Abstract
Background and Aims:
Post-reperfusion syndrome (PRS) is a serious haemodynamic event during liver transplantation (LT), which increases early graft dysfunction and mortality. This study aimed to test the efficacy and safety of norepinephrine (NE) boluses to prevent PRS during orthotopic LT.
Methods:
This feasibility phase II trial prospectively recruited a single arm of 40 patients undergoing living donor LT. The intervention was an escalated protocol of NE boluses starting at 20 µg. The primary outcome was the incidence of PRS. The secondary outcomes were arrhythmia, electrocardiographic (EKG) ischaemic changes, mean pulmonary pressure after reperfusion, 3-month survival and 1-year survival.
Results:
PRS occurred in 28 (70%) cases [95% confidence interval (CI) 54% to 83%, P < 0.001], with a relative risk reduction of 0.22 when compared to our previous results (90%). Twelve cases developed transient EKG ischaemic changes. All EKG ischaemic changes returned to baseline after correction of hypotension. There was no significant arrhythmia or bradycardia (95% CI 0 to 0.9). After reperfusion, the mean pulmonary artery pressure was not significantly higher than the normal limit (20 mmHg) (P = 0.88). The 3-month survival was 0.95 (95% CI 0.83 to 0.99), and the 1-year survival was 0.93 (95% CI 0.8 to 0.98).
Conclusion:
Our findings suggest that NE boluses starting with 20 μg is feasible and effective in lowering the risk of PRS during living donor LT. Additionally, NE boluses were not associated with significant myocardial ischaemic events, arrhythmia or a rise in pulmonary pressure.
Keywords: Feasibility studies, liver transplantation, living donor, norepinephrine, reperfusion injury
INTRODUCTION
Liver transplantation (LT) has become the standard treatment for most cases of advanced liver disease.[1] Post-reperfusion syndrome (PRS) increases the risk of graft dysfunction and mortality.[2,3] Despite the versatile approaches, the incidence of PRS is still variable and unacceptably high.[4]
Intravenous (IV) infusion is the standard method of norepinephrine (NE) delivery. However, PRS, despite its usual time-limited nature, is an acute severe event; the acute titration rate of a NE infusion is neither precisely measured nor standardised; other bolus vasopressors have limitations, among which are reduced potency (ephedrine), tachycardia (epinephrine) and bradycardia (phenylephrine); NE infusions usually require additional vasoactive drug boluses to control PRS. Some authors reported IV 8–32 µg NE boluses after a case of severe PRS.[5] NE IV boluses (maximum 12 µg) were investigated during caesarean delivery to prevent post-spinal hypotension.[6]
In this study, we hypothesised that escalating IV NE boluses starting at 20 µg would prevent PRS during living donor LT (LDLT) without significant adverse effects.
METHODS
We conducted this prospective, open-label, single-arm trial between December 2018 and July 2019 in accordance with the standards of the Helsinki Declaration, 2013. The institutional review board (IRB) approved the study (vide approval number R.18.11.323 dated 21 November 2018). The study was registered at clinicaltrial.gov (No. NCT03773276, dated 3 December 2018). All patients gave their written informed consent during the preoperative visits and consented to using the data for educational, research and publication purposes.
We included 40 consecutive recipients of either gender aged 18–65 years who underwent LDLT with a right lobe graft and an estimated graft weight-to-recipient weight ratio of 0.8–1.4. We excluded patients with acute fulminant hepatitis, hepato-renal syndrome, hepato-pulmonary syndrome, chronic hypertension, long-standing diabetes mellitus (>10 years), moderate- to-severe valvular heart disease, preoperative serum creatinine (S. Cr) >1.4 mg/kg, dialysis within 3 months before surgery, re-transplantation or massive blood transfusion (more than five red blood cell [RBC] units) before portal clamping.
Besides the standard monitoring, we used invasive arterial blood pressure monitoring and pulmonary artery catheters with continuous cardiac output (CCO) monitoring (CCO/mixed venous oxygen saturation [SvO2], Edwards Life Science, Irvine, CA, USA). We aimed to keep the intraoperative mean arterial blood pressure (MAP) ≥65 mmHg using IV NE infusions, if needed, after appropriate fluid replacement (pulse pressure variation <10% using Carescape B850; GE Healthcare, USA). The intraoperative fluid infusions used were Ringer's acetate as crystalloid maintenance, albumin 4% as a colloid replacement and RBCs [to keep haemoglobin (Hb) ≥7 g/dl]. In recipients, the explanted liver was extracted while preserving the inferior vena cava (piggyback technique). The right hepatic vein was unclamped, followed by the portal vein, and the graft preservative [histidine–tryptophan–ketoglutarate (HTK)] was washed into the systemic circulation. We started either 500 ml of 4% albumin infusion or packed RBCs (if the an-hepatic Hb <7 g/dl) with a pressure of about 250 mmHg using a 14 to 16-gauge peripheral venous cannula after portal vein unclamping (reperfusion). We used the right atrial port of the pulmonary artery catheter for injecting the NE IV boluses, flushing 5 ml of saline after each bolus. After portal vein de-clamping, we injected a 20 µg NE IV bolus when the MAP dropped by at least 10% of the basal reading. The basal reading was taken immediately before portal vein de-clamping, with as little surgical manipulation as possible.
