ABSTRACT
Background
Intradialytic hypotension (IDH) is a common hemodialysis complication causing adverse outcomes. Despite the well-documented associations of ambient temperatures with fluid removal and pre-dialysis blood pressure (BP), the relationship between ambient temperature and IDH has not been adequately studied.
Methods
We conducted a cohort study at a tertiary hospital in southern Taiwan between 1 January 2016 and 31 October 2021. The 24-h pre-hemodialysis mean ambient temperature was determined using hourly readings from the weather station closest to each patient's residence. IDH was defined using Fall40 [systolic BP (SBP) drop of ≥40 mmHg] or Nadir90/100 (SBP <100 if pre-dialysis SBP was ≥160, or SBP <90 mmHg). Multivariate logistic regression with generalizing estimating equations and mediation analysis were utilized.
Results
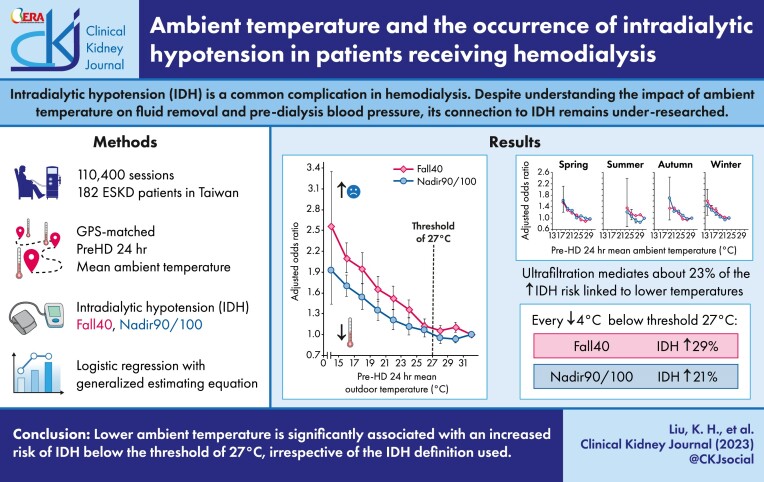
The study examined 110 400 hemodialysis sessions from 182 patients, finding an IDH prevalence of 11.8% and 10.4% as per the Fall40 and Nadir90/100 criteria, respectively. It revealed a reverse J-shaped relationship between ambient temperature and IDH, with a turning point around 27°C. For temperatures under 27°C, a 4°C drop significantly increased the odds ratio of IDH to 1.292 [95% confidence interval (CI) 1.228 to 1.358] and 1.207 (95% CI 1.149 to 1.268) under the Fall40 and Nadir90/100 definitions, respectively. Lower ambient temperatures correlated with higher ultrafiltration, accounting for about 23% of the increased IDH risk. Stratified seasonal analysis indicated that this relationship was consistent in spring, autumn and winter.
Conclusion
Lower ambient temperature is significantly associated with an increased risk of IDH below the threshold of 27°C, irrespective of the IDH definition. This study provides further insight into environmental risk factors for IDH in patients undergoing hemodialysis.
Keywords: ambient temperature, hemodialysis, intradialytic hypotension, seasonal variation, threshold
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Intradialytic hypotension (IDH) is recognized as a common and potentially fatal complication during hemodialysis, yet there is no consensus on its definition.
Ambient temperatures are linked to blood pressure and ultrafiltration, both closely related to IDH, though not conclusively studied in this context.
This study adds:
The research demonstrates a reverse J-shaped relationship between ambient temperature and IDH, identifying approximately 27°C as a critical threshold, consistent across multiple IDH definitions.
Below 27°C, each 4°C decrease in temperature is associated with up to a 29% increased risk of IDH.
Lower temperatures before hemodialysis are associated with higher ultrafiltration, which mediates 23% of the cold temperature's impact on IDH.
Potential impact:
Clinicians should consider environmental temperature as a factor in the care of hemodialysis patients and utilize optimal fluid management to partially mitigate cold-related IDH.
Coping strategies and physiological mechanisms underlying low-temperature risks necessitate further research as they are critical to understanding and addressing IDH.
INTRODUCTION
As climate change intensifies heat and cold, evaluating the potential health impacts of temperature exposure is crucial [1, 2]. Ambient temperature has been linked to an increased risk of cardiovascular morbidity and mortality [3–7]. Literature has reported that patients with end-stage kidney disease are susceptible to the hazards of temperature exposure [8, 9], partly relating to their high burden of cardiovascular disease and comorbidities [10].
Intradialytic hypotension (IDH), a common complication of hemodialysis, is associated with vascular access dysfunction [11], inadequate hemodialysis [12], morbidities [13–15] and mortality [13, 14, 16, 17]. Identification of associated risk factors is vital for improved patient care [14, 18–21]. However, the potential impact of ambient temperature on IDH remains understudied, despite these patients being particularly vulnerable to temperature challenges.
Cold temperatures affect blood pressure (BP) and water balance, with prior studies showing that dialysis patients in cold conditions have more fluid removal [ultrafiltration (UF)] and vasoconstriction [22, 23]. This leads to higher pre-dialysis BP and a diminished ability to counter fluid deficits, potentially causing IDH [22, 24].
Moreover, vasoconstriction may increase the heart's workload and oxygen demand, potentially disrupting steady circulation in susceptible patients [25]. Although low temperatures are linked to high UF and higher pre-dialysis BP—both risk factors for IDH—studies investigating the impact of ambient temperature on IDH are limited. Furthermore, previous studies on the health effects of temperature had methodological weaknesses; for example, they used ambient temperature data from a single weather station near the hemodialysis center or nearby airport, which may not accurately reflect patients’ actual residential temperature exposure [8, 9, 22, 26, 27].
In this study, we examined the association between 24-h mean ambient temperature obtained from Global Positioning System (GPS)-matched weather stations prior to each hemodialysis session. The objectives of the analysis were to (i) examine the correlation between ambient temperature and IDH; (ii) determine whether there is a threshold ambient temperature associated with IDH; (iii) identify the IDH definition most relevant to ambient temperature; and (iv) investigate any potential seasonal variations in this association. Findings from this study would contribute to understanding the potential influence of ambient temperature on IDH, which may aid in developing interventions to ameliorate the adverse effects of low temperatures on patients undergoing hemodialysis.
