Abstract
Background:
The use of cesarean section (CS) worldwide has increased to unprecedented levels. In Ethiopia, the CS delivery rate is above the rate recommended by the WHO. The postoperative pain experience is moderate to severe in most patients during their postoperative period. The administration of intravenous dexamethasone is thought to have an analgesic effect after surgery even though the analgesic profile of preoperatively administered dexamethasone is less addressed.
Objective:
This study aimed to assess the postoperative analgesic effect of preoperative intravenous dexamethasone for patients undergoing cesarean delivery under spinal anesthesia at Dilla University Referral Hospital, Southern Ethiopia.
Methodology:
A double-blinded randomized controlled trial (RCT) was done on 112 patients undergoing elective CS under spinal anesthesia who were allocated randomly into normal saline and dexamethasone groups. Total analgesic consumption, time to first analgesic request, and postoperative pain score with the numerical rating scale (NRS) were followed for 24 h in both groups. Shapiro–Wilk tests were used to check normality. Independent samples t-test was used for the comparison of means between groups, Mann–Whitney U test for non-normally distributed data, and χ 2 test for categorical variables, and P-value <0.05 was considered statistically significant with a power of 80%.
Result:
The finding of this study showed that the postoperative pain score of the dexamethasone group was significantly lower than the normal saline group at 2, 4, 6, 12, 18, and 24 h with a statistically significant P-value <0.05. There was also a significant difference in the time to the first rescue analgesic request between the two groups, with the dexamethasone group (median=347.5 min) and the normal saline group (median=230 min) with P=0.001.
Conclusion and recommendation:
The authors conclude that preoperative administration of 8 mg of dexamethasone prolongs the first analgesic request time, decreases postoperative tramadol and diclofenac consumption, and decreases the postoperative pain score. The authors recommend that researchers conduct further RCTs with a different dose of dexamethasone and on a multicenter basis.
Keywords: cesarean delivery, cesarean section, dexamethasone, intravenous, postoperative analgesia
Background
Highlights
Preoperative administration of 8 mg of dexamethasone prolongs the first analgesic request time.
Postoperative pain score of the dexamethasone group was significantly lower than the normal saline group.
The mean total analgesic consumption in 24 h in the normal saline group was higher than in the dexamethasone group.
Cesarean section (CS) is the birth of a fetus through incisions in the abdominal and uterine walls. Its rate is increasing worldwide to unprecedented levels. The latest data from 150 countries indicates CS delivery is increasing both in the least and most developed regions. The largest absolute increases occurred in Latin America (22.8–42.2%) and Africa (2.9–7.4%)1. In Ethiopia, the CS delivery rate is above the rate recommended by the World Health Organization. The prevalence of CS in Dessie, Addis Ababa, and Hawassa was reported to be 47.6%, 21.4%, and 49.3%, respectively2–4.
The revised International Association for the Study of Pain (IASP) defines pain as ‘An unpleasant sensory and emotional experience associated with actual or potential tissue damage’5. Incidence of moderate to severe pain intensity in the first 24 h after surgery and pain with its effective management remains an issue among surgical patients6. According to the US Institute of Medicine, 80% of patients who undergo surgery report postoperative pain, of which 88% report moderate, severe, or extreme pain levels7. In Ethiopia, moderate to severe pain was reported in 85 (57%) patients in the immediate postoperative period and 117 (78%) in the first 12 h, while female gender and incision length more than 10 cm were identified as independent risk factors for postoperative pain severity8.
Similarly, the prevalence of moderate to severe postoperative pain after CS was high with 85.5% within the first 24 postoperative hours9. Evidence also supports that the postoperative pain after CS is much higher than previous10. Severe acute postoperative pain is the most common independent risk factor for the development of chronic post-CS pain11,12. The incidence of chronic pain after CS is higher in patients with high postoperative pain scores than in patients with lower pain scores13,14.
Postoperative pain can affect and be associated with increased length of hospital stay, morbidity, functional impairment, prolonged duration of opioid use, delayed recovery time, decreased patient mobilization after surgery, and affect patient satisfaction15,16. The use of a procedure-specific, multimodal perioperative pain management provides a rational basis for enhanced postoperative pain control, optimization of analgesia, decrease in adverse effects, and improved patient satisfaction17–19.
Different adjuvants and intrathecal opioids could be used with spinal anesthesia (SA) to reduce postoperative pain for patients undergoing cesarean delivery (CD) However, a complication related to those drugs is a concern while using them for postoperative pain20,21 and led to the mandatory alternative option for non‑opioid analgesics in postoperative pain associated with CS22–24. Even though nonsteroidal anti-inflammatory drugs (NSAIDs) are used to improve postoperative pain relief after CD, potential side effects like bleeding are the most common concern25,26. On the other hand, preoperative gabapentin used as multimodal analgesia reduces post-CS pain and increases maternal satisfaction. However, it is associated with severe sedation27. Administration of neostigmine as a neuraxial adjuvant enhances postoperative analgesia, but it has been associated with a high incidence of prolonged motor blockade, nausea, and vomiting even with a spinal dose.