Additional NE IV boluses were administered as follows: We withheld NE boluses if the MAP rose to 65 mmHg (the lowest target level). We injected another 20 µg NE bolus if the MAP was maintained or began to rise (but did not reach 65 mmHg) more than 10 seconds after the previous bolus. If the MAP continued to fall after 10 seconds, we increased the NE bolus dose by 10 µg every 10 seconds until it reached 100 µg (for each dose). If the MAP remained below 65 mmHg for over 1 minute, we administered the scheduled NE bolus (maximum, 100 μg) plus 10 µg adrenaline boluses. During the NE bolus administration, we did not withhold or change the rate of the NE infusion. All patients received intravenous 500 mg methylprednisolone at the start of the warm ischaemia. After hepatic artery anastomosis and unclamping, we administered 500 mg mycophenolate mofetil through the nasogastric tube and IV 20 mg basiliximab. In the intensive care unit (ICU), patients received oral tacrolimus starting the day after the operation (adjusting the dose targeting a serum level of 5–10 ng/ml) and mycophenolate mofetil 500 mg after 4 days.
We collected participants' demographic data, disease characteristics, comorbidities and basal laboratory results. The supplementary material (online)details the anaesthesia and surgical techniques, immunosuppression and data collected.
The primary outcome was the incidence of PRS (defined as a 30% drop in the MAP when compared to the MAP immediately before portal de-clamping sustained for 1 minute within the first 5 minutes after portal de-clamping).[7] We also recorded the duration of hypotension (MAP below 20% of the basal value (before incision) or below 65 mmHg), significant arrhythmias or bradycardia requiring treatment) and ischaemic changes on electrocardiogram (ECG) (a horizontal or down-sloping ST segment depression of ≥1 mm in lead II or 1.5 mm slowly up-sloping ST segment depression or an ST segment elevation of at least 1.5 mm in lead V5) during reperfusion and NE boluses.[8] The rates of use of pre-reperfusion and post-reperfusion NE infusions were also recorded. We recorded the intraoperative haemodynamics and electrolytes at six time points (T): (T1) immediately before skin incision, (T2) at the beginning of the an-hepatic phase (portal vein clamping), (T3) 5 minutes before portal reperfusion, (T4) 5 minutes after portal unclamping, (T5) 5 minutes after hepatic arterial de-clamping and (T6) during skin closure.
Early acute kidney injury (AKI) was defined as a 0.3 mg/dl increase from the baseline S. Cr in the early 48 postoperative hours.[9] AKI was staged as stages 1, 2 and 3. We recorded warm ischaemia, cold ischaemia, total ischaemia, surgery durations, blood component transfusions and the total intraoperative urine output (UO). Data on the laboratory assessments of the graft function were collected up to 90 days after transplantation. ICU and hospital length of stay, early postoperative surgical complications (28 days), 3-month survival and 1-year survival were also anlaysed. The 90th-day observations were collected within 3 days before or 90th postoperative day.
The historical control was taken from the study conducted at the same centre and published in a peer-reviewed journal in 2017.[10] This study's inclusion or exclusion criteria and primary outcome definition were nearly identical (PRS). We chose the group corresponding to our current study regarding washing the graft into the circulation. The sample size was increased from 25 to 40 participants while the trial was ongoing [after obtaining IRB approval] to increase the power (1–β) of the study for detecting the difference in the risk of PRS (the primary outcome) to approximately 0.9. The power of the study was calculated using G*Power (version 3.1.4 for Windows) with a true proportion = 0.7, null proportion = 0.9, two-tailed α error = 0.05 and a sample size of 40 participants. We presented continuous data as the mean (standard deviation (SD)) or the median (interquartile range (IQR)) according to the normality of the data as verified using the Shapiro–Wilk test, histogram and quantile-quantile (Q-Q) plot. To improve the comparability of variables at different time points, we reported data using the median (IQR) if the variable was non-normally distributed at any time point. We reported the incidence of PRS (our primary outcome) as a proportion and its 95% confidence interval (CI). We tested its statistical significance using the one-sample exact test (Clopper–Pearson method) as compared with 0.9 (the proportion of PRS in the historical control from our centre).[10] We presented categorical outcomes as frequencies and proportions with 95% CIs calculated using the binomial exact test and continuous outcomes as mean values and 95% CIs. We used the Chi-square and Fisher's exact test to explore the proportion of transient ischaemic changes on ECG and postoperative AKI among those who developed PRS and those who did not. We also explored the incidence of PRS in patients who started pre-reperfusion NE infusion and those who did not use Fisher's exact test. Then, we compared the mean doses of the NE boluses, pre-reperfusion MAP and systemic vascular resistance (SVR) in both groups (those who received NE infusion and those who did not) using the independent-samples t-test, reporting the mean difference (MD) and 95% CI. Then, we compared the post-reperfusion reading of the mean pulmonary artery pressure (MPAP) with 20 mmHg (the upper limit of the normal MPAP) using a two-sided one-sample t-test. We analysed the data using the Statistical Package for the Social Sciences (SPSS) software version 26. A two-tailed P ≤ 0.05 was considered statistically significant.