MATERIALS AND METHODS
Study design
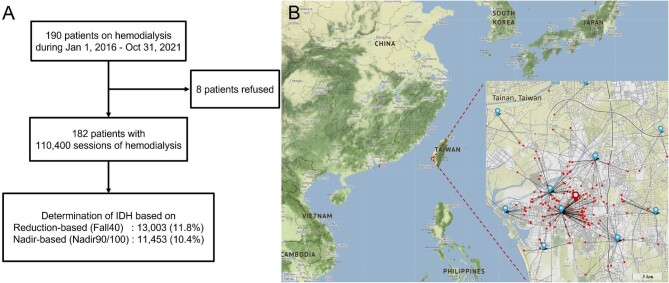
The Assessing Temperature Effect on Hemodialysis (ATEMPT-HD) study was an observational cohort study designed to investigate the association between temperature and hemodialysis outcomes. The study was conducted at the hemodialysis center of National Cheng Kung University Hospital, a tertiary teaching hospital in Tainan, Taiwan. In the study period between 1 January 2016 and 31 October 2021, a total of 190 patients underwent dialysis. Of these, 182 patients (95.8%) were included in the study analysis, including 17 deceased patients. Written informed consent was obtained from all living patients. The study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-110-327) and adhered to the principles of the Declaration of Helsinki. The number of patients and the corresponding number of hemodialysis sessions are depicted in Fig. 1A.
Figure 1:
(A) Patient enrollment and geographic information. Patient enrollment and prevalence of IDH, (B) geographic information of southern Taiwan and the distance between the 21 weather stations of the CWB (blue marks) and the 182 home addresses assessed (red dots). Only 10 weather stations were shown in this figure due to the scale of figure. The dialysis center is shown as a red mark. The figure was generated by Python 3.8.9 with the extension library, Folium 0.12.1.
Temperature assessment
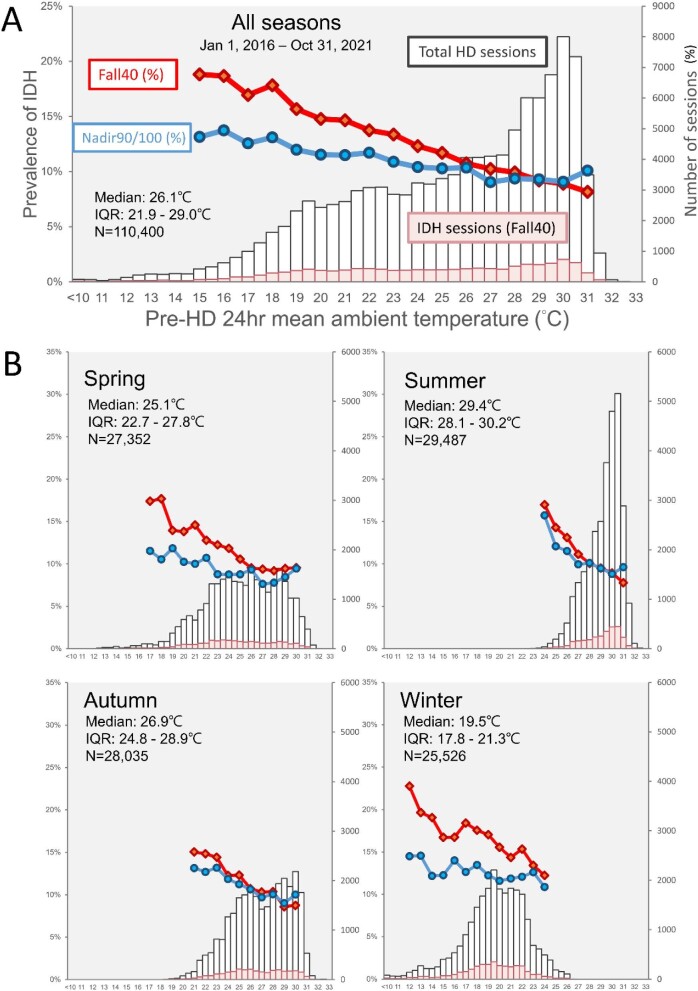
We obtained hourly ambient temperature measurements from the Central Weather Bureau (CWB) of Taiwan (https://opendata.cwb.gov.tw). The address of each patient's home was inserted into the GPS and coordinated according to its latitude and longitude. The closest weather station was determined based on the shortest great circle distance, calculated using the Haversine formula [28]. Temperature data were obtained from the weather station nearest to the patient's address (Fig. 1B). A total of 56 weather stations were affiliated with CWB in southern Taiwan (the study area), and 21 of them provided temperature readings for this study (Supplementary data, Table S1). We extracted the hourly temperature records for the 24 h preceding each hemodialysis session and calculated the mean ambient temperature over this period (i.e. pre-HD 24-h mean ambient temperature). The selection of 24 h is based on literature, which has shown that cold temperatures impact mortality with the strongest association on the same day [29]. Temperature distribution during hemodialysis sessions is shown in Fig. 2A and by season in Fig. 2B. IDH prevalence is also depicted based on specific temperatures.
Figure 2:
Distribution of mean ambient temperature during all hemodialysis sessions, both with and without IDH (in a stacked bar chart, refer to the right vertical axis for session numbers) and the prevalence of IDH in specific temperatures (in a line chart, refer to the left vertical axis) (A) for the entire observation period from 1 January 2016 to 31 October 2021, or (B) broken down by four seasons.
Determination of IDH
The determination of IDH was based on multiple criteria in the primary and sensitivity analyses. We adopted the reduction-based criteria (Fall40), which defines IDH as a reduction in systolic BP (SBP) of ≥40 mmHg (pre-HD SBP minus minimum SBP, ≥40 mmHg). We also used the nadir-based criteria (Nadir90/100), which defines IDH as the occurrence of intradialytic SBP <90 mmHg in patients with a pre-HD SBP <160 mmHg or SBP <100 mmHg in patients with a pre-HD SBP ≥160 mmHg. Moreover, we conducted sensitivity analyses with another five definitions of IDH to confirm the temperature–IDH relationship. Detailed definitions of IDH are shown in Supplementary data, Table S2. BP was measured before and every 60 min during hemodialysis, resulting in at least five records per 4-h session. Each session was then classified as either IDH or non-IDH. The prevalence of IDH according to the definitions of IDH is shown in Supplementary data, Table S2.
