Abstract
Purpose of Review
To review the role of ocular surface epithelial (corneal and conjunctival) ion transporters in the pathogenesis and treatment of dry eye disease (DED).
Recent Findings
Currently, anti-inflammatory agents are the mainstay of DED treatment, though there are several agents in development that target ion transport proteins on the ocular surface, acting by pro-secretory or anti-absorptive mechanisms to increase the tear fluid Film volume. Activation or inhibition of selected ion transporters can alter tear fluid osmolality, driving water transport onto the ocular surface via osmosis. Several ion transporters have been proposed as potential therapeutic targets for DED, including the cystic fibrosis transmembrane conductance regulator (CFTR), calcium-activated chloride channels (CaCCs), and the epithelial sodium channel (ENaC).
Summary
Ocular surface epithelial cell ion transporters are promising targets for pro-secretory and anti-absorptive therapies of DED.
Keywords: Dry eye disease, Ion transport, Ocular surface electrophysiology, Ocular surface epithelium, Cornea, Conjunctiva
Introduction
The most recent model of ocular surface ion transporters was published in 2012 [1]. Since then, new therapies and targets have been identified. This review will highlight these developments and put forth an updated model to account for potential novel drug targets.
Ion Transporters
Ion transporters are proteins that lie in the apical and basolateral membranes of epithelial cells and facilitate the influx and efflux of ions, playing a crucial role in the maintenance of cell homeostasis. The movement of ions across the epithelium establishes transepithelial chemical, electrical, and osmotic gradients that in turn influence water movement [2].
Mechanisms of Ion Transport
Ion channels use a variety of mechanisms to move ions across the cell membrane. Active transport uses the energy input of ATP hydrolysis to transport molecules between extracellular and intracellular environments against an electrochemical gradient. Passive transport utilizes the potential energy in a concentration or electrochemical gradient. This includes simple diffusion, membrane carrier-mediated facilitated diffusion, and the movement of water through membrane pores, such as aquaporin (AQP). Osmosis is movement of water stimulated by transepithelial osmotic gradients [2].
Systemic Diseases Due to Ion Transport Dysfunction
Dysfunction of ion transporters can lead to a variety of diseases, and a key example of such is cystic fibrosis (CF). CF is a genetic disease characterized by loss of function mutations in cystic fibrosis transconductance membrane regulator (CFTR), a cAMP-activated chloride channel expressed in many tissues [3]. Dysregulation of CFTR leads to abnormally viscous mucus that results in a variety of comorbidities such as chronic pulmonary inflammation and infection, pancreatic exocrine insufficiency, and male infertility, among others [3]. Of note, some CF patients exhibit low tear film stability on the ocular surface due to the impaired CFTR chloride secretion [4]. As opposed to the loss of function of CFTR in CF, the enterotoxins of Vibrio cholerae (producing cholera) and certain Escherichia coli strains (traveler’s diarrhea) hyperactivate CFTR causing secretory diarrhea characterized by enhanced salt and water loss in feces [2, 5]. CFTR, along with multiple other ion transporters (Table 1), are expressed on the ocular surface epithelium and play a role in tear production and ocular surface epithelial protection [1, 6, 7••, 8••].
Table 1.