RESULTS
Figure 1 shows the flow of patient recruitment. Tables 1 and 2 represent the patients' baseline characteristics.
Figure 1.
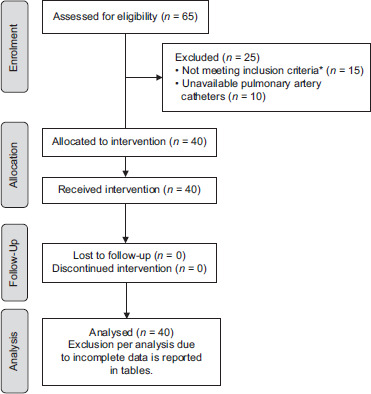
Study flow chart. *Excluded cases were three cases <18 years, three massive bleeding before reperfusion, seven cases with diabetes mellitus (≥10 years), one moderate pulmonary hypertension (mean pulmonary artery pressure = 45 mmHg), and one acute hepatitis
Table 1.
Descriptive characteristics of the study recipients and donors
| Intervention group (n=40) | Historical control[10] (n=40) | |
|---|---|---|
| Age (years) | 49 (12.5) | 50.9 (7.6) |
| Male patients | 25 (62.5%) | 34 (85%) |
| BMI | 27.5 (3.9 | - |
| MELD | 14.95 (5) | 15.4 (4) |
| Cause of ESLD | ||
| HCV cirrhosis | 18 (45%) | 22 (55%) |
| HCC | 16 (40%) | 18 (45%) |
| Autoimmune hepatitis | 6 (15%) | 0 |
| Comorbidities | ||
| No | 23 (57.5%) | - |
| Hypertension | 1 (2.5%) | |
| Diabetes | 11 (27.5%) | |
| Diabetes and hypertension | 2 (5%) | |
| Others* | 2 (5%) | |
| Missing | 1 (2.5%) | |
| Donor age (years) | 27.5 (6.8) | 25.9 (4.2) |
| Actual GRWR | 1.23 (0.199) | 0.94 (0.18) |
Data are expressed as mean (standard deviation) and number (%). BMI: Body mass index, BSA: Body surface area, MELD: Model for end-stage liver disease, ESLD: End-stage liver disease, HCV: Hepatitis C virus, HCC: Hepatocellular carcinoma, GRWR: Graft weight-to-recipient weight ratio, n: Number. *One case was cardiac (mild mitral regurgitation), and the other was hypothyroidism
Table 2.
Baseline laboratory tests (n=40), mean±SD
| Laboratory test | Results |
|---|---|
| Haemoglobin (g/dl) | 10.6 (2.2) |
| Platelets (k/μL) | 49.5 [37 to 70] |
| White blood cell count (k/μL) | 2.4 [1.9 to 3.6] |
| Albumin (g/dl) | 2.9 (0.6) |
| Total bilirubin (mg/dl) | 2.3 [1.7 to 3.25] |
| Direct bilirubin (mg/dl) | 1 [0.65 to 1.35] |
| INR | 1.5 [1.3 to 1.7] |
| AST (U/l) | 41.5 [33 to 62.5] |
| ALT (U/l) | 26 [21 to 46] |
| S. Cr (mg/dl) | 0.7 [0.6 to 0.85] |
| CRP (ml/l) | 0 [0 to 8] |
Data are expressed as mean (standard deviation) or median (interquartile range). INR: International normalised ratio, ALT: Alanine transaminase, AST: Aspartate transaminase, S. Cr: Serum creatinine, CRP: C-reactive protein, n: Number, SD: Standard deviation
PRS occurred in 28 (70%) cases (0.7, 95% CI 0.54 to 0.83, P < 0.001), with a relative risk reduction of 0.22 when compared to our previous results (0.9).[10] The mean (SD) surgery duration was 567 (77) minutes. Intraoperative haemodynamics are shown in Figure 2 and Supplemental Table (S1). Serum electrolyte levels are shown in Supplemental Table (S2).
Figure 2.