Clinical covariates and dialysis records
Clinical covariates were collected and categorized into three groups: demographic and comorbidity information, hemodialysis records of each session and antihypertensive medications. Demographic and comorbidity data, such as age, sex, diabetes mellitus, hypertension, coronary artery disease and Charlson Comorbidity Index [30] were obtained by reviewing patient medical charts. Hemodialysis records of each session included information such as hemodialysis vintage (months), pre-HD SBP (mmHg), pre-HD body weight (kg), dry weight (kg), UF weight (kg), blood flow (mL/min), dialysate flow (mL/min), dialysate average temperature (°C), conductivity [dialysate sodium (mEq/L)], dialysate calcium (mEq/L) and monthly hemoglobin; all of these were extracted from the hospital electronic medical records. The UF weight to dry weight ratio (UF/DW) was calculated and presented as a percentage (%). Antihypertensive medications were categorized monthly into five groups: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, alpha-blockers, calcium channel blockers and vasodilators. Additionally, data on the use of intravenous iron supplements (IV_iron) were extracted (see Supplementary data, Table S3). Under the Taiwan National Health Insurance program, prescriptions for more than one drug in the same category are restricted. Therefore, we coded each category as either present or absent.
Statistical analyses
Data were presented as mean ± standard deviation (SD) or median with interquartile range for continuous variables and numbers and percentages for categorical variables. Characteristics of hemodialysis sessions were compared using standardized difference, a measure that is not sensitive to sample size [31]. A difference <0.1 was considered negligible.
The risk of IDH associated with ambient temperature was assessed using binary logistic regression. To account for the inter-correlations among hemodialysis sessions of the same patient, we applied the generalizing estimating equation (GEE) method with the first-order autoregressive (AR-1) as the working correlation matrix. The odds ratio (OR) for risk of IDH per four degrees (around 1 SD) in temperature was presented, considering the SD of the pre-HD 24-h mean ambient temperature was 4.4°C.
Sequential adjustments for covariates were made in the multivariable logistic regression models. We plotted the trend of crude and covariate-adjusted ORs of IDH per 2°C increase (0.5 SD of temperature) to visualize the temperature–IDH relationship and determine whether a threshold existed. If a threshold was identified, we further fitted and tested the interaction term of “above and below the threshold” and “pre-HD 24-hour mean ambient temperature.” A mediation analysis assessed the effects of temperature and UF/DW on IDH, adjusting for covariates. It used patient-specific random effects for repeated measures and Quasi-Bayesian confidence intervals (CIs) from 1000 bootstrap simulations [32].
To assess whether season may modify the effect of ambient temperature on IDH, we conducted a test for interaction between season and ambient temperature. Subsequently, subgroup analyses were conducted to assess the association of temperature with IDH by season. Seasons were categorized as spring (March–May), summer (June–August), autumn (September–November) and winter (December–February). Statistical analysis was conducted using SPSS and R. A P-value <.05 was considered statistically significant.
RESULTS
Prevalence of IDH in sessions by multiple definitions
We analyzed 110 400 hemodialysis sessions from 182 patients with 611 136 BP records. The prevalence of IDH in these sessions was estimated at 11.8% and 10.4% based on the reduction-based (Fall40) and nadir-based (Nadir90/100) criteria, respectively (Fig. 2A, Supplementary data, Table S2). The prevalence of IDH varied with ambient temperature, and the lowest and highest estimates were observed in hot (>31°C) and cold temperatures (<15°C) regardless of the IDH definition: 8.2% and 18% for Fall40 and 10.1% and 13.1% for Nadir90/100, respectively (Fig. 2A).
Characteristics of IDH sessions
The differences in session characteristics and medication use between sessions with IDH and without IDH are shown in Table 1. The characteristic with the greatest standardized difference was pre-HD SBP for both definitions. Interestingly, pre-HD SBP was higher in IDH sessions defined by the Fall40 criteria (155 in IDH vs 134 mmHg in non-IDH) but was lower (121 in IDH vs 138 mmHg in non-IDH) in IDH sessions determined by Nadir90/100. IDH sessions also tended to have higher UF/DW than non-IDH sessions for both criteria, 4.91% vs 3.61% in Fall40 and 4.63% vs 3.66% in Nadir90/100. Age and sex were also differently distributed between the definitions, with IDH sessions defined by Fall40 being younger and male, and IDH sessions defined by Nadir90/100 being older and female (Table 1).
Table 1:
Characteristics of hemodialysis sessions according to IDH definitions.