Ocular surface ion transporters
| Gene | Transporter name | Location | Putative function | |
|---|---|---|---|---|
| 1 | TRP | TRPV1, TRPV2, TRPV4, TRPA1, TRPM8 | Apical | Non-selective cation channel |
| ASIC | ASIC | Acid-sensing cation channel | ||
| 2 | SLC8A1 | NCX | Apical | Na+/Ca2+ exchanger |
| 3 | SLC10A6 | SOAT | Apical | Na+-dependent organic anion transporter |
| 4 | ATP2B1/ATP2C1 | Mg2+/Ca2+-ATPase | Apical | ATP-activated transport of Ca2+ and Mg2+ |
| 5 | CFTR | CFTR | Apical | cAMP-activated Cl− channel |
| CLCN2 | Clc-2 | |||
| 6 | P2RY2 | P2Y2 | Apical | Purinergic GPCR, indirectly activates CaCC via increasing intracellular Ca2+ |
| 7 | CLCA | CaCC | Apical | Ca2+-activated Cl− channel |
| 8 | SCNN1A/G/B | ENaC | Apical | Amiloride-sensitive Na+ channel |
| 9 | KCN | Kv | Apical | Voltage-gated K+ channel |
| 10 | ATP12A | H+/K+-ATPase | Apical | ATP-activated exchange of H+/K+ |
| 11 | AQP5 | AQP-5 | Apical/Basolateral | Water channel protein* |
| 12 | SLC12A1 | NKCC | Basolateral | Electroneutral Na+/K+/2Cl− cotransporter |
| 13 | ATP1A1/ATP1B1 | Na+/K+-ATPase | Basolateral | ATP-activated exchange of 3 Na+/2 K+ |
| 14 | SLC9A1 | NHE | Basolateral | Na+/H+ exchanger |
| 15 | SLC12A6 | KCC | Basolateral | K+/Cl− cotransporter |
| 16 | SLC4 | CBE | Basolateral | Cl−/HCO3− exchanger |
| 17 | KCNQ | Kv7 | Basolateral | cAMP-activated K+ channel |
| 18 | KCNN | KCa | Basolateral | Ca2+-activated K+ channel |
Not an ion transporter, but critical for movement of water
Dry Eye Disease
The Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) defines dry eye disease (DED) as, “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [9]. DED can be classified as evaporative (e.g. meibomian gland dysfunction), aqueous deficient (e.g. Sjögren’s syndrome (SS)), or mixed. DED is estimated to affect 6.8% of adults in the United States. The global prevalence is estimated to be as high as 50%, with estimations reaching as high as 75% in specific populations [10]. Tear hyperosmolarity and instability are major offenders in DED by damaging the epithelia directly, inducing inflammation, and causing insufficient tear production and excessive tear evaporation [10]. Most FDA-approved therapies for DED target only the inflammation and have limited clinical efficacy [11–13]. The ocular surface, comprised of the cornea and conjunctiva, is lined by stratified epithelial cells expressing ion transport proteins that facilitate fluid secretion or absorption to regulate tear fluid volume and osmolarity. These ocular surface ion transporters thus present an attractive target to develop drugs to treat DED.
Ocular Surface Ion Transporters
Major ion channels that are functionally expressed in ocular surface epithelial cells include the CFTR chloride channel and the epithelial sodium channel (ENaC) on the apical membrane, which are involved in fluid secretion and absorption, respectively [14–18]. Additional ion channels expressed on the apical membrane include calcium-activated chloride channels (CaCCs), and potassium channels. The basolateral membrane (facing the corneal stroma) expresses potassium channels, an electroneutral Na+/K+/2Cl− cotransporter (NKCC1), and a sodium-potassium pump (Na+/K+-ATPase), the latter providing the energy to drive fluid secretion. There is paracellular ion transport as well. To create the electrochemical driving force for apical chloride secretion, and hence fluid secretion into the tear film, the basolateral membrane transporters act in concert to maintain a cell interior negative membrane potential and a high concentration of potassium in the cytoplasm, a low concentration of sodium, and a concentration of chloride that is above its electrochemical equilibrium potential for its transport onto the ocular surface when CFTR or CaCCs are open [7••].
Our updated diagram of ion transporters on the ocular surface epithelium includes the original ion transporters recognized in 2012 with the addition of P2Y2, ASIC, Clc-2, and additional potassium channels (Table 1 and Fig. 1A) [1]. Transporter gene names, locations (apical or basolateral), and putative functions are listed in Table 1. The roles of CFTR and ENaC in tear fluid secretion and absorption, respectively, are well established. However, less is known for the other ion transporters. NHE8, a Na+/H+ exchanger expressed on the basolateral membrane, is suggested to play a role in tear production and ocular epithelial protection [19]. Clc-2, a non-CFTR cAMP-activated chloride channel expressed on the apical membrane, may play a similar chloride secretory role to CFTR in many tissues, including the eye [20]. ASIC, a voltage-insensitive acid-sensing cation (mainly sodium) channel, is implicated in increasing tear and blinking rates and reducing ocular neuropathic pain [8••]. While their exact etiologic roles remain unclear, various potassium channels (e.g. voltage-gated, cAMP-activated, and calcium-activated) are expressed in the ocular surface epithelium and may also play a role in lacrimal gland secretion [21].