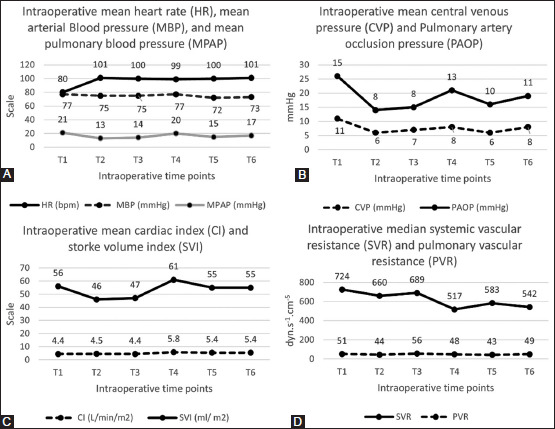
Intraoperative haemodynamic parameters. Panel A: Intraoperative HR, MAP and MPAP. Panel B: Intraoperative CVP and PAOP. Panel C: Intraoperative CI and SVI. Panel D: Intraoperative SVR and PVR. SD: standard deviation, (T1): immediately before skin incision, (T2): at the beginning of the an-hepatic (portal vein clamping), (T3): 5 minutes before portal reperfusion, (T4): 5 min after portal unclamping, (T5): 5 min after hepatic arterial de-clamping, (T6): at the time of skin closure, HR: heart rate, bpm: beats per minute, CI: cardiac index, SVI: stroke volume index, MAP: mean arterial blood pressure, MPAP: mean pulmonary artery pressure, CVP: central venous pressure, PAOP: pulmonary artery occlusion pressure, SVR: systemic vascular resistance, PVR: pulmonary vascular resistance
Twelve (30%) participants developed transient ischaemic changes on ECG (0.3, 95% CI 0.17 to 0.47) (Figure 3). The mean MPAP reading after reperfusion was 19.8 ± 6.4 mmHg, which was not significantly different from 20 mmHg (MD = −0.2, 95% CI −2.24 to 1.84, P = 0.884).
Figure 3.
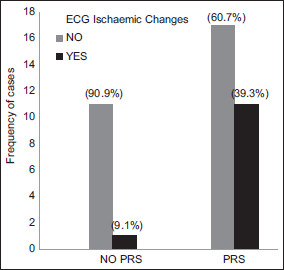
Number of patients who developed transient ST ischaemic changes during the reperfusion phase due to the post-reperfusion syndrome (PRS)
Patients received, on average, 5.2 (1.5) l of crystalloid and 4.5 (1.8) l of colloid infusions. The total intraoperative UO was 166 (89) ml/h, and the fluid balance was +1.4 l [0 to 2.3]. Twenty-four (60%) patients received packed RBC transfusions with a median transfusion amount of one unit (0 to 2.5), while five (12.5%) received plasma [range, 0 to 7 units]. No patient received platelet units in this cohort.
The mean (SD) warm ischaemia, cold ischaemia and an-hepatic durations were 37 (11) min, 28 (11) min and 71 (16) min. Sixteen patients (40%) received pre-reperfusion NE infusions, and they all continued in the intraoperative post-reperfusion period, and one patient commenced NE infusions in the post-reperfusion phase. Supplemental Table (S3) shows the graft ischaemic times in the intervention and historical control groups. Patients received a median of 220 [135 to 265] µg NE boluses. Epinephrine was needed in 20 cases (50%), with a median of 10 µg [0 to 40].
There were no cases of significant arrhythmia or bradycardia that required treatment (0, 95% CI 0–0.09). Hypotension (<20% from the basal value or <65 mmHg) continued for 91 (52.5) seconds on average during the first 5 minutes after portal vein de-clamping.
Patients who needed pre-reperfusion NE infusion received higher doses of NE boluses during reperfusion, 267.5 µg versus 174.6 (MD = 92.9, 95% CI 13 to 172.8, P = 0.024). PRS occurred in 13/16 (81.25%) among patients who received pre-reperfusion NE versus 15/24 (62.5%) in patients who did not, P = 0.297. Nevertheless, there were insignificant differences in pre-reperfusion MAP and SVR between both groups: 76.4 mmHg in patients who received NE infusions versus 73.1 mmHg (MD = 3.3, 95% CI −2.3 to 8.9, P = 0.238) and 744.3 Dyn/s/cm5 in patients who received pre-reperfusion NE infusions and 677.2 Dyn/s/cm5 in those who did not (MD = 67.1, 95% CI −55.9 to 190, P = 0.276).
Two participants needed intraoperative hepatic artery single re-anastomosis after ultrasonographic Doppler flow assessment (0.05, 95% CI 0.01 to 0.17). Patients were discontinued from postoperative mechanical ventilation after a median (IQR) of 1.3 [1–3.4] hours. Ten patients (25%) developed early AKI (0.25, 95% CI 0.13 to 0.41), five as stage 1 and four as stage 2. Ninety per cent of those who developed AKI had a history of PRS; however, this finding was not statistically significant (P = 0.231).
The median (IQR) durations of ICU stay and hospital stay were 6 (5–7) days and 16.5 (13–22) days, respectively. The 3-month overall survival was 95% (0.95, 95% CI 0.83 to 0.99), while the 1-year overall survival was 93% (0.93, 95% CI 0.8 to 0.98).
Two recipients died early on the 7th and 10th postoperative days with graft dysfunction (portal vein thrombosis) and sepsis, respectively, while a third one died after 97 days post-transplantation with pneumonia. Two patients (8%) developed early postoperative portal vein thrombosis: one died, and the other needed surgical re-exploration and thrombectomy. The three deceased patients experienced PRS in the intraoperative period. Table 3 shows the postoperative laboratory results in the first 3 postoperative months.
Table 3.