Intravenous injection of dexamethasone is thought to reduce postoperative pain, though analgesic effect after surgery and the preoperative administration of this drug produces less variation of effects on pain outcomes28,29. Kinds of literature also support the local infiltration, intrathecal, and systemic administration of dexamethasone for postoperative analgesia. It is also thought to reduce the incidence of postoperative nausea and vomiting in patients undergoing CS and lower extremity orthopedic surgery under SA30–32.
The scarcity of well-controlled trials on the effect of preoperative intravenous dexamethasone on postoperative pain after CS in a developing country, easy availability, safety, and fewer side effects of this drug is an initiative for this study. There is also a conflict in the report on the effect of preoperative dexamethasone on postoperative pain on visual analog pain score and the reduction of postoperative rescue analgesic requests after CS33. Therefore, the result of our study will be used as baseline data for further research and to show possible alternative postoperative pain management modalities for CS in resource-constrained settings.
The main aim of this study was to investigate the effect of preoperative intravenous dexamethasone on postoperative pain for patients undergoing elective CS.
Methodology
A double-blind randomized controlled trial (RCT) was conducted from 10 January 2022 to 10 January 2023. After receiving an ethical clearance from Dilla University institutional review board with protocol unique no: 008/19-10, all ASA II parturient mothers who underwent elective CD under SA were included in the study, while patients with a history of diabetes mellitus, heart failure, preeclampsia, eclampsia, patients unable to self-report acute pain, cognitive impairment (dementia, Alzheimer’s disease), and acute/chronic pain diagnosis were excluded from this study. This study was reported in line with the 2010 consort guideline for clinical trials at http://www.consortstatement.org and also registered according to the Declaration of Helsinki 201334 on the research registry and has a unique identifying number of the researchregistry8544 (https://www.researchregistry.com/browse-the-registry#home/). The study was also registered on pan African clinical trial with a unique identification number of PACTR202310828674007.
Sample size and sampling procedure
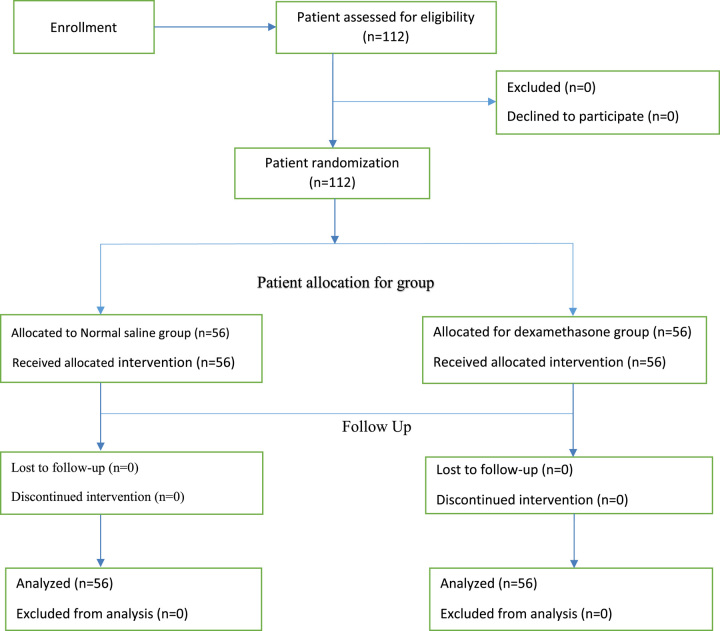
The sample size was calculated based on the previous study done in Iran that shows the mean pain score of μ1=1, SD1=1 for the normal saline group and μ2=2.3, SD2=2.2 for the dexamethasone group for 24 h35 to obtain the largest sample size. By using α (alpha)=0.05 and 80% power (β=0.2), a priori power analysis with G power 3.1.9.2 version software was used to calculate a sample size to be 102. An additional 10% was added to the enrollment to offset the potential loss to follow-up and assuming a balanced design, the total sample size became 112 participants with 56 in each group. Based on situational analysis with exclusion criteria for 12 months before the study period, about 450 patients underwent elective CS at Dilla University Referral Hospital. Thus, our K=N/n is 4, which is our skipping interval. Based on our inclusion criteria, each patient had a 25% equal chance to be a study subject during the 12 months of our data collection. The first patient was recruited by lottery method and then one patient from four consecutive patients was included by systematic random sampling method from the expected 450 patients or more during our data collection period until the required sample size was achieved. The enrollment of the participants in the trial was explained in line with the Consort 2010 flow diagram (http://www.consort-Flow%20Diagram.doc) (Fig. 1).