| Reduction-based (Fall40) | Nadir-based (Nadir90/100) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Total number | All sessions | Non-IDH (n = 97 397) | IDH (n = 13 003) | Standardized difference | Non-IDH (n = 98 947) | IDH (n = 11 453) | Standardized difference |
| Pre-HD 24 h. mean temp. (°C), mean (SD) | 110 400 | 25.2 (4.4) | 25.3 (4.3) | 24.1 (4.7) | 0.272 | 25.2 (4.4) | 24.7 (4.6) | 0.128 |
| Demographic data | ||||||||
| Age (years), mean (SD) | 110 400 | 62.9 (13.7) | 62.9 (13.7) | 63.3 (13.3) | 0.035 | 62.3 (13.6) | 68.6 (13.4) | 0.470 |
| Male, n (%) | 110 400 | 55 644 (50.4) | 47 837 (49.1) | 7807 (60.0) | 0.221 | 51 518 (52.1) | 4126 (36.0) | 0.327 |
| DM, n (%) | 110 400 | 48 180 (43.6) | 40 020 (41.1) | 8160 (62.8) | 0.444 | 42 179 (42.6) | 6001 (52.4) | 0.197 |
| HTN, n (%) | 110 400 | 83 010 (75.2) | 72 725 (74.7) | 10 285 (79.1) | 0.105 | 75 602 (76.4) | 7408 (64.7) | 0.259 |
| CAD, n (%) | 110 400 | 27 222 (24.7) | 24 380 (25.0) | 2842 (21.9) | 0.075 | 24 163 (24.4) | 3059 (26.7) | 0.052 |
| CCI, mean (SD) | 110 400 | 7.0 (2.8) | 7.0 (2.9) | 7.4 (2.6) | 0.146 | 6.9 (2.8) | 8.0 (2.7) | 0.407 |
| Hemodialysis records | ||||||||
| HD vintage (months), mean (SD) | 110 400 | 59 (53) | 60 (54) | 52 (46) | 0.163 | 59 (53) | 62 (58) | 0.050 |
| UF/DW (%), mean (SD) | 108 635 | 3.76 (1.80) | 3.61 (1.74) | 4.91 (1.85) | 0.726 | 3.66 (1.77) | 4.63 (1.83) | 0.539 |
| Pre-HD SBP (mmHg), mean (SD) | 110 395 | 136 (22) | 134 (21) | 155 (22) | 0.987 | 138 (21) | 121 (27) | 0.702 |
| Blood flow (mL/min), mean (SD) | 110 084 | 275 (36) | 275 (35) | 277 (38) | 0.040 | 277 (35) | 260 (34) | 0.483 |
| Dialysate flow (mL/min), mean (SD) | 110 379 | 564 (113) | 560 (110) | 597 (123) | 0.317 | 566 (114) | 547 (100) | 0.176 |
| Dialysate avg. temp. (°C), mean (SD) | 110 231 | 36.1 (0.4) | 36.1 (0.4) | 36.0 (0.4) | 0.244 | 36.1 (0.4) | 35.9 (0.4) | 0.448 |
| Conductivity (mEq/L), mean (SD) | 109 769 | 140.0 (0.6) | 140.0 (0.6) | 140.0 (0.7) | 0.031 | 140.0 (0.7) | 140.0 (0.5) | 0.033 |
| Dialysate calcium (mEq/L), n (%) | 110 384 | |||||||
| 1.8 | 1490 (1.3) | 1336 (1.4) | 154 (1.2) | 0.017 | 1428 (1.4) | 62 (0.5) | 0.091 | |
| 2.5 | 58 946 (53.4) | 52 021 (53.4) | 6925 (53.3) | 0.003 | 52 712 (53.3) | 6234 (54.4) | 0.023 | |
| 3.0 | 42 286 (38.3) | 37 230 (38.2) | 5056 (38.9) | 0.014 | 37 755 (38.2) | 4531 (39.6) | 0.029 | |
| 3.5 | 7662 (6.9) | 6794 (7.0) | 868 (6.7) | 0.012 | 7037 (7.1) | 625 (5.5) | 0.068 | |
| Hb, mean (SD) | 110 374 | 10.6 (1.0) | 10.6 (1.0) | 10.8 (1.0) | 0.188 | 10.6 (1.0) | 10.7 (1.0) | 0.150 |
| Antihypertensives and iron use, n (%) | 107 867 | |||||||
| ACEi/ARB | 27 204 (25.2) | 24 320 (25.0) | 2884 (22.2) | 0.066 | 26 216 (26.5) | 988 (8.6) | 0.483 | |
| Beta-blockers | 31 692 (29.4) | 27 943 (28.7) | 3749 (28.8) | 0.003 | 29 755 (30.1) | 1937 (16.9) | 0.314 | |
| Alpha-blockers | 11 912 (11.0) | 10 823 (11.1) | 1089 (8.4) | 0.092 | 11 530 (11.7) | 382 (3.3) | 0.320 | |
| Calcium channel blocker | 40 708 (37.7) | 37 099 (38.1) | 3609 (27.8) | 0.221 | 39 594 (40.0) | 1114 (9.7) | 0.748 | |
| Vasodilator | 8712 (8.1) | 7951 (8.2) | 761 (5.9) | 0.091 | 8289 (8.4) | 423 (3.7) | 0.198 | |
| IV_iron | 110 400 | 1569 (1.4) | 1392 (1.4) | 177 (1.4) | 0.000 | 1457 (1.5) | 112 (1.0) | 0.158 |
Data presented as mean (SD) in the continuous variables, and numbers (n) with the percentage (%) in categorical variables.
A standardized difference of <0.1 was considered a negligible difference between groups.
Temp., temperature; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; CCI, Charlson Comorbidity Index; HD, hemodialysis; avg, average; Hb, hemoglobin; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; IV_iron, intravenous iron infusion.
Relationship between ambient temperature and IDH
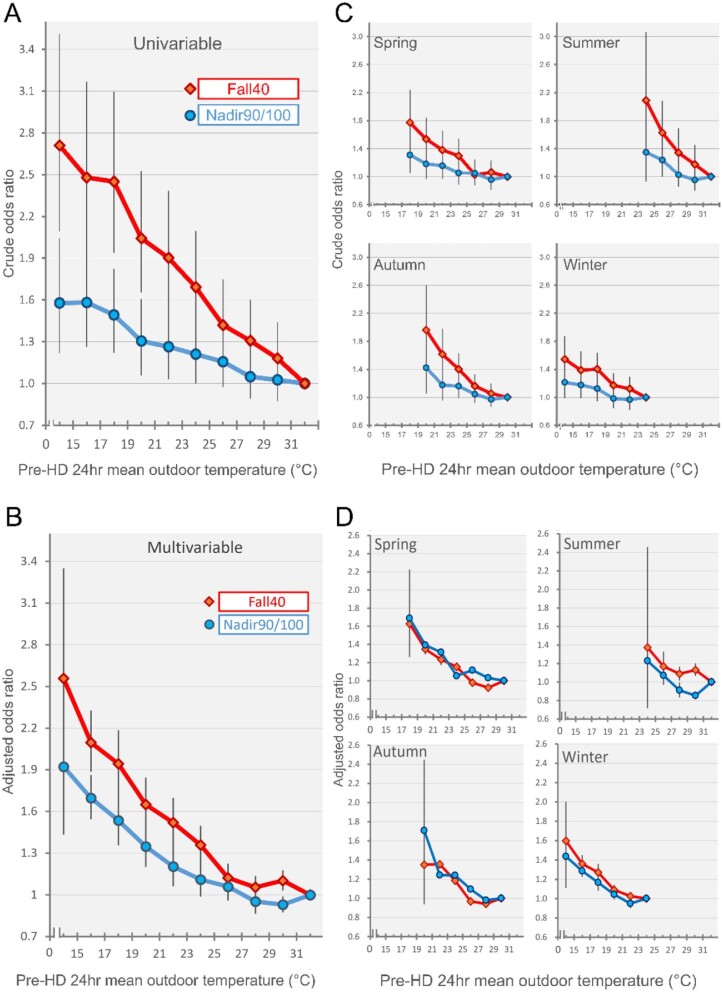
We observed that the relationship between ambient temperature and IDH was not linear but exhibited a reverse J-shaped pattern, indicating a possible inflection point around 27°C. The linear relationship was found only in temperatures below 27°C (Fig. 3A and B). We performed a test for interaction and found significant results with P-values of .001 and .033 for both Fall40 and Nadir90/100 definitions, suggesting that the effect of temperature on the risk of IDH was significantly different between temperatures above and below 27°C (Table 2). However, using 26°C and 28°C as alternative cut-off points, the Nadir90/100 definition showed no consistent, significant interaction. We then conducted stratified analyses. For temperatures above 27°C, the ORs of IDH were 0.924 (95% CI 0.772 to 1.105) for the Fall40 definition and 1.010 (95% CI 0.830 to 1.228) for the Nadir90/100 definition. In contrast, for temperatures below 27°C, the OR of IDH per 4°C decrease was significantly increased at 1.292 (95% CI 1.228 to 1.358) and 1.207 (95% CI 1.149 to 1.268) for the Fall40 and Nadir90/100 definitions, respectively. Therefore, all subsequent analyses were limited to temperatures <27°C.