Laboratory results in the postoperative 3 months (n=40)
| Day 1 | Day 2 | Day 7 | Day 28 | Day 90 | |
|---|---|---|---|---|---|
| S. Cr (mg/dl) | 0.7 [0.6–1.1] | 0.7 [0.6–1.1] | 0.7 [0.6–0.9] | 0.9 [0.7–1.2]*3 | 0.9 [0.7–1.2]*9 |
| INR | 1.9 [1.5–2.2] | 1.5 [1.4–1.7] | 1.3 [1.1–1.5] | 1.0 [1.0–1.3]*3 | 1.0 [1.0–1.1]*9 |
| Albumin (g/dl) | 2.9±0.6 | 2.9±0.4 | 3.1±0.4 | 4±0.5*3 | 4.3±0.4*8 |
| T. bil. (mg/dl) | 2.6 [1.7–3.9] | 2.2 [1.6–2.8] | 3.5 [1.8–5.8] | 1.1 [0.8–1.6]*3 | 0.7 [0.6–0.95)*8 |
| D. bil. (mg/dl) | 1.4 [0.95–2.1] | 1.2 [1.0–2] | 2.7 [1.0–4.9] | 0.6 [0–1.1]*3 | 0 [0–0.25]*8 |
| AST (U/l) | 200 [120–441] | 116 [89–334] | 41 [29–60] | 22 [21–36]*3 | 31 [21–41]*8 |
| ALT (U/l) | 274 [145–523] | 242 [144–492] | 67 [52–144] | 22 [20–33]*3 | 31 [21–50]*8 |
| CRP (ml/l) | 36 [27–56] | 41 [28–53] | 24 [17–39] | 0 [0–16]*3 | 0 [0–3]*8 |
| GGT (U/l) | 22 [17–39] | 21 [15–39] | 79 [53–126] | 75 [58–126]*13 | 57 [29–128]*15 |
| LDH (U/l) | 557 [440–839] | 469 [376–609] | |||
| Lactate (mmol/l) | 2.3 [1.8–2.8] | 1.5 [1.2–1.8] | |||
| pH | 7.45±0.06 | 7.44±0.45 | |||
| Urine output (ml) | 2150 [1845–2000] | 2750 [2000–3280] |
Data are expressed as mean (standard deviation) or median (interquartile range). INR: International normalised ratio, T. bil.: Total bilirubin, D. bil.: Direct bilirubin, ALT: Alanine transaminase, AST: Aspartate transaminase, S. Cr: Serum creatinine, CRP: C-reactive protein, GGT: γ-glutamyl transferase, LDH: Lactate dehydrogenase. *nmissed observation, where n=number of missed observations
DISCUSSION
We found a 20% absolute risk reduction in PRS compared with our previous results.[10] We did not find any significant tachy- or brady-arrhythmia. However, ECG revealed ischaemic changes in 30% of our study participants, reversible after the hypotension correction. The post-reperfusion MPAP was not significantly higher than the normal ranges. Twenty-five per cent of our participants developed early postoperative AKI, with 95% surviving for 3 months and 93% surviving for at least 1 year post-transplantation.
During PRS, the pulmonary resistance and pressure increase, while the corresponding systemic parameters decrease.[2] This differential response suggests that NE increases the SVR with minimal or no effects on the pulmonary circulation. With some concerns of reperfusion-associated bradycardia and reduced cardiac contractility,[2] NE might also be the ideal drug; NE has some cardiac β1 effects.[11] NE infusion during LT is common. Some authors reported the administration of NE IV boluses up to 32 µg on the baseline infusion for a case of post-reperfusion vasoplegia.[5]
We began our research on this topic with a single-arm trial because we believed that our new practice would benefit all patients rather than deprive a group of patients, as suggested in other new therapies.[12,13] NE boluses, as per the study protocol, significantly reduced the incidence of PRS to 70% (P < 0.001) compared with our previous results (90%).[10] The incidence of PRS varies between centres and may not differ significantly between living donor and deceased donor LT.[4,14] We do not use portocaval shunts during LT and use HTK as a preservative solution; this may explain our high incidence of PRS.[15,16]
Regarding the safety of NE boluses, we did not notice any significant arrhythmia or bradycardia during reperfusion. ECG ischaemic changes occurred in 12 of our 40 patients. All ischaemic changes were reversed on correcting either the hypotension or the PRS. Therefore, these ischaemic changes are probably related to the demand or supply discrepancy and not the vasopressor effects of NE. The post-reperfusion MPAP was, on average, 20 mmHg and did not exceed the basal MPAP (21 mmHg after the induction of anaesthesia). By contrast, the PVR reduced after reperfusion. This suggests that NE boluses did not adversely affect the pulmonary circulation pressures. We also investigated the possible effects of NE boluses on hepatic artery anastomosis. The small incidence (5%) of intraoperative re-anastomosis does not seem to be related to the NE boluses.
Our NE injection protocol was escalating. We started at 20 µg and adapted the doses according to the MAP every 10 seconds. As we aimed to prevent PRS, we started injecting NE boluses once the MAP either reduced by 10% or dropped to less than 65 mmHg, whichever happened first, before the 30% decline (the threshold for PRS by definition). Besides, we did not commence before a decline in the blood pressure for fear of unsuccessful anastomosis and, in turn, no reperfusion. We used epinephrine as a rescue vasoactive drug if NE did not enable us to reach the MAP after 1 minute.