Figure 1.
Flow diagram of the participants in the study.
Blinding and randomization
After meeting the inclusion criteria, patients were randomly allocated to the normal saline and dexamethasone group by lottery method. Randomization was conducted by drawing one of the two labels from a sealed envelope containing either label ‘01’ or ‘02’, where ‘01’ stands for the normal saline group and ‘02’ for the dexamethasone group. By using the operation schedule as a sampling frame, the first patient was allocated to a group with a lottery method. Group I took 2 ml of normal saline, which was labeled as 01 with opaque paper, and Group II took 2 ml of 8 mg dexamethasone, which was labeled as 02 and was immediately administered before surgery after SA. Both the anesthesia clinician and the data collector were blinded.
Data collection process and analysis procedure
After a pretest was done on 5% of the total sample size, which was not included in the actual analysis processes, all patients who fulfilled eligibility criteria were informed about the benefits, risks, and objectives of the study the night before surgery by the principal investigator. On the morning of surgery, both NPO status and informed consent were checked in addition to the special consent that a patient signed to be involved in the research. Data collection was carried out by a standardized and validated questionnaire, which consisted of general patient characteristics information, and it was prepared in English and filled by a BSc anesthetist who was already blinded to label coded syringes. Patient medical charts, vital sign charts, monitors, and patient interviews were used as the data source. A description of the objectives of the study, the benefits and risks, and the consent form were prepared in English and Amharic languages. Patients who met the inclusion criteria and underwent elective CS under SA were recruited into the study during the data collection period.
After completion of data collection, the data were checked for errors and the data were coded. The coded data were entered into the SPSS version 25 statistical package, and descriptive statistics, tables, figures, and narratives were used to summarize the data. The normality distribution for both groups was assessed by visual inspection.
Statistical tests and visual inspection were employed to check the normal distribution of data by the Shapiro–Wilk test, histogram, Q–Q plot, box plot, kurtosis, and skewness and homogeneity of variance were assessed by using Levene’s test. Analytic statistics were calculated by independent samples t-test for comparison of means between groups, Mann–Whitney U test for non-normally distributed data, and χ 2 for categorical variables. After Levene’s test for equality of variances was done, Welch’s t-test was used in case the assumption of homogeneity of variances was violated.
P-value <0.05 was considered statistically significant. Normally distributed numerical data were presented as mean±standard deviation, non-normally distributed numerical data were presented by median interquartile range (IQR), and categorical data were presented as numbers (percentage).
Results
Sociodemographic data
Fifty-six patients were included in each study group in which there was no lost follow-up. There was no statistically significant difference between the two groups in terms of age, weight, and BMI with P>0.05 (Table 1). The mean height of the normal saline group in meters (1.65±0.05) was comparable to that of the dexamethasone group in meters (1.66±0.05). There was also no significant difference between the two groups concerning gravida, parity, preoperative diagnosis, and gestational age (P-value >0.05). A common indication for CS was two and one previous CS scar with no statistical difference between the two groups. There was no significant difference between the groups in terms of preoperative diagnosis, educational status, gestational age, and previous history of CS (Tables 1 and 2).
Table 1.
Demographic characteristics of the study participants who underwent elective cesarean section
| Patient characteristics | Normal saline (n=56) | Dexamethasone (n=56) | Difference | 95% CI | P |
|---|---|---|---|---|---|
| Age (years) | 26.51±2.58 | 26.99±2.76 | −0.536 | −1.569 to 0.498 | 0.144 |
| Weight (kg) | 63.49±3.88 | 62.82±3.37 | 0.3214 | −1.0736 to 1.7165 | 0.145 |
| Height (m) | 1.65±0.05 | 1.66±0.05 | −0.00714 | −0.02703 to 0.01275 | 0.288 |
| BMI (kg/m2) | 23.65±1.49 | 23.42±1.23 | 0.10536 | −0.40319 to 0.61391 | 0.164 |
| Educational status | |||||
| Never attended school | 17 (30.4) | 20 (35.7) | 0.435 | ||
| Primary school | 19 (33.9) | 13 (23.2) | |||
| High school | 16 (28.6) | 15 (26.8) | |||
| Higher institution | 4 (7.1) | 8 (14.3) | |||
Values are presented as mean±SD, independent student t-test, frequency(%), χ 2 test.
P<0.05 is considered statistically significant.
Table 2.
