovascular systems and preliminary evidence for synergy. Altern. Med. Rev. 2010;15:199–222. [PubMed] [Google Scholar]
- 35.Van der Meijden K., van Essen H.W., Bloemers F.W., Schulten E.A., Lips P., Bravenboer N. Regulation of CYP27B1 mRNA Expression in Primary Human Osteoblasts. Calcif. Tissue Int. 2016;99:164–173. doi: 10.1007/s00223-016-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimitrov V., Barbier C., Ismailova A., Wang Y., Dmowski K., Salehi-Tabar R., Memari B., Groulx-Boivin E., White J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology. 2021;162:bqaa218. doi: 10.1210/endocr/bqaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi M., Weitzmann M.N. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int. J. Mol. Med. 2012;29:934–938. doi: 10.3892/ijmm.2012.900. [DOI] [PubMed] [Google Scholar]
- 38.Han X., Zhu N., Wang Y., Cheng G. 1,25(OH)2D3 inhibits osteogenic differentiation through activating β-catenin signaling via downregulating bone morphogenetic protein 2. Mol. Med. Rep. 2020;22:5023–5032. doi: 10.3892/mmr.2020.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J., Sun B., Wang W., Han X., Liu H., Du J., Feng W., Liu B., Amizuka N., Li M. Histochemical examination of the effects of high-dose 1,25(OH)2D3 on bone remodeling in young growing rats. J. Mol. Histol. 2016;47:389–399. doi: 10.1007/s10735-016-9681-4. [DOI] [PubMed] [Google Scholar]
- 40.Miller G., Matthews S.P., Reinheckel T., Fleming S., Watts C. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J. 2011;25:1606–1617. doi: 10.1096/fj.10-172312. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Kang L., Chen C., Guo J., Du L., Zhou D., Li G., Zhang Y., Mi X., Zhang M., et al. Loss of legumain induces premature senescence and mediates aging-related renal fibrosis. Aging Cell. 2022;21:e13574. doi: 10.1111/acel.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khamiseh G., Vaziri N.D., Oveisi F., Ahmadnia M.R., Ahmadnia L. Vitamin D absorption, plasma concentration and urinary excretion of 25-hydroxyvitamin D in nephrotic syndrome. Proc. Soc. Exp. Biol. Med. 1991;196:210–213. doi: 10.3181/00379727-196-43181. [DOI] [PubMed] [Google Scholar]
- 43.Miller M.S., Rudinsky A.J., Klamer B.G., Chew D.J., Parker V.J. Association between vitamin D metabolites, vitamin D binding protein, and proteinuria in dogs. J. Vet. Intern. Med. 2020;34:2468–2477. doi: 10.1111/jvim.15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddad J.G., Jr. Transport of vitamin D metabolites. Clin. Orthop. Relat. Res. 1979:249–261. doi: 10.1097/00003086-197907000-00040. [DOI] [PubMed] [Google Scholar]
- 45.Bouillon R., Vandoren G., Van Baelen H., De Moor P. Immunochemical measurement of the vitamin D-binding protein in rat serum. Endocrinology. 1978;102:1710–1715. doi: 10.1210/endo-102-6-1710. [DOI] [PubMed] [Google Scholar]
- 46.Safadi F.F., Thornton P., Magiera H., Hollis B.W., Gentile M., Haddad J.G., Liebhaber S.A., Cooke N.E. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Investig. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanhooke J.L., Prahl J.M., Kimmel-Jehan C., Mendelsohn M., Danielson E.W., Healy K.D., DeLuca H.F. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc. Natl. Acad. Sci. USA. 2006;103:75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusunoki Y., Matsui I., Hamano T., Shimomura A., Mori D., Yonemoto S., Takabatake Y., Tsubakihara Y., St-Arnaud R., Isaka Y., et al. Excess 25-hydroxyvitamin D3 exacerbates tubulointerstitial injury in mice by modulating macrophage phenotype. Kidney Int. 2015;88:1013–1029. doi: 10.1038/ki.2015.210. [DOI] [PubMed] [Google Scholar]
- 49.Theodoropoulos C., Demers C., Petit J.L., Gascon-Barre M. High sensitivity of rat hepatic vitamin D3-25 hydroxylase CYP27A to 1,25-dihydroxyvitamin D3 administration. Am. J. Physiol. Endocrinol. Metab. 2003;284:E138–E147. doi: 10.1152/ajpendo.00303.2002. [DOI] [PubMed] [Google Scholar]
- 50.Gascon-Barré M., Demers C., Mirshahi A., Néron S., Zalzal S., Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034–1042. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- 51.Adams J.S., Rafison B., Witzel S., Reyes R.E., Shieh A., Chun R., Zavala K., Hewison M., Liu P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014;144 Pt A:22–27. doi: 10.1016/j.jsbmb.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi M., Grabner A., Wolf M. Importance of Extra-Renal CYP24A1 Expression for Maintaining Mineral Homeostasis. J. Endocr. Soc. 2021;5((Suppl. 1)):A234. doi: 10.1210/jendso/bvab048.476. [DOI] [Google Scholar]
- 53.Masuda S., Byford V., Arabian A., Sakai Y., Demay M.B., St-Arnaud R., Jones G. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–834. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.