Cardiac output measurement with the thermodilution method is inaccurate during the reperfusion phase.[17] Therefore, we delayed our invasive haemodynamic measurements by 5 minutes after reperfusion until thermal equilibrium.
Despite the high incidence of PRS in this study, the overall survival after transplantation was appreciable, with the 3-month and 1-year overall survivals being 95% and 93%, respectively. This might attract attention to the duration of hypotension during the reperfusion phase and not only the incidence of PRS. However, it is still unknown whether the duration or the degree of hypotension affects post-transplantation morbidity and mortality more.[4]
The study has limitations: first, the sample size is small, which might be acceptable for a feasibility study with adequate power. Second, we did not use a parallel control, as we believed that all patients should benefit from the intervention. Besides, we used a published historical control at the same centre with almost the same inclusion criteria and definition of PRS.[10] The fluid management protocol and the PRS management were different between both groups, and the study is not a comparative study in the first place. Third, we did not record the lowest MAP during reperfusion, nor did we record reactive hypertension or the highest mean pulmonary pressures after NE boluses. Fourth, the generalisability of our results cannot be guaranteed due to the many exclusion criteria and small sample size.
Future randomised studies should be conducted to find out the best dose and protocol (e.g., de-escalating protocol) for the administration of NE boluses.
CONCLUSION
During LDLT, escalating NE boluses starting at 20 µg was feasible in reducing the risk of PRS without causing significant ischaemia, arrhythmia or a significant rise in pulmonary arterial pressure.
Study data availability
De-identified data may be requested with reasonable justification from the authors (email to the corresponding author) and shall be shared after approval as per the authors' Institution policy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the Egyptian Knowledge Bank—Enago—for providing proofreading of the article (www.enago.com). Additionally, we acknowledge Dr. Kareem M. Abozeid as a collaborator for helping in collecting data and Kareem M. Abozeid, MSc., Assistant Lecturer of Anaesthesiology and Intensive Care and Pain Medicine at Mansoura University, Faculty of Medicine, Egypt.
SUPPLEMENTARY MATERIAL (FOR ONLINE)
Methodology supplement
Anaesthesia Technique: In the operating room, we applied standard monitors in pulse oximetry, non-invasive blood pressure and electrocardiography (ECG). After premedication with 0.01 mg/kg of midazolam, anaesthesia was induced by fentanyl 2 µg/kg, propofol 1–2 mg/kg and rocuronium bromide 0.8–1 mg/kg. After tracheal intubation, we inserted an arterial catheter in the radial artery of the non-dominant hand after performing the modified Allen test. Then, we inserted a continuous thermal fibre-optic pulmonary artery catheter for monitoring pulmonary artery pressures and cardiac output (Continuous cardiac output [CCO]/ mixed venous oxygen saturation [SvO2]; Edwards Life Science, Irvine, CA, USA). Anaesthesia was maintained by sevoflurane in 40% oxygen with a continuous infusion of fentanyl 0.5–1 µg/kg/h and rocuronium bromide 200–400 µg/kg/h. Mechanical ventilation was started in pressure-control volume-guarantee mode, with tidal volume (8 ml/kg, predicted body weight), rate started at 12 bpm and then adjusted to keep PCO2 between 33 and 40 mmHg (concerning the pH). Positive end-expiratory pressure (PEEP) was adjusted initially at 5 cmH2O and then individualised according to the case. Recruitment manoeuvres were performed as needed.
Surgical Technique: The donor surgical team excised the right liver lobe. Then, a surgeon flushes the graft with 3–4 litres of cold histidine–tryptophan–ketoglutarate (HTK) (Custodial, Bensheim, Germany) via antegrade flushing of the portal vein to get completely clear fluid without flushing via the hepatic artery. Both recipient and donor operations were synchronised to minimise graft preservation time.
In the intensive care unit (ICU): We kept the pulmonary artery catheter in place for haemodynamic monitoring in case of instability and exchanged it with a central venous catheter 48 hours after insertion. We adopted early tracheal extubation in the ICU once the patient is haemodynamically stable, with pH ≥7.3 and adequate consciousness and muscle power (train-of-four ratio ≥0.9). We kept glucose between 110 and 180 mg/dl in the intraoperative and ICU periods using intravenous insulin infusion or glucose 10% boluses as appropriate. We monitored urine output (UO) hourly in the intraoperative and ICU periods.
Immunosuppression: All patients received intravenous 500 mg methylprednisolone at the start of the warm ischaemia. After hepatic artery anastomosis and unclamping, we administered 500 mg mycophenolate mofetil through the nasogastric tube and i.v. 20 mg basiliximab. In the ICU, patients received oral tacrolimus starting the day after the operation (adjusting the dose targeting a serum level of 5–10 ng/ml) and mycophenolate mofetil 500 mg after 4 days.
Data collection
Preoperative data: We collected the preoperative data 24 hours before the operation. It includes (1) age, sex, weight, height, model for end-stage liver disease (MELD) score and Child–Pugh classification; (2) medical comorbidities (hypertension, cardiac diseases and diabetes mellitus); (3) cause of the end-stage liver disease (ESLD); (4) the donor age and gender; (5) liver function tests: serum albumin, bilirubin, international normalised ratio (INR), aspartate transaminase (AST) and alanine transaminase (ALT); (6) serum creatinine (S. Cr); and (7) C-reactive protein (CRP).