Surgical characteristics of patients who underwent elective cesarean section
| Characteristics | Normal saline, N (%) | Dexamethasone (%) | P |
|---|---|---|---|
| Preoperative diagnosis | |||
| Two cesarean sections | 24 (42.9) | 19 (33.9) | 0.74 |
| One cesarean section | 10 (17.9) | 12 (21.4) | |
| Fetal macrosomia | 10 (17.9) | 12 (21.4) | |
| Oligohydramnios | 7 (12.5) | 5 (8.9) | |
| Opted for cesarean section | 5 (8.9) | 8 (14.3) | |
| Gravid | |||
| 1 | 7 (12.5) | 5 (8.9) | 0.409 |
| 2 | 13 (23.2) | 21 (37.5) | |
| 3 | 20 (35.2) | 18 (32.1) | |
| 4 | 16 (28.6) | 12 (21.4) | |
| Parity | |||
| Primiparous | 6 (10.7) | 5 (8.9) | 0.751 |
| Multiparous | 50 (89.3) | 51 (91.1) | |
| Previous history of CS | |||
| Yes | 34 (60.7) | 31 (55.35) | 0.445 |
| No | 22 (39.3) | 25 (44.64) | |
| Gestational age | |||
| Term | 49 (87.5) | 50 (89.3) | 0.768 |
| Post-term | 7 (12.5) | 6 (10.7) | |
Values are presented as frequency(%), χ 2 test.
P<0.05 is considered statistically significant.
Level of sensory block, Bromage scale, baseline respiratory rate (RR), and intraoperative blood loss were comparable between the two groups. The proportion of postoperative nausea vomiting (PONV) was high in normal saline groups even though statistically not significant (Table 3).
Table 3.
Block and clinical characteristics of patients who underwent elective cesarean
| Clinical characteristics | Normal saline | Dexamethasone | P |
|---|---|---|---|
| Spinal injection to skin incision time in minutes | 5±1.335 | 5.52±1.888 | 0.097 |
| Time from skin incision to baby out in minutes | 5.96±3.693 | 5.20±1.793 | 0.165 |
| Level of Bromage scale | 3 (3–3) | 3 (3–3) | |
| Level of sensory block | 6 (6–6) | 6 (6–6) | |
| The intraoperative fluid used in ml | 1954.17±99.91 | 1951.69±102.72 | 0.844 |
| Intraoperative blood loss in ml | 507.5±84.71 | 518.25±78.66 | 0.292 |
| Baseline RR | 19.45±2.45 | 18.86±1.72 | 0.144 |
| Baseline SpO2 | 97.41±1.005 | 95.59±1.05 | 0.199 |
| PONV | |||
| Nausea | 21 (37.5) | 12 (21.4) | 0.095 |
| Vomiting | 11 (19.6) | 9 (16.1) | |
Values are presented as mean±SD, independent t-test and frequency (%), χ 2 test and median IQR, Mann–Whitney U test.
IQR, interquartile range; RR, respiratory rate.
P<0.05 is considered statistically significant.
Primary outcome results
The time to first analgesia request
A Mann–Whitney U test revealed a significant difference in the time to the requirement of first rescue analgesia for the dexamethasone group (median=347.5 min, n=56) and the normal saline group (median=230 min, n=56), in which the result in dexamethasone group is significantly higher than the normal saline group with P-value=0.001. In this study, we found that there was a significant difference between the groups in the median time for the first analgesic requirement, with the dexamethasone group median of 347.5 (320–360) and normal saline group median of 230 (192.5–240), with a statistically significant difference of U=3016, Z=8.455, and P=0.001.
Total analgesic consumption in 24 h
The mean total consumption of tramadol in 24 h in the normal saline group was higher (108.04±24.821) than in the dexamethasone group (97.32±11.360), a statistically significant difference of 10.714 (95% CI, 3.451–17.978), t (77.075)=2.937, and P=0.004. The mean total consumption of diclofenac in 24 h in the normal saline group was higher (104.91±21.543) than in the dexamethasone group (92.86±30.790), a statistically significant difference of 12.054 (95% CI, 2.089–22.018), t (98.438)=2.400, and P=0.018.
Results for postoperative pain score
A Mann–Whitney U test revealed a significant difference in the postoperative pain with the numerical rating scale (NRS) score both at rest and voluntary coughing at 2, 4, 6, 12, 18, and 24 h between normal saline and dexamethasone groups (Table 4).
Table 4.