Figure 3:
Relationship between ambient temperature and risk of IDH compared with the subsets of highest temperature. (A) displays the crude OR, while (B) shows the adjusted OR with adjustments made for age, sex, diabetes, coronary artery disease, Charlson Comorbidity Index, UF/DW, pre-HD SBP, blood flow, dialysate flow, dialysate mean temperature, conductivity, dialysate calcium, hemoglobin and antihypertensive medication use. Additionally, the relationship between environmental temperature and the risk of IDH is further examined by season in (C) and (D), with crude and adjusted ORs, respectively, using the same adjustments as in (B).
Table 2:
Logistic regression with GEE methods for risk of IDH per 4°C decrease above and below the threshold (27°C).
| Stratified by temperature | |||||
|---|---|---|---|---|---|
| Overall | ≤27°C | >27°C | |||
| Definition | (n = 105 895)a | (n = 59 751)a | (n = 46 144)a | P interaction b | |
| Fall40 | Adjustedc OR | 1.230 | 1.292 | 0.924 | |
| 95% CI | 1.175–1.289 | 1.228–1.358 | 0.772–1.105 | ||
| P-value | <.001 | <.001 | .39 | .001 | |
| Nadir90/100 | Adjustedc OR | 1.181 | 1.207 | 1.010 | |
| 95% CI | 1.126–1.238 | 1.149–1.268 | 0.830–1.228 | ||
| P-value | <.001 | <.001 | .92 | .033 | |
The number was reduced because of missing data.
Interaction for ambient temperature and temperature above/below the threshold 27°C.
Adjusted for age, sex, diabetes, coronary artery disease, CCI, HD vintage, UF/DW, pre-HD SBP, blood flow, dialysate flow, dialysate average temperature, conductivity, dialysate calcium, hemoglobin, antihypertensive medications and iron infusion.
Temperature–IDH relationship after multivariable adjustment
In temperatures below the threshold of 27°C, we conducted univariate and multivariable logistic regression analyses with adjustments for demographic data, dialysis-related variables and medications in Models 1–3 accordingly (Table 3). With IDH defined by Fall40, the crude and adjusted ORs were similar across the univariate and adjusted Models 1–3 with a change in OR <3%. Conversely, the OR increased from 1.123 in the crude OR to 1.207 in the fully adjusted OR, representing a 7% increase if IDH was defined by Nadir90/100. The fully adjusted models showed that each 4°C decrease from 27°C to 6.2°C was associated with an adjusted OR of 1.292 (95% CI 1.228 to 1.358) for Fall40 and 1.207 (95% CI 1.149 to 1.268) for Nadir90/100.
Table 3:
Univariate and multivariable logistic regression with GEE methods for risk of IDH per 4°C decrease below the threshold (27°C).
| Definition | Univariate | Model 1a | Model 2b | Model 3c | |
|---|---|---|---|---|---|
| Fall40 | OR | 1.244 | 1.257 | 1.273 | 1.292 |
| 95% CI | 1.186–1.305 | 1.197–1.319 | 1.210–1.338 | 1.228–1.358 | |
| P-value | <.001 | <.001 | <.001 | <.001 | |
| Nadir90/100 | OR | 1.123 | 1.121 | 1.185 | 1.207 |
| 95% CI | 1.078–1.169 | 1.074–1.170 | 1.131–1.242 | 1.149–1.268 | |
| P-value | <.001 | <.001 | <.001 | <.001 |
Model 1 adjusted for age, sex, diabetes, hypertension, coronary artery disease and Charlson Comorbidity Index.
Model 2 adjusted for covariates in Model 1 and additionally adjusted for hemodialysis vintage, UF/DW, pre-HD SBP, blood flow, dialysate flow, dialysate average temperature, conductivity and dialysate calcium.
Model 3 further adjusted for antihypertensive medications and iron infusion, but omitted covariate of hypertension to avoid collinearity.
Causal mediation analysis
Fluid removal (UF/DW) substantially mediated the effect of temperature on IDH risk, accounting for 23.16% (95% CI 21.23% to 25.40%) of the total effect (Supplementary data, Table S4). Both direct and mediated effects were significant.
Temperature effect on IDH across the four seasons
Analysis of interaction indicates that season significantly modifies the relationship between ambient temperature and IDH, according to the covariate-adjusted Nadir90/100 criteria (interaction P-value = .029). However, this interaction is not significant when assessed using the Fall40 criteria (interaction P-value = .728). After stratifying by four seasons, the low ambient temperature remained significantly associated with IDH in all seasons except summer. The adjusted ORs (95% CIs) of IDH defined by Fall40 were 1.267 (1.167 to 1.375), 0.863 (0.440 to 1.694), 1.368 (1.176 to 1.591) and 1.227 (1.136 to 1.325) in the spring, summer, autumn and winter, respectively. The corresponding ORs for the Nadir90/100 definition were 1.251 (1.134 to 1.381), 0.886 (0.409 to 1.919), 1.207 (1.010 to 1.443) and 1.182 (1.093 to 1.277) (Table 4, Fig. 3C and D).
Table 4:
Univariate and multivariable logistic regression for risk of IDH per 4°C decrease in four seasons (≤27°C).
| Sessions with temperature ≤27°C (n = 62 234) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||||||
| Definition | Spring (n = 18 781) | Summer (n = 3565) | Autumn (n = 14 362) | Winter (n = 25 526) | Spring (n = 17 372)b | Summer (n = 3271)b | Autumn (n = 13 383)b | Winter (n = 23 259)b | |
| Fall40 | OR | 1.278 | 1.260 | 1.415 | 1.189 | 1.267 | 0.863 | 1.368 | 1.227 |
| 95% CI | 1.196–1.366 | 0.753–2.108 | 1.232–1.625 | 1.119–1.264 | 1.167–1.375 | 0.440–1.694 | 1.176–1.591 | 1.136–1.325 | |
| P-value | <.001 | .38 | <.001 | <.001 | <.001 | .67 | <.001 | <.001 | |
| P interaction c | .343 | .728 | |||||||
| Nadir90/100 | OR | 1.136 | 0.884 | 1.158 | 1.110 | 1.251 | 0.886 | 1.207 | 1.182 |
| 95% CI | 1.050–1.229 | 0.510–1.533 | 1.006–1.333 | 1.047–1.177 | 1.134–1.381 | 0.409–1.919 | 1.010–1.443 | 1.093–1.277 | |
| P-value | .002 | .66 | .041 | <.001 | <.001 | .76 | .038 | <.001 | |
| P interaction c | .134 | .029 | |||||||
Adjusted for age, sex, diabetes, coronary artery disease, Charlson Comorbidity Index, hemodialysis vintage, UF/DW, pre-HD SBP, blood flow, dialysate flow, dialysate average temperature, conductivity, dialysate calcium, hemoglobin, antihypertensive medications and iron infusion.