Fig. 1.
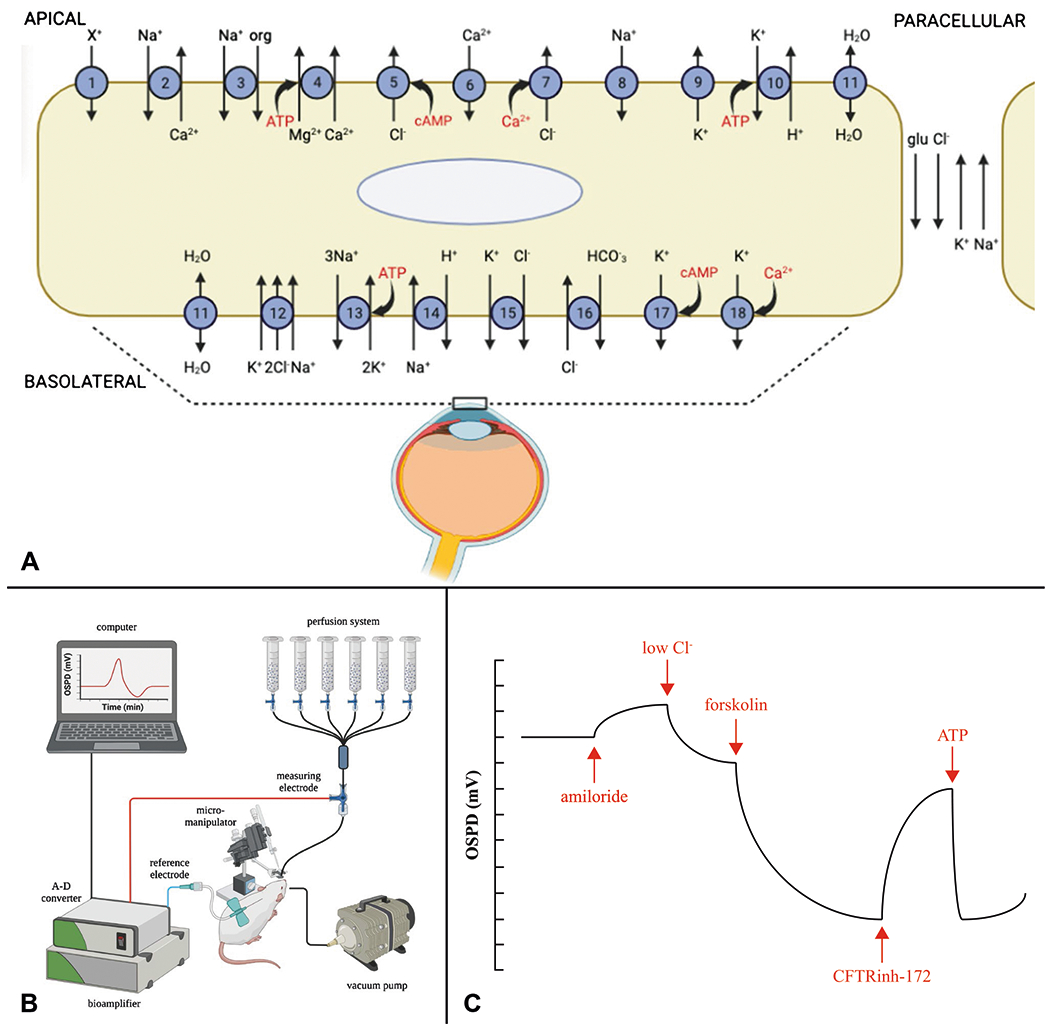
A Schematic diagram of membrane proteins in the ocular surface epithelium (cornea and conjunctiva) involved in the movement of ions via ion channels, pumps, transporters, and receptors [1, 16]. See Table 1 for detailed descriptions. B Schematic of OSPD setup showing the multi-syringe perfusion system to deliver fluid to bathe the ocular surface and the electrical system with measuring electrode in contact with the ocular surface (through the perfusate), subcutaneous reference electrode, and high impedance bioamplifier to measure the mV electrical potential. C Representative OSPD tracing showing PD changes from sequential perfusion exchanges in a typical protocol to study chloride transport
Therapeutic Ocular Surface Ion Transport Targets
Currently, there are no FDA-approved drugs for DED that target ion transporters. However, there are several drugs in various stages of clinical development including drugs approved outside the USA. Pro-secretory drugs include VSJ-110, a small-molecule triazine CFTR activator with nanomolar potency currently in phase 2 clinical trial. VSJ-110 stimulates secretion of chloride from the apical membrane and has been shown to increase tear secretion and reverse epithelial damage in mice and rabbits [22•, 22–26]. Another pro-secretory drug is diquafosol, a purinergic P2Y2 agonist currently approved in Japan, which indirectly stimulates chloride secretion via activation of CaCCs [27–29]. Diquafosol submitted New Drug Approval (NDA) to the FDA in 2003 but was denied FDA approval following phase 3 clinical trial where it failed to meet its primary endpoints of reduced corneal staining and increased corneal clearing [30]. A phase 4 clinical trial for diquafosol is currently recruiting. An anti-absorptive drug in development, P-321 (SHP-659), is an ENaC inhibitor that increased tear production in normal and DED mice [31, 32]. A phase 2 clinical trial from 2016 failed to show a significant difference between P-321 and placebo on various outcome measures. Notably, ENaC inhibition with amiloride demonstrated minimal electrophysiological effect in our recent in vivo human pilot study, which may explain the apparent lack of efficacy of P-321 in the USA clinical trials [7••]. See Table 2 for additional details on therapeutic ocular surface ion transport targets.
Table 2.
Dry eye disease ion transport therapies
| Therapy name | Mechanism of action | Clinical trial |
Relevant references | |
|---|---|---|---|---|
| Identifier | Phase | |||