Comparison of postoperative pain using 11-point NRS score (0–10) at rest and voluntary coughing for the patients who underwent elective cesarean section
| Time interval | @15 min | @2nd hour | @4th hour | @6th hour | @12th hour | @18th hour | @24th hour |
|---|---|---|---|---|---|---|---|
| Resting NRS | |||||||
| Normal saline | 0 0 |
1 (0–1) | 2 (1–3) | 3 (2–4) | 4 (3–4) | 4 (2.25–4) | 3 (3–4) |
| Dexamethasone | 0 0 |
0 (0–1) | 1 (1–2) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–4) |
| P | 0.317 | 0.041* | 0.0030* | 0.005* | 0.001* | 0.009* | 0.0130* |
| Coughing NRS | |||||||
| Normal saline | 0 0 |
1 (1–2) | 2 (1–3) | 4 (3–4) | 4 (2–4.75) | 4 (3–4) | 4.5 (3–6) |
| Dexamethasone | 0 | 1 (0–1) | 1 (1–2) | 3 (2.25–4) | 3 (2–3) | 3 (3–4) | 4 (3–4.75) |
| P | 0.99 | 0.019* | 0.0011* | 0.0001* | 0.001* | 0.004* | 0.012* |
Values are presented as median IQR, Mann–Whitney U test.
IQR, interquartile range; NRS, numerical rating scale.
*P<0.05 is considered statistically significant.
Secondary outcome variables
There was no significant difference between the two groups in terms of respiratory rate, SPO2, and PONV. The proportion of PONV was higher in the normal saline group than in the dexamethasone group even though it was not statistically significant (Table 3). There was also no significant difference between the two groups in hemodynamic parameters like heart rate, systolic blood pressure, and mean arterial pressure (Figs 2–4).
Figure 2.
Mean heart rate for patients who underwent elective cesarean section.
Figure 4.
Mean arterial pressure for patients who underwent elective cesarean section.
Figure 3.
Mean systolic blood pressure for patients who underwent elective cesarean section.
Discussion
In this study, the median time to first analgesic request was prolonged in dexamethasone as compared with normal saline, the postoperative pain score decreased both at rest and on voluntary coughing, and total tramadol and diclofenac consumption was reduced in the dexamethasone group as compared with the normal saline group for patients who underwent elective CS under SA. Anti-inflammatory action and inhibition of phospholipase A2 by dexamethasone results in decreased production of various inflammatory mediators that play a major role in amplifying and maintenance of pain perception, which reduces postoperative pain by dexamethasone theoretically36.
Our finding indicates there was a significant difference between the groups in the median time for the first analgesic requirement, which is consistent with a study done in India by Shalu et al.37 on the effect of intravenous dexamethasone on the duration of SA in CS, which shows the mean time to first rescue analgesic request was 8.67 h in the dexamethasone group and 4.40 h in the normal saline group.
The finding of this study is also in line with a study done by Melese et al.38 in Ethiopia, which shows that there is a significant difference in the requirement of first rescue analgesia between the dexamethasone group (median=6.58 h, n=32) and the non-dexamethasone group (median=4.1 h, n=32). Those findings are also comparable to a study done in India39 which shows a significant prolongation of the first analgesic request time in the dexamethasone group (297.83±29.56) than in the normal saline group (175.50±29.17). Another finding of their study indicates that there is a statistical difference between the dexamethasone group and the normal saline group, in which the incidence of nausea and vomiting is higher in the normal saline group than the dexamethasone group, which is inconsistent with our finding. This inconsistency may be due to the reason that in our study we gave metoclopramide, which reduces postoperative nausea and vomiting.
Our finding indicates that the total tramadol consumption in the normal saline group was also higher (108.04±24.821) than the dexamethasone group (97.32±11.360), with a statistically significant difference of 10.714 (95% CI, 3.451–17.978), t (77.075)=2.937, and P=0.004. The 24-h mean total diclofenac consumption was higher in the normal saline group (104.91±21.543) as compared with the dexamethasone group (92.86±30.790), and there was a statistically significant difference of 12.054 (95% CI, 2.089–22.018), t (98.438)=2.400, and P=0.018. This finding is consistent with the RCT done in Iran to assess the effect of intravenous dexamethasone on postoperative pain after CS, which shows that there is a statistically significant difference in terms of total morphine consumption between the dexamethasone (average of 4 mg) and control (average of 8 mg) groups35. This result is in line with a study done in Ethiopia, which shows that there is a significant difference in total tramadol consumption of dexamethasone and non-dexamethasone groups. Contrary to our finding, there is no statistically significant difference in terms of diclofenac consumption between the dexamethasone and the non-dexamethasone groups. The difference in study design and sample size may be the probable reason for this variability38.