The number is reduced because of missing data.
Interaction for season and ambient temperature.
Sensitivity analysis
We conducted sensitivity analyses incorporating an additional five definitions outlined in Supplementary data, Table S2. The temperature–IDH relationship based on these definitions revealed similar patterns (Supplementary data, Fig. S1). The characteristics of hemodialysis sessions according to various IDH definitions in sensitivity analysis are shown in Supplementary data, Table S5. The threshold was evident in all definitions with Pinteraction < .05 (Supplementary data, Table S6). We repeated multivariable logistic regression and found the ORs to be similar in magnitude to those obtained using the Fall40 and Nadir90/100 definitions outlined in Supplementary data, Table S7.
DISCUSSION
The current study found that low ambient temperature is associated with an increased risk of IDH. More specifically, for every 4°C drop in temperature, the OR of IDH increased by 1.29 and 1.21 for the reduction-based and nadir-based definitions, respectively. Furthermore, our analysis revealed a statistically significant threshold of 27°C across all seven predetermined IDH definitions, with the reduction-based definition (Fall40) being particularly relevant for cold temperatures. Moreover, ambient temperature's impact on IDH was 23% mediated by changes in UF (UF/DW). The relationship between temperature and IDH was consistent across all seasons except summer. These results have important implications for the management and care of hemodialysis patients, as well as for developing interventions and policies related to cold weather exposure.
In this study, we identified a threshold temperature of 27°C for the temperature–IDH relationship in southern Taiwan. An analysis of mortality in 11 Eastern US cities revealed that temperature was the most significant weather-related factor affecting mortality. Southern cities, which have similar latitudes to Taiwan, had a higher risk of mortality at lower temperatures, while lower risk at higher temperatures [33]. The study found threshold temperatures of 27.06°C and 27.18°C in Tampa and Miami, similar to a previously identified threshold of 27°C for cardiovascular mortality in Taiwan [6]. Although the threshold temperature associated with IDH risk may vary in different regions, the findings from our study might be generalized to regions with similar latitudes. Further studies across different regions and latitudes are needed.
The IDH definition is currently lacking consensus [13, 14, 34]. We used two primary definitions, both of which were reported to have a significant association with mortality in different patient populations. Shoji et al. [17] found that a reduction in systolic blood pressure SBP of more than 40 mmHg (Fall40) was associated with 2-year mortality in a Japanese population. Flythe et al. [16] reported that Nadir90/100 was consistently associated with mortality in two large cohorts in the USA. Our analysis found Fall40 to be more strongly associated with cold temperatures. Cold temperatures are associated with high UF rates, which are a risk factor for SBP decline and thus more likely to meet the criteria of Fall40. In addition, cold temperatures are linked to higher pre-HD SBP [35, 36], which may make individuals less likely to reach Nadir90/100 criteria, as the lowest intradialytic SBP determines it. Our results suggest that Fall40 is more sensitive to cold ambient temperatures. The potential impact of different definitions on the relationship between IDH and temperature should be further explored in future studies.
Previous research has shown that there are seasonal variations in hemodialysis initiation [37], reductions in intradialytic BP [27], heart failure [38, 39], acute myocardial infarctions [40] and overall mortality [41], with the highest risks observed in winter. Our study differentiates between “winter” and “low temperatures” as they are related but not contextually identical. We conducted a stratified analysis to examine the difference in the temperature–IDH relationship in four seasons. The results indicate that cold temperature remained a risk factor for IDH across seasons in spring, autumn and winter. This provided reassurance that certain season-related factors such as lifestyle, comorbidity and diet would not confound the relationship obverted in this study. In addition, such findings also challenged the perception that winter is the only season associated with an increased risk of IDH (Table 4, Fig. 3D).
The analysis showed no significant association in the summer, possibly due to the hot and stable weather of southern Taiwan's summer, which has a small temperature variation with an interquartile range of around 2.1°C (Fig. 2B). This lack of temperature variation may facilitate temperature acclimation, potentially reducing the risk of IDH [42–45]. Interestingly, the spring and autumn seasons had the highest adjusted ORs and most dispersed temperature distribution, aligning with the idea of temperature acclimation.
Our findings demonstrate a correlation between lower temperatures and increased IDH risk, with a negative relationship between temperature and UF (UF/DW) (Supplementary data, Fig. S2). Mediation analysis found that approximately one-quarter of the IDH risk from cold temperature was attributable to increased UF, suggesting that controlling UF volumes may offer an opportunity to partially mitigate the influence of low temperatures on IDH (Supplementary data, Table S4). Besides, previous research has established the efficacy of indoor heating as a means of combating cold outdoor temperatures. One randomized controlled trial evaluated the effect of intensive room heating on BP in healthy Japanese participants during the winter months [46]. The results showed that intensive heating was associated with a significant reduction in morning SBP and a suppression of morning pressure surges compared with weak heating. However, further research is needed to ascertain the potential benefits of this intervention for dialysis patients.
Our study has several strengths. First, the large sample size of over 110 000 dialysis sessions improves the assessment of temperature exposure. The study matched each patient to temperature data from the nearest weather station. Second, we have collected detailed parameters of dialysis, such as UF, dialysate temperature, dialysate calcium and hemodialysis vintage, which are all important factors related to IDH. Adjustments for these parameters largely reduced the potential for confounding. Third, as most dialysis patients have hypertension, we adjusted for antihypertensive medications, which may significantly impact IDH. Fourth, we included living and deceased patients from our dialysis center, reducing the potential selection and survivorship bias. Lastly, the temporal relationship between temperature exposure and IDH is clear, as the temperature is traced back according to the timing of each session.
Despite all the efforts, this study has limitations. One limitation is that we could only investigate ambient temperature, not indoor temperature. However, a prior study found a strong correlation between outdoor and indoor temperatures when the outdoor temperature exceeded 12.7°C [47]. Moreover, when cold waves strike, the actual temperature patients are exposed to indoors may not be as cold as the recorded outdoor temperature, introducing potential exposure misclassification. This could potentially bias our results toward the null, however the study still presents significant findings. While this study incorporates UF normalized to dry weight (UF/DW), the inclusion of bioimpedance measurements would offer a more detailed representation of body fluid changes, which can be considered in future studies. Additionally, there may be confounding factors, such as patients’ adaptive behaviors to cold, that were not accounted for. However, we believe that stratifying the data by season has partially controlled for these variables. Another limitation is that this study was conducted in Tainan, Taiwan, a subtropical climate city. Therefore, it is unclear whether the findings apply to places with different climates or latitudes. Further research is needed to assess the generalizability of the findings.