| Diquafosol | Purinergic P2Y2 receptor agonist | NCT04668118 | 4 | [8••, 33, 34•, 35••, 36–49] |
| Tivanisiran (SYL1001) | siRNA TRPV1 inhibitor | NCT04819269 | 3 | [35••, 50, 51] |
| ALY688 | Adiponectin receptor agonist | NCT04899518 | 3 | [52–54] |
| RGN-259 (Tb4) | ATP-responsive purinergic receptor P2X4 agonist | NCT03937882 | 3 | [35••, 55–57] |
| VSJ-110 | CFTR activator | NCT04622345 | 2 | [22•, 23–25] |
| P-321 (SHP-659) | ENaC inhibitor | NCT02824913, NCT02831387 | 2 | [32, 58•] |
| AR-15512 | TRPM8 agonist | NCT04498182 | 2 | [59–63•] |
| Cact-3 | CFTR activator | NA | NA | [64•] |
| Isorhamnetin | CFTR activator | NA | NA | [65•] |
| Cryosim-3 (C3) | TRPM8 agonist | NA | NA | [8••, 61] |
| Pituitary Adenylate Cyclase-Activating Peptide (PACAP) | Increases cAMP, PKA, and AQP-5 | NA* | NA* | [8••, 66, 67•, 68–70] |
| 2-Guanidine-4-Methylquinazoline (GMQ) | ASIC3 agonist | NA | NA | [8••, 71] |
No DED-related clinical trial
Experimental Approaches
A variety of mechanisms are used to measure ion transport and elucidate the location of ion transporters on the ocular surface. Likewise, a variety of clinical tests are used to measure the tear film.
Ocular Surface Potential Difference (OSPD) Measurement
Clinical evaluation of ocular surface health typically involves slit lamp examination with fluorescein and lissamine green (LG) staining, tear breakup time (TBUT), Schirmer’s test, corneal sensation, MMP-9 levels, and tear fluid osmolarity. As a direct measure of ocular surface function, we exploited the millivolt (mV) electrical potentials generated by ion transport across the ocular surface epithelium. The concept of ocular surface potential difference (OSPD) was originally introduced for studies of sodium and chloride transport in experimental animals [16, 72], and more recently, applied to humans [7••]. The OSPD signal arises from the activities of various ion transporters in ocular surface epithelia, as depicted in Fig. 1A and Table 1. By manipulating experimental conditions, such as using selective transport modulators (activators or inhibitors), ion substitution, or genetic knockout/knockdown, it is possible to systematically characterize the ion transport pathways in vivo. Quantitative interpretation of OSPD data can be facilitated by mathematical modeling [16]. The OSPD method has broad potential applications in studying normal ocular surface physiology and disease, characterizing ocular surface ion transport pathways, and testing investigational drugs in animals and humans.