Our finding is inconsistent with the study done in Iran on the effect of 0.1 mg/intravenous administration of dexamethasone and normal saline on postoperative pain for the patient undergoing inguinal herniorrhaphy after the administration of intrathecal anesthesia with meperidine 15 mg. In this study, the median IQR for total diclofenac consumption in milligrams was 32.5 (0–225), which is statistically significant (P<0.05). Most likely, this inconsistency might be due to the difference in the administration of 15 mg of intrathecal meperidine and 75 mg of intramuscular diclofenac injection every 6 h, and there were also differences in surgical procedure and population20.
A RCT done in South Korea shows that single intravenous administration of dexamethasone during the preoperative period does not reduce epidural patient-controlled analgesic opioid consumption between the control and dexamethasone infusion groups40. This finding is contrary to our finding, which shows there is a significant difference between normal saline and dexamethasone groups in terms of tramadol and diclofenac consumption. The probable cause for the inconsistency of our result may be due to the heterogeneity of the study population, the surgical procedure, and the technique of postoperative pain management.
The finding of this study on total consumption of diclofenac was also in line with a study done in India by Memon et al. to assess the effect of single-dose intravenous dexamethasone on postoperative pain in patients undergoing lower segment CS under SA. It shows the requirement of analgesic injection of diclofenac sodium is 62±28.4 mg in the dexamethasone group and 132±74.2 mg in the saline group31.
Our finding also indicates that there are statistically significant decrements in NRS score both at rest and voluntary coughing in the dexamethasone group at 2, 4, 6, 12, 18, and 24 h. This is because steroids have anti-inflammatory action resulting in decreased production of various inflammatory mediators that play a major role in amplifying and maintenance of pain perception and inhibition of phospholipase A2, as well as changes in cell function induced by glucocorticoid receptor activation36.
A RCT done in Brazil by Cardoso et al. on the effect of dexamethasone on the prevention of postoperative nausea, vomiting, and pain after CS shows that pain score with movement is lower among patients who received dexamethasone at 1, 6, 12, and 24 h than the control group41. Contrary to our finding, the proportion of patients at 2 and 3 h was comparable in their finding between control and dexamethasone, which was not statistically significant. The probable reason for this inconsistency with our result may be due to the administration of morphine as an adjuvant for SA that we were not using, which affects postoperative pain6.
Our result is also comparable with a study done in Ethiopia, which shows that there are statistically significant decrements in pain scores both at rest and voluntary coughing in the dexamethasone group at 3, 6, 18, and 24 h between the two groups38. This current study is also in line with a study done in Egypt, which shows a higher visual analog scale (VAS) score among women in the placebo group than in the dexamethasone groups at 2, 4, 6, 12, and 24 h with a significant difference between the groups42.
Strengths of the study
This is a double-blinded RCT, and the homogeneity of the population in both groups concerning sociodemographic aspects is among the strengths of the study.
Limitations of the study
Not measuring blood serum glucose as an outcome, the size of the incision, and the types of incision were not controlled, and the shorter period of postoperative follow-up and being a single center are the limitations of the study. The analysis did not also encompass potential fetal outcomes in this RCT.
Conclusion and recommendations
Conclusion
Preoperative administration of 8 mg of dexamethasone for parturient undergoing CS prolongs the first analgesia request time, decreases 24-hour postoperative pain score, and decreases total tramadol and diclofenac consumption.
Recommendations
We recommend that clinicians administer preoperative 8 mg of intravenous dexamethasone to be used as an alternative multimodal analgesic drug to reduce postoperative pain, prolong first analgesic request time, and decrease opioid consumption after CS. We also recommend that researchers perform a further study on a different analgesic dose of dexamethasone with larger sample sizes, multicenter RCT with adequate postoperative follow-up period.
Ethical approval
Ethical clearance was obtained from the College of Medicine and Health Sciences of Dilla University institutional review board with protocol unique no: 008/19-10 before the start of the study. The purposes and the importance of the study were clearly explained, and written informed consent was obtained from each study participant.
Consent
Written informed consent was obtained from the patient for the publication of this study and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
There is no financial support from any institution to conduct this study.
Author contribution
All authors have made substantial contributions to the conception, design, analysis, and interpretation of data and participated in the critical review and editing of the manuscript drafts for scientific merit and depth.
Conflicts of interest disclosure
There are no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Research Registry.
Unique identifying number or registration ID: researchregistry8544.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-the-registry#home/
Guarantor
Seyoum Hailu.
Data availability statement
All datasets used and analyzed during this study are available from the first author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
We would like to thank Dilla University for sponsoring this research project.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 28 November 2023
Contributor Information
Mesfin Gurmu, Email: mesfingurmu92@gmail.com.
Hailemariam Mulugeta, Email: hmerry1990@gmail.com.
Abebayehu Zemedkun, Email: abe.z01n@gmail.com.
Timsel Girma, Email: timsikebron15@gmail.com.