CONCLUSION
Our study provides important insights into the relationship between low ambient temperature and IDH. Low ambient temperature before hemodialysis is associated with an increased risk of IDH regardless of IDH definitions. Future studies should investigate the potential benefits of interventions such as indoor heating for dialysis patients and the generalizability of these findings to other latitudes and climates. In addition, clinicians should consider environmental temperature a potential risk factor in patient care.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU). We would like to acknowledge the contributions of Tsung-Han Wu, from the Department of Computer Science and Information Engineering at National Cheng Kung University, for his expertise in GPS matching and data merging. Furthermore, we are grateful for the insightful advice provided by Jian-An Wang, from the Institute of Data Science at the College of Medicine at National Cheng Kung University, on the data visualization of the matching process.
Contributor Information
Kuan-Hung Liu, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wei-Hsiang Chang, Department of Food Safety/ Hygiene and Risk Management, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Research Center of Environmental Trace Toxic Substances, National Cheng Kung University, Tainan, Taiwan.
Edward Chia-Cheng Lai, School of Pharmacy, Institute of Clinical Pharmacy and Pharmaceutical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Pei-Chen Tsai, Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan, Taiwan.
Bin Hsu, Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yu-Hsuan Yang, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wei-Ren Lin, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Tzu-Shan Huang, Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Fang-Yi Su, Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan, Taiwan.
Jung-Hsien Chiang, Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan, Taiwan.
Chung-Yi Li, Department of Public Health, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Public Health, College of Public Health, China Medical University, Taichung, Taiwan; Department of Healthcare Administration, College of Medical and Health Science, Asia University, Taichung, Taiwan.
Yau-Sheng Tsai, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Junne-Ming Sung, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
FUNDING
This work was supported by grants from the National Science and Technology Council (NSTC 112-2314-B-006-088-MY3), the National Cheng Kung University Hospital (NCKUH-11106021) and the Ministry of Science and Technology of Taiwan (MOST-111-2314-B-006-068). The funding sources did not influence the study design, analysis, interpretation or approval of the manuscript.
AUTHORS’ CONTRIBUTIONS
K.-H.L., J.-M.S., Y.-S.T. and C.-Y.L. conceptualized and designed the study. T.-S.H., P.-C.T., F.-Y.S., and J.-H. C. performed data curation and GPS matching. J.-H.Jiang provided supervision for data curation and matching process. K.-H.L. and B.H. conducted the main statistical analysis, assisted by P.-C.T., W.-R.L. and F.-Y.S. C.-Y.L., W.-H.C., E.C.-C.L. and Y.-S.T. advised on statistical aspects and interpretation of the data. K.-H.L. wrote the original draft of the manuscript. C.-Y.L., J.-M.S., W.-H.C., E.C.-C.L. and Y.-H.Y. reviewed and edited the manuscript. All the authors participated in reviewing the manuscript and approved the final version to be published.
DATA AVAILABILITY STATEMENT
The data sharing is beyond the scope of the authorization from the patient's informed consent and from the Institutional Review Board.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1. Deschenes O. Temperature, human health, and adaptation: a review of the empirical literature. Energy Econ 2014;46:606–19. 10.1016/j.eneco.2013.10.013 [DOI] [Google Scholar]
- 2. McMichael AJ. Globalization, climate change, and human health. N Engl J Med 2013;368:1335–43. 10.1056/NEJMra1109341 [DOI] [PubMed] [Google Scholar]
- 3. Bai L, Li Q, Wang Jet al. . Increased coronary heart disease and stroke hospitalisations from ambient temperatures in Ontario. Heart 2018;104:673–9. 10.1136/heartjnl-2017-311821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng J, Su H, Xu Zet al. . Extreme temperature exposure and acute myocardial infarction: elevated risk within hours? Environ Res 2021;202:111691. 10.1016/j.envres.2021.111691 [DOI] [PubMed] [Google Scholar]
- 5. Fukuda H, Ninomiya H, Ueba Yet al. . Impact of temperature decline from the previous day as a trigger of spontaneous subarachnoid hemorrhage: case-crossover study of prefectural stroke database. J Neurosurg 2019;133:374–82. 10.3171/2019.4.JNS19175 [DOI] [PubMed] [Google Scholar]
- 6. Yang LT, Chang YM, Hsieh THet al. . Associations of ambient temperature with mortality rates of cardiovascular and respiratory diseases in Taiwan: a subtropical country. Acta Cardiol Sin 2018;34:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohammadi D, Zare Zadeh M, Zare Sakhvidi MJ.. Short-term exposure to extreme temperature and risk of hospital admission due to cardiovascular diseases. Int J Environ Health Res 2021;31:344–54. 10.1080/09603123.2019.1663496 [DOI] [PubMed] [Google Scholar]
- 8. Remigio RV, Turpin R, Raimann JGet al. . Assessing proximate intermediates between ambient temperature, hospital admissions, and mortality in hemodialysis patients. Environ Res 2022;204:112127. 10.1016/j.envres.2021.112127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Remigio RV, Jiang C, Raimann Jet al. . Association of extreme heat events with hospital admission or mortality among patients with end-stage renal disease. JAMA Netw Open 2019;2:e198904. 10.1001/jamanetworkopen.2019.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cozzolino M, Mangano M, Stucchi Aet al. . Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018;33:iii28–34. 10.1093/ndt/gfy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang TI, Paik J, Greene Tet al. . Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 2011;22:1526–33. 10.1681/ASN.2010101119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ronco C, Brendolan A, Milan Met al. . Impact of biofeedback-induced cardiovascular stability on hemodialysis tolerance and efficiency. Kidney Int 2000;58:800–8. 10.1046/j.1523-1755.2000.00229.x [DOI] [PubMed] [Google Scholar]
- 13. Sars B, van der Sande FM, Kooman JP.. Intradialytic hypotension: mechanisms and outcome. Blood Purif 2020;49:158–67. 10.1159/000503776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanbay M, Ertuglu LA, Afsar Bet al. . An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J 2020;13:981–93. 10.1093/ckj/sfaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoccali C, Benedetto FA, Tripepi Get al. . Cardiac consequences of hypertension in hemodialysis patients. Semin Dial 2004;17:299–303. 10.1111/j.0894-0959.2004.17331.x [DOI] [PubMed] [Google Scholar]