Intraoperative data: We recorded the intraoperative cardiac index (CI), stroke volume index (SVI), MAP, mean pulmonary arterial pressure (MPAP), pulmonary artery occlusion pressure (PAOP), systemic vascular resistance (SVR), pulmonary vascular resistance (PVR) and serum Na, K, ionised Ca and Cl at six times: (T1) immediately before skin incision, (T2) at the beginning of the an-hepatic phase (portal vein clamping), (T3) 5 minutes before portal reperfusion, (T4) 5 min after portal unclamping, (T5) 5 min after hepatic arterial de-clamping and (T6) at the time of skin closure.
Postoperative data: Early acute kidney injury (AKI) incidence was defined as a 0.3 mg/dl increase from the baseline S. Cr in the early 48 postoperative hours.12 AKI was staged as stages 1, 2 and 3.12 In the postoperative period, S. Cr was measured at arrival to ICU, 1st, 2nd, 7th, 28th and 3 months postoperative with measuring of the 24-hour UO in the first 2 days. We staged AKI as follows: stage 1—when S. Cr rises 1.5–1.9 times baseline or >0.3 mg/dl increase from the baseline; stage 2—when S. Cr rises 2–2.9 times baseline; and stage 3—when S. Cr rises three times the baseline or increase to >4 mg/dl or the initiation of renal replacement therapy.12 We recorded warm ischaemia, cold ischaemia, total ischaemia and operative times, blood component transfusions and the total intraoperative UO. Laboratory assessment of the graft function included INR, AST, ALT, γ-glutamyl transferase (GGT), albumin and bilirubin at the 1st, 2nd, 7th and 28th days and 90th day postoperatively. We have also recorded pH, lactate and lactate dehydrogenase (LDH) on the first 2 postoperative days. ICU and hospital length of stay, early postoperative surgical complications (28 days), 3-month survival and 1-year survival were also reported. The 90-day observations were collected 3 days before or after the 90th postoperative day. The follow-up data were completed for mortality at the 3-month and 1-year time points.
Table S1.
Intraoperative haemodynamic parameters (n=40)
| T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|
| HR (bpm) | 80 (14) | 101 (15) | 100 (14) | 99 (13) | 100 (13) | 101 (13) |
| CI (L/min/m2) | 4.4 (1.3) | 4.5 (1.0) | 4.4 (1.1) | 5.8 (1.2) | 5.4 (1.3) | 5.4 (1.2) |
| SVI (ml/m2) | 56 (18) | 46 (13) | 47 (17) | 61 (23) | 55 (18) | 55 (15) |
| MBP (mmHg) | 77 (73-85) | 75 (72-81) | 75 (70-82) | 77 (73-93) | 72 (66-78) | 73 (68-83) |
| MPAP (mmHg) | 21 (6) | 13 (4) | 14 (4) | 20 (6) | 15 (5) | 17 (5) |
| CVP (mmHg) | 11 (4) | 6 (3) | 7 (3) | 8 (4) | 6 (3) | 8 (3) |
| PAOP (mmHg) | 15 (5) | 8 (4) | 8 (3) | 13 (6) | 10 (4) | 11 (4) |
| SVR (dyn.s-1.cm-5) | 724 (575-959) | 660 (562-826) | 689 (538-842) | 517 (459-698) | 583 (455-698) | 542 (416-714) |
| PVR (dyn.s-1.cm-5) | 51 (33-63) | 44 (34-63) | 56 (37-82) | 48 (34-68) | 43 (26-67) | 49 (28-67) |
Data are expressed as mean (standard deviation) or median (interquartile range). (T1): Immediately before skin incision, (T2): At the beginning of the an-hepatic (portal vein clamping), (T3): 5 min before portal reperfusion, (T4): 5 min after portal unclamping, (T5): 5 min after hepatic arterial de-clamping, (T6): At the time of skin closure, HR: Heart rate, bpm: Beat per minute, CI: Cardiac index, SVI: Stroke volume index, MBP: Mean arterial blood pressure, MPAP: Mean pulmonary artery pressure, CVP: Central venous pressure, PAOP: Pulmonary artery occlusion pressure, SVR: Systemic vascular resistance, PVR: Pulmonary vascular resistance, N: Number of patients
Table S2.
Intraoperative electrolytes (n=40)
| T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|
| Na (mEq/L) | 138 (4) | 137 (3) | 136 (5) | 137 (5) | 137 (3) | 137 (3) |
| K (mEq/L) | 3.2 (0.4) | 3.8 (0.5) | 3.8 (0.5) | 3.7 (0.5) | 4.0 (0.5) | 4.2 (0.7) |
| Ionised Ca (mEq/L) | 0.9 (0.14) | 1.05 (0.2) | 1.04 (0.14) | 1.05 (0.22) | 1.13 (0.13) | 1.08 (0.7) |
| CL (mEq/L) | 109 (5) | 112 (5) | 110 (7) | 110 (6) | 111 (5) | 112 (4) |
Data are expressed as mean (standard deviation). (T1): Immediately before skin incision, (T2): At the beginning of the an-hepatic (portal vein clamping), (T3): 5 min before portal reperfusion, (T4): 5 min after portal unclamping, (T5): 5 min after hepatic arterial de-clamping, (T6): At the time of skin closure, Na: Serum sodium, K: Serum potassium, Ca: Ionised calcium, CL: Serum chloride, n: Number of patients
Table S3.