Belete Destaw, Email: baleanst@gmail.com.
Muhiddin Tadessa, Email: muhiddinhassen@gmail.com.
Yayeh Adamu, Email: yayehadamu02@gmail.com.
Seyoum Hailu, Email: seyoumhailu44@gmail.com.
References
- 1.Betran AP, Ye J, Moller A-B, et al. The increasing trend in caesarean section rates: global, regional and national estimates. PLoS One 2016;11:e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wondie AG, Zeleke AA, Yenus H, et al. Cesarean delivery among women who gave birth in Dessie town hospitals, Northeast Ethiopia. PLoS One 2019;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenaw Z, Kassa ZY, Kassahun G, et al. Maternal preference, mode of delivery and associated factors among women who gave birth at public and private hospitals in Hawassa city, Southern Ethiopia. Ann Glob Health 2019;85:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yisma E, Smithers LG, Lynch JW, et al. Cesarean section in Ethiopia: prevalence and sociodemographic characteristics. J Matern Neonatal Med 2019;32:1130–1135. [DOI] [PubMed] [Google Scholar]
- 5.Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian P, Ramasamy S, Ng KH, et al. Pain experience and satisfaction with postoperative pain control among surgical patients. Int J Nurs Pract 2016;22:232–238. [DOI] [PubMed] [Google Scholar]
- 7.Simon LS. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. J Pain Palliat Care Pharmacother 2012;26:197–198. [Google Scholar]
- 8.Admassu WS, Hailekiros AG, Abdissa ZD. Severity and risk factors of post-operative pain in University of Gondar Hospital, Northeast Ethiopa. J Anesth Clin Res 2016;7:675. [Google Scholar]
- 9.Demelash G, Woldegerima Y, Hailekiros A, et al. Postoperative pain after cesarean section at University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia, 2019, a cross-sectional follow-up study Research Square 2020. doi: 10.21203/rs.3.rs-124364/v1 [DOI] [Google Scholar]
- 10.Marcus H, Gerbershagen HJ, Peelen LM, et al. Quality of pain treatment after caesarean section: results of a multicentre cohort study. Eur J Pain 2015;19:929–939. [DOI] [PubMed] [Google Scholar]
- 11.Gordon DB, De Leon-Casasola OA, Wu CL, et al. Research gaps in practice guidelines for acute postoperative pain management in adults: findings from a review of the evidence for an American pain society clinical practice guideline. J Pain 2016;17:158–166. [DOI] [PubMed] [Google Scholar]
- 12.Yimer H, Woldie H. Incidence and associated factors of chronic pain after caesarean section: a systematic review. J Obstet Gynaecol Can 2019;41:840–854. [DOI] [PubMed] [Google Scholar]
- 13.Sommer M, de Rijke JM, van Kleef M, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur J Anaesthesiol 2008;25:267–274. [DOI] [PubMed] [Google Scholar]
- 14.Orrico T, Cançado DB, Omais M, et al. Chronic pain after cesarean section. Influence of anesthetic/surgical technique and postoperative analgesia. Rev Bras Anestesiol 2012;62:762–774. [DOI] [PubMed] [Google Scholar]
- 15.Nikolajsen L, Sorensen HC, Jensen TS. Chronic pain following caeserean section. Acta Anaesthesiol Scand 2004;48:111–116. [DOI] [PubMed] [Google Scholar]
- 16.Lundborg C. Why postoperative pain remains a problem. J Pain Palliat Care Pharmacother 2015;29:300–302. [DOI] [PubMed] [Google Scholar]
- 17.Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am 2015;95:301–318. [DOI] [PubMed] [Google Scholar]
- 18.Gerbershagen HJ, Aduckathil S, van Wijck AJM, et al. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118:934–944. [DOI] [PubMed] [Google Scholar]
- 19.Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97:534–540. [DOI] [PubMed] [Google Scholar]
- 20.Movafegh A, Soroush AR, Navi A, et al. The effect of intravenous administration of dexamethasone on postoperative pain, nausea, and vomiting after intrathecal injection of meperidine. Anesth Analg 2007;104:987–989. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe EE, Molitor RJ, Arendt KW, et al. Intrathecal Morphine versus Intrathecal Hydromorphone for Analgesia after Cesarean Delivery: a Randomized Clinical Trial. Anesthesiology 2022;132:1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato R, Shimamoto H, Terui K, et al. Delayed respiratory depression associated with 0.15 mg intrathecal morphine for cesarean section: a review of 1915 cases. J Anesth 2008;22:112–116. [DOI] [PubMed] [Google Scholar]