OSPD is measured using a high-impedance voltmeter/bioamplifier connected to a computer system using an analog-to-digital converter. A head stage is used to connect the measuring and reference electrodes to the bioamplifier. The measuring electrode contacts the ocular surface using a perfusion catheter whose tip is immersed in fluid bathing the ocular surface while the reference electrode is inserted subcutaneously. Solution exchange is accomplished using a gravity or peristaltic pump perfusion system (Fig. 1B). We adapted the system to measure OSPD in human subjects in which the head is stabilized using a slit lamp, with the tip of the perfusion catheter positioned under direct visualization in a fluid pocket created by eversion of the lower lid without contacting the ocular surface [7••]. Unlike nasal PD studies that often have considerable noise due to difficulty achieving and maintaining optimal measuring electrode positioning, the OSPD system provides a low-noise, robust signal that is related quantitatively to the activities of ion transport pathways at the ocular surface.
Our group has used OSPD measurements from sequential perfusion exchanges to study chloride transport pathways on the ocular surface (Fig. 1C). After determination of baseline OSPD using a high chloride solution that mimics the tear film, five solution exchanges are done to isolate ENaC, CFTR, and CaCCs functions. A high chloride solution containing the ENaC inhibitor, amiloride, produces minimal depolarization, suggesting minimal ENaC activity. A low chloride solution that probes basal transcellular and paracellular chloride transport pathways produces a rapid, modest hyperpolarization. Adding the cAMP agonist, forskolin, produces a more gradual but larger hyperpolarization due to activation of CFTR and potentially other cAMP-dependent ion channels. The potent and selective CFTR inhibitor, CFTRinh-172, produces a rapid and near complete reversal of the forskolin-induced hyperpolarization. Finally, the calcium agonist, ATP, produces a rapid hyperpolarization followed by slow depolarization due to transient elevation in cytoplasmic calcium, which activates CaCCs and calcium-activated potassium channels.
Short-Circuit Current (Isc) Measurement
The transport of ions from the basolateral and apical sides of epithelial membranes creates a transepithelial voltage (Vte), generally recorded using an Ussing chamber. Measurements of Vte are often referred to as open-circuit recordings and can be useful for studying the secretory and absorptive channels such as CFTR and ENaC, respectively. When Vte is held constant at 0 mV, it is possible to measure the charge flow, known as short-circuit current (Isc) [73]. In animal corneal and conjunctival specimens, Isc demonstrated the activating and inhibiting effects to forskolin and CFTRinh-172, respectively, on CFTR channels [27, 74, 75].
Whole-Cell Patch-Clamp Measurement
Whole-cell patch clamp measurements yield biophysical information about single ion channel function by characterizing current flow through voltage and ligandgated ion channels. These measurements offer functional information regarding specific pharmacological agents by capturing the effect on ionic current following their administration [76]. Recordings are obtained by inserting a glass pipette into the cell membrane and applying suction, forming a seal between the pipet and membrane thus creating the whole-cell configuration. Pipettes contain an ionic solution that mimics the intracellular environment and connects to a recording electrode [77]. After achieving whole-cell configuration, cells are pulsed with hyperpolarizing and depolarizing voltages to induce currents. This technique was recently utilized to identify the modulatory activity of novel CFTR activator compounds relative to that produced by maximal forskolin activation [64•].
cAMP Measurement
As a secondary messenger, cAMP has numerous downstream effects including CFTR-mediated chloride secretion and activation of protein kinase A (PKA). Therefore, cAMP measurements offer insight into the mechanism of therapeutic agents on the ocular surface. Effective measurements can be obtained using commercial kits [78]. These kits utilize a competitive enzyme immunoassay which can be used to measure cAMP levels in tear fluid, ocular surface epithelial cells, and the lacrimal gland [66].
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) utilizes antibodies to target and localize antigens in a particular tissue [79]. IHC can localize ion transporters to the apical or basolateral membranes of epithelial cells based on the pattern of staining. For example, using immunofluorescence microscopy, chloride channels such as CFTR and Clc-2 have been identified in the apical membrane of human corneal epithelium [18].