- 16. Flythe JE, Xue H, Lynch KEet al. . Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 2015;26:724–34. 10.1681/ASN.2014020222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shoji T, Tsubakihara Y, Fujii Met al. . Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 2004;66:1212–20. 10.1111/j.1523-1755.2004.00812.x [DOI] [PubMed] [Google Scholar]
- 18. Gul A, Miskulin D, Harford Aet al. . Intradialytic hypotension. Curr Opin Nephrol Hypertens 2016;25:545–50. 10.1097/MNH.0000000000000271 [DOI] [PubMed] [Google Scholar]
- 19. Stefánsson BV, Brunelli SM, Cabrera Cet al. . Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol 2014;9:2124–32. 10.2215/CJN.02680314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sands JJ, Usvyat LA, Sullivan Tet al. . Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int 2014;18:415–22. 10.1111/hdi.12138 [DOI] [PubMed] [Google Scholar]
- 21. Davenport A. Why is intradialytic hypotension the commonest complication of outpatient dialysis treatments? Kidney Int Rep 2023;8:405–18. 10.1016/j.ekir.2022.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung AK, Yan G, Greene Tet al. . Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol 2002;13:2345–52. 10.1097/01.ASN.0000026611.07106.A7 [DOI] [PubMed] [Google Scholar]
- 23. Flythe JE, Assimon MM, Wenger JBet al. . Ultrafiltration rates and the quality incentive program: proposed measure definitions and their potential dialysis facility implications. Clin J Am Soc Nephrol 2016;11:1422–33. 10.2215/CJN.13441215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alba BK, Castellani JW, Charkoudian N.. Cold-induced cutaneous vasoconstriction in humans: function, dysfunction and the distinctly counterproductive. Exp Physiol 2019;104:1202–14. 10.1113/EP087718 [DOI] [PubMed] [Google Scholar]
- 25. Wilson TE, Gao Z, Hess KLet al. . Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 2010;298:R1627–33. 10.1152/ajpregu.00099.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duranton F, Kramer A, Szwarc Iet al. . Geographical variations in blood pressure level and seasonality in hemodialysis patients. Hypertension 2018;71:289–96. 10.1161/HYPERTENSIONAHA.117.10274 [DOI] [PubMed] [Google Scholar]
- 27. Uchiyama K, Shibagaki K, Yanai Aet al. . Seasonal variation and predictors of intradialytic blood pressure decline: a retrospective cohort study. Hypertens Res 2021;44:1417–27. 10.1038/s41440-021-00714-1 [DOI] [PubMed] [Google Scholar]
- 28. Robusto CC. The Cosine-Haversine formula. Am Math Mon 1957;64:38–40. 10.2307/2309088 [DOI] [Google Scholar]
- 29. Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology 2001;12:662–7. [DOI] [PubMed] [Google Scholar]
- 30. Charlson ME, Pompei P, Ales KLet al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 31. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tingley D, Yamamoto T, Hirose Ket al. . mediation: R package for causal mediation analysis. J Stat Soft 2014;59:1–38. 10.18637/jss.v059.i05 [DOI] [Google Scholar]
- 33. Curriero FC, Heiner KS, Samet JMet al. . Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol 2002;155:80–7. 10.1093/aje/155.1.80 [DOI] [PubMed] [Google Scholar]
- 34. Assimon MM, Flythe JE.. Definitions of intradialytic hypotension. Semin Dial 2017;30:464–72. 10.1111/sdi.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartwig SV, de Souza Hacon S, de Oliveira BFAet al. . The effect of ambient temperature on blood pressure of patients undergoing hemodialysis in the Pantanal-Brazil. Heliyon 2021;7:e07348. 10.1016/j.heliyon.2021.e07348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Z, Lan S, Chen Cet al. . Seasonal variation: a non-negligible factor associated with blood pressure in patients undergoing hemodialysis. Front Cardiovasc Med 2022;9:820483. 10.3389/fcvm.2022.820483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Obi Y, Kalantar-Zadeh K, Streja Eet al. . Seasonal variations in transition, mortality and kidney transplantation among patients with end-stage renal disease in the USA. Nephrol Dial Transplant 2017;32:ii99–105. 10.1093/ndt/gfw379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boulay F, Berthier F, Sisteron Oet al. . Seasonal variation in chronic heart failure hospitalizations and mortality in France. Circulation 1999;100:280–6. 10.1161/01.CIR.100.3.280 [DOI] [PubMed] [Google Scholar]
- 39. Stewart S, McIntyre K, Capewell Set al. . Heart failure in a cold climate. Seasonal variation in heart failure-related morbidity and mortality. J Am Coll Cardiol 2002;39:760–6. 10.1016/S0735-1097(02)01685-6 [DOI] [PubMed] [Google Scholar]
- 40. Nagarajan V, Fonarow GC, Ju Cet al. . Seasonal and circadian variations of acute myocardial infarction: findings from the Get With The Guidelines-Coronary Artery Disease (GWTG-CAD) program. Am Heart J 2017;189:85–93. 10.1016/j.ahj.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 41. Usvyat LA, Carter M, Thijssen Set al. . Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin J Am Soc Nephrol 2012;7:108–15. 10.2215/CJN.03880411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Daanen HA, Van Marken Lichtenbelt WD.. Human whole body cold adaptation. Temperature 2016;3:104–18. 10.1080/23328940.2015.1135688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yurkevicius BR, Alba BK, Seeley ADet al. . Human cold habituation: physiology, timeline, and modifiers. Temperature 2022;9:122–57. 10.1080/23328940.2021.1903145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leppäluoto J, Korhonen I, Hassi J.. Habituation of thermal sensations, skin temperatures, and norepinephrine in men exposed to cold air. J Appl Physiol 2001;90:1211–8. 10.1152/jappl.2001.90.4.1211 [DOI] [PubMed] [Google Scholar]
- 45. Stewart S, Keates AK, Redfern Aet al. . Seasonal variations in cardiovascular disease. Nat Rev Cardiol 2017;14:654–64. 10.1038/nrcardio.2017.76 [DOI] [PubMed] [Google Scholar]
- 46. Saeki K, Obayashi K, Iwamoto Jet al. . Influence of room heating on ambulatory blood pressure in winter: a randomised controlled study. J Epidemiol Community Health 2013;67:484–90. 10.1136/jech-2012-201883 [DOI] [PubMed] [Google Scholar]
- 47. Nguyen JL, Schwartz J, Dockery DW.. The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 2014;24:103–12. 10.1111/ina.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing is beyond the scope of the authorization from the patient's informed consent and from the Institutional Review Board.