- 23.Oderda GM, Senagore AJ, Morland K, et al. Opioid-related respiratory and gastrointestinal adverse events in patients with acute postoperative pain: prevalence, predictors, and burden. J Pain Palliat Care Pharmacother 2019;33:82–97. [DOI] [PubMed] [Google Scholar]
- 24.Ali S, Athar M, Ahmed SM. Basics of CPB. Indian J Anaesth 2019;49:257–262. [Google Scholar]
- 25.Towers CV, Shelton S, van Nes J, et al. Preoperative cesarean delivery intravenous acetaminophen treatment for postoperative pain control: a randomized double-blinded placebo control trial. Am J Obstet Gynecol 2018;218:353.e1–353.e4. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho B, Chu L, Fuller A, et al. Valdecoxib for postoperative pain management after cesarean delivery: a randomized, double-blind, placebo-controlled study. Anesth Analg 2006;103:664–670. [DOI] [PubMed] [Google Scholar]
- 27.Moore A, Costello J, Wieczorek P, et al. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg 2011;112:167–173. [DOI] [PubMed] [Google Scholar]
- 28.Hermans V, De Pooter F, De Groote F, et al. Effect of dexamethasone on nausea, vomiting, and pain in paediatric tonsillectomy. Br J Anaesth 2012;109:427–431. [DOI] [PubMed] [Google Scholar]
- 29.Olofsson CI, Legeby MH, Nygårds EB, et al. Diclofenac in the treatment of pain after caesarean delivery: an opioid-saving strategy. Eur J Obstet Gynecol Reprod Biol 2000;88:143–146. [DOI] [PubMed] [Google Scholar]
- 30.Maged AM, Deeb WS, Elbaradie S, et al. Comparison of local and intra venous dexamethasone on post operative pain and recovery after caeseream section. A randomized controlled trial. Taiwan J Obstet Gynecol 2018;57:346–350. [DOI] [PubMed] [Google Scholar]
- 31.Memon N, Bagga J. Effect of single-dose intravenous dexamethasone on postoperative pain and postoperative nausea and vomiting in patients undergoing lower segment cesarean section under spinal anesthesia. Asian J Med Sci 2022;13:31–37. [Google Scholar]
- 32.Kadur SN, Ahmed F, Purohit A, et al. The effect of intravenous dexamethasone on postoperative pain, nausea and vomiting after intrathecal pethidine and bupivacaine in lower limb orthopedic surgery. Anesth Pain Intensive Care 2015;19:254–259. [Google Scholar]
- 33.Ituk U, Thenuwara K. The effect of a single intraoperative dose of intravenous dexamethasone 8 mg on post-cesarean delivery analgesia: a randomized controlled trial. Int J Obstet Anesth 2018;35:57–63. [DOI] [PubMed] [Google Scholar]
- 34.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 35.Shahraki AD, Feizi A, Jabalameli M, et al. The effect of intravenous dexamethasone on post‑cesarean section pain and vital signs: a double-blind randomized clinical trial. J Res Pharm Pract 2013;2:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K, Hsu CC, Chia YY. Effect of dexamethasone on postoperative emesis and pain. Br J Anaesth 1998;80:85–86. [DOI] [PubMed] [Google Scholar]
- 37.Shalu PS, Ghodki PS. To study the efficacy of intravenous dexamethasone in prolonging the duration of spinal anesthesia in elective cesarean section. Anesth Essays Res 2017;11:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melese E, Tesfaye A, Getachew L, et al. Analgesic effect of intravenous dexamethasone prior to spinal anesthesia among parturient undergo cesarean section at Gandhi Memorial Hospital, Addis Ababa, Ethiopia, Prospective Cohort Study, 2019. J Anesth Clin Res 2019;10:1–7. [Google Scholar]
- 39.Parthasarathy P, Babu K, Rao RSR, et al. The effect of single-dose intravenous dexamethasone on postoperative pain and postoperative nausea and vomiting in patients undergoing surgery under spinal anesthesia: a double‑blind randomized clinical study. Anesth Essays Res 2018;12:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joung K-W, Kim HR, Kim W-J, et al. Preoperative dexamethasone for acute post-thoracotomy analgesia: a randomized, double-blind, placebo-controlled study. BMC Anesthesiol 2018;18:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardoso MMS, Leite AO, Santos EA, et al. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: a randomised, placebo-controlled, double-blind trial. Eur J Anaesthesiol 2013;30:102–105. [DOI] [PubMed] [Google Scholar]
- 42.Maged AM, Deeb WS, Elbaradie S, et al. Comparison of local and intra venous dexamethasone on post operative psain and recovery after caeseream section. A randomized controlled trial. Taiwan J Obstet Gynecol 2018;57:346–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and analyzed during this study are available from the first author upon reasonable request.