Tear Film Osmolarity
The ocular environment needs regulated tear flow, which is driven by osmolarity. Measurements of tear film osmolarity, usually acquired by measuring the freezing point depression or tear fluid electrical conductivity, have been historically difficult to acquire [80]. Despite the challenge of measuring tear osmolarity, tear hyperosmolarity is a hallmark feature of DED [81]. Tear osmolarity is considered one of the best predictors of DED severity when compared to Schirmer’s test, meibomian gland grading, TBUT, and corneal and conjunctival staining [82, 83]. Measurements have been facilitated through development of technologies such as the I-PEN Tear Osmolarity System (I-MED Pharma: Quebec City, Canada). This device detects and measures tear film osmolarity on orbital tissues bathed in tear film, such as the palpebral conjunctiva, in approximately two seconds. Another method for determining tear film osmolarity is the TearLab Osmolarity System (TearLab: San Diego, CA), which requires only 50 nL of tear film to determine osmolarity. Both systems indirectly measure tear film osmolarity based on electrical impedance.
Tear Volume
Schirmer’s test evaluates aqueous tear production. The test is carried out by applying paper strips to the inferior temporal aspect of both conjunctival sacs of the patient. After 5 minutes, the length of the wetted paper is measured [84]. A measurement of less than 5 mm of strip wetting after 5 minutes is diagnostic for aqueous deficiency [85••]. The phenol red thread (PRT) test is less invasive than Schirmer’s test. It involves placing a soft thread treated with phenol red (phenolsulfonphthalein), a pH indicator, on the ocular surface for 15 seconds. The thread will turn red when contacted by alkaline tears, and the length of the red (wet) portion of the thread is measured. A length of 20 mm in 15 seconds is considered normal and anything less than 11 mm is criteria for DED [84]. Another effective way to quantitatively gauge aqueous production is the measurement of the tear meniscus height and cross-sectional volume of tears. Tear meniscus heights between 0.1-0.2 mm indicate mild DED and values <0.1 mm indicate moderate to severe DED [85••].
Corneal Fluorescein Staining
Corneal fluorescein staining provides insight into the severity of DED and the structural condition of the epithelium by highlighting loss of tight junctions. Fluorescein is a dark orange dye applied to the lower cul-de-sac of the eye and distributed across the ocular surface via blinking. The eye is subsequently examined under cobalt blue light where the density and extent of staining are assessed [64•]. Staining is commonly scored with the Oxford or National Eye Institute (NEI) scale [86].
Animal Models
Animal models are useful to elucidate the pathology of DED and develop therapeutic agents. Dry eye can be experimentally induced in mice via subcutaneous injection of 5 mg/mL of scopolamine hydrobromide three times per day for 14 days while placed in a desiccating environment with continuous airflow (15 L/min), 35% humidity, and a constant temperature of 25°C [64•]. Using this scopolamine-induced DED model, tear volume levels are significantly reduced with resultant increased corneal staining scores [64•, 65•]. Another method of inducing DED is lacrimal duct cautery (LDC). The extraorbital lacrimal gland is exposed via linear skin incisions and the lacrimal duct is ablated with high temperature cautery. LDC produces a DED model with an instant and marked decrease in tear volume with concomitant increase in corneal staining [22•, 23]. Models can also be induced through genetic knockdown of AQP expressed in the corneal epithelium, specifically AQP1, AQP3, and AQP5 [87, 88].
In addition to DED mouse models described, there are also multiple mouse models specific to SS. Mice deficient in thrombospondin-1 (TSP-1), a matricellular glycoprotein that modulates cell migration and plays a critical role in wound healing, develop SS and the accompanying ocular surface dryness [89–91]. TSP-1 mice lines are generated through homologous recombination in embryonic stem cells that disrupt TSP-1 genes [92]. A recent review evaluated multiple SS animal models and determined the non-obese diabetic (NOD) model, which demonstrates decreased glandular secretion and lymphocytic infiltration, to be most optimal for studying SS pathogenesis and drug testing [89, 93].