Operative times and ischaemic times
| Intervention group (n=40) | Historical control[8] (n=40) | |
|---|---|---|
| Operative time (min) | 567 (77) | 610 (47) |
| Warm ischaemia (min) | 37 (11) | 36.6 (9) |
| Cold ischaemia (min) | 28 (11) | 34 (12.6) |
| An-hepatic time (min) | 71 (16) | - |
Data are expressed as mean (standard deviation). n: Number of patients
REFERENCES
- 1.Burra P, Burroughs A, Graziadei I, Pirenne J, Valdecasas JC, Muiesan P, et al. EASL Clinical practice guidelines: Liver transplantation. J Hepatol. 2016;64:433–85. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 Suppl 3):54–5. [PubMed] [Google Scholar]
- 3.Siniscalchi A, Gamberini L, Bardi T, Laici C, Ravaioli M, Bacchi Reggiani ML, et al. Post-reperfusion syndrome during orthotopic liver transplantation, which definition best predicts postoperative graft failure and recipient mortality? J Crit Care. 2017;41:156–60. doi: 10.1016/j.jcrc.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Manning MW, Kumar PA, Maheshwari K, Arora H. Post-reperfusion syndrome in liver transplantation-an overview. J Cardiothorac Vasc Anesth. 2020;34:501–11. doi: 10.1053/j.jvca.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 5.An SS, Henson CP, Freundlich RE, McEvoy MD. Case report of high-dose hydroxocobalamin in the treatment of vasoplegic syndrome during liver transplantation. Am J Transplant. 2018;18:1552–5. doi: 10.1111/ajt.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S, Shen X, Liu S, Yang J, Wang X. Efficacy and safety of norepinephrine versus phenylephrine for the management of maternal hypotension during cesarean delivery with spinal anesthesia: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e14331. doi: 10.1097/MD.0000000000014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Jr, Pinsky MR. Postreperfusion syndrome: Hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154–60. doi: 10.1016/0883-9441(93)90021-c. [DOI] [PubMed] [Google Scholar]
- 8.London MJ, Hollenberg M, Wong MG, Levenson L, Tubau JF, Browner W, et al. Intraoperative myocardial ischemia: Localization by continuous 12-lead electrocardiography. Anesthesiology. 1988;69:232–41. [PubMed] [Google Scholar]
- 9.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–9. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 10.Yassen AM, Elsarraf WR, Elmorshedi MA, Abdel Wahab M, Salah T, Sultan AM, et al. Short-term effects of extracorporeal graft rinse versus circulatory graft rinse in living donor liver transplantation. A prospective randomized controlled trial. Transpl Int. 2017;30:725–33. doi: 10.1111/tri.12968. [DOI] [PubMed] [Google Scholar]
- 11.Ngan Kee WD, Lee SWY, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–45. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 12.Gehan EA, Freireich EJ. Non-randomized controls in cancer clinical trials. N Engl J Med. 1974;290:198–203. doi: 10.1056/NEJM197401242900405. [DOI] [PubMed] [Google Scholar]
- 13.Lasagna L. Historical controls: The practitioner's clinical trials. N Engl J Med. 1982;307:1339–40. doi: 10.1056/NEJM198211183072110. [DOI] [PubMed] [Google Scholar]
- 14.Chung IS, Kim HY, Shin YH, Ko SJ, Gwak MS, Sim WS, et al. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation: Post-reperfusion syndrome. Clin Transplant. 2012;26:539–43. doi: 10.1111/j.1399-0012.2011.01568.x. [DOI] [PubMed] [Google Scholar]
- 15.Paugam-Burtz C, Kavafyan J, Merckx P, Dahmani S, Sommacale D, Ramsay M, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: Outcome and predictors: Postreperfusion Syndrome. Liver Transpl. 2009;15:522–9. doi: 10.1002/lt.21730. [DOI] [PubMed] [Google Scholar]
- 16.Ko JS, Kim GS, Gwak MS, Yang M, Kim HK, Shin JK, et al. Greater hemodynamic instability with a histidine-tryptophan-ketoglutarate solution than the University of Wisconsin solution during the reperfusion period in living donor liver transplantation. Transplant Proc. 2008;40:3308–10. doi: 10.1016/j.transproceed.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Böttiger BW, Sinner B, Motsch J, Bach A, Bauer H, Martin E. Continuous versus intermittent thermodilution cardiac output measurement during orthotopic liver transplantation. Anaesthesia. 1997;52:207–14. doi: 10.1111/j.1365-2044.1997.079-az0077.x. [DOI] [PubMed] [Google Scholar]