Conclusion
DED remains a major unmet need, with a significant USA and global prevalence. Current therapies primarily target inflammation despite tear film homeostasis and osmolarity playing key etiological roles. The modulation of ion transporters on the ocular surface epithelium therefore represents an attractive target for the development of pro-secretory and anti-absorptive therapies that aim to increase tear fluid secretion. We have summarized the current limited knowledge of ocular surface ion transport mechanisms and promising ion transport-related therapeutic targets. The full extent of ion transport mechanisms on the ocular surface remains to be elucidated. Methods such as OSPD measurements are exciting novel approaches to further investigate and identify ion transporters and their respective modulators.
Funding
This work was supported by grants EY033859, EY031372, and EY013574 from the National Institutes of Health/National Eye Institute, Research to Prevent Blindness Career Development Award (N.D.P.), and an All May See Foundation grant. This work was made possible in part by the Research to Prevent Blindness Unrestricted Grant to the University of California San Francisco, Department of Ophthalmology
Footnotes
Conflict of Interest N.D.P. and A.S.V. are named inventors on patent applications on ocular surface potential difference measurements owned by the University of California San Francisco. O.C. and A.S.V. are named inventors on patent applications on CFTR activators for dry eye disease and other indications, owned by the University of California San Francisco and licensed to Vanda Pharmaceuticals.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Zhao M, Chalmers L, Cao L, Vieira AC, Mannis M, Reid B. Electrical signaling in control of ocular cell behaviors. Prog Retin Eye Res. 2012:31(1):65–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devor DC, Hamilton KL. Ion channels and transporters of epithelia in health and disease. New York, Imprint: Springer; 2016. [Google Scholar]
- 3.Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet. 2021;397(10290):2195–211. [DOI] [PubMed] [Google Scholar]
- 4.Mrugacz M, Minorowska A, Bakunowicz-Lazarczyk A, Zywalewska N. Dry eye syndrome in children with cystic fibrosis. Med Wieku Rozwoj. 2004:8(4 Pt 1):865–70. [PubMed] [Google Scholar]
- 5.Cil O, Phuan PW, Gillespie AM, Lee S, Tradtrantip L, Yin J, et al. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins. FASEB J. 2017:31(2):751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin MH, Kim JK, Hu J, Verkman AS. Potential difference measurements of ocular surface na+ absorption analyzed using an electrokinetic model. Invest Ophthalmol Vis Sci. 2006;47(1):306–16. [DOI] [PubMed] [Google Scholar]
- 7.••.Pasricha ND, Smith AJ, Levin MH, Schallhorn JM, Verkman AS. Ocular surface potential difference measured in human subjects to study ocular surface ion transport. Transl Vis Sci Technol. 2020:9(11):20. [DOI] [PMC free article] [PubMed] [Google Scholar]; A human clinical pilot study describing the experimental procedure of human OSPD and demonstrating safety and reliability of OSPD measurements in healthy controls and CF patients.
- 8.••.Yang S, Wu Y, Wang C, Jin X. Ocular surface ion-channels are closely related to dry eye: key research focus on innovative drugs for dry eye. Front Med (Lausanne). 2022:9:830853 [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of various ion transporters on the ocular surface and the clinical and basic research results of ion channel regulatory compounds related to DED.
- 9.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–12. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–65. [DOI] [PubMed] [Google Scholar]
- 11.de Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008:71(6 Suppl):89–95. [DOI] [PubMed] [Google Scholar]
- 12.Lee JD, Kim HY, Park JJ, Oh SB, Goo H, Cho KJ, et al. Metabolomics approach to biomarkers of dry eye disease using (1) H-NMR in rats. J Toxicol Environ Health A. 2021;84(8):313–30. [DOI] [PubMed] [Google Scholar]
- 13.Lollett IV, Galor A. Dry eye syndrome: developments and lifitegrast in perspective. Clin Ophthalmol. 2018:12:125–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Nakkash L, Hu S, Li M, Hwang TC. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296(2):464–72. [PubMed] [Google Scholar]
- 15.Yu D, Thelin WR, Rogers TD, Stutts MJ, Randell SH, Grubb BR, et al. Regional differences in rat conjunctival ion transport activities. Am J Physiol Cell Physiol. 2012;303(7):C767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin MH, Verkman AS. Aquaporins and CFTR in ocular epithelial fluid transport. J Membr Biol. 2006:210(2):105–15. [DOI] [PubMed] [Google Scholar]
- 17.Turner HC, Bernstein A, Candia OA. Presence of CFTR in the conjunctival epithelium. Curr Eye Res. 2002;24(3):182–7. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Zhang XD, Liu X, Chen TY, Zhao M. Chloride channels and transporters in human corneal epithelium. Exp Eye Res. 2010;90(6):771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Zhao Y, Li J, Wang M, Lian F, Gao M, et al. Loss of NHE8 expression impairs ocular surface function in mice. Am J Physiol Cell Physiol. 2015;308(1):C79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oak AA, Chu T, Yottasan P, Chhetri PD, Zhu J, Du Bois J, et al. Lubiprostone is non-selective activator of cAMP-gated ion channels and Clc-2 has a minor role in its prosecretory effect in intestinal epithelial cells. Mol Pharmacol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almassy J, Diszhazi G, Skaliczki M, Marton I, Magyar ZE, Nanasi PP, et al. Expression of BK channels and Na(+)-K(+) pumps in the apical membrane of lacrimal acinar cells suggests a new molecular mechanism for primary tear-secretion. Ocul Surf. 2019:17(2):272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•.Chen X, Lee S, Zhang T, Duan T, Pasricha ND, Schallhorn JM, et al. Nanomolar potency aminophenyltriazine cftr activator reverses corneal epithelial injury in a mouse model of dry eye. J Ocul Pharmacol Ther. 2020:36(3):147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]; An original study demonstrating the efficacy of CFTRact-K089 (now known as VSJ-110), a nanomolar potent CFTR activator, in reversing corneal epithelial damage in a lacrimal duct cautery aqueous-deficient mouse model of DED.
- 23.Flores AM, Casey SD, Felix CM, Phuan PW, Verkman AS, Levin MH. Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease. FASEB J. 2016:30(5):1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felix CM, Lee S, Levin MH, Verkman AS. Pro-secretory activity and pharmacology in rabbits of an aminophenyl-1,3,5-Triazine CFTR activator for dry eye disorders. Invest Ophthalmol Vis Sci. 2017:58(11):4506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Phuan PW, Felix CM, Tan JA, Levin MH, Verkman AS. Nanomolar-potency aminophenyl-1,3,5-triazine activators of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel for prosecretory therapy of dry eye diseases. J Med Chem. 2017:60(3):1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verkman AS, Galietta LJV. Chloride transport modulators as drug candidates. Am J Physiol Cell Physiol. 2021:321(6):C932–C46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Kuang K, Yerxa B, Wen Q, Rosskothen H, Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl(−) and fluid secretion. Am J Physiol Cell Physiol. 2001;281(2):C595–602. [DOI] [PubMed] [Google Scholar]
- 28.Murakami T, Fujihara T, Horibe Y, Nakamura M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004;36(2):89–93. [DOI] [PubMed] [Google Scholar]
- 29.Byun YS, Yoo YS, Kwon JY, Joo JS, Lim SA, Whang WJ, et al. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp Eye Res. 2016:143:89–97. [DOI] [PubMed] [Google Scholar]
- 30.Order Instituting Cease and Desist Proceedings, Making Findings, and Imposing Cease-And-Desist Order Pursuant to Section 21C of the Securities Exchange Act of 1934, In the Matter of Inspire Pharmaceuticals, Inc., et al. , Admin. Proceeding File No. 3-13264 (Securities and Exhange Comm’n Sep. 30, 2008) 2008. [Available from: https://www.sec.gov/litigation/admin/2008/34-58690.pdf. [Google Scholar]
- 31.Boyer J, Johnson MR, Ansede J, Donn K, Boucher R, Thelin W. P-321, a novel long-acting epithelial sodium channel (ENaC) blocker for the treatment of dry eye disease. Invest Ophthalmol Vis Sci. 2013;54(15):957. [Google Scholar]
- 32.Thelin WR, Johnson MR, Hirsh AJ, Kublin CL, Zoukhri D. Effect of topically applied epithelial sodium channel inhibitors on tear production in normal mice and in mice with induced aqueous tear deficiency. J Ocul Pharmacol Ther. 2012;28(4):433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eom Y, Kim HM. Clinical effectiveness of diquafosol ophthalmic solution 3% in Korean patients with dry eye disease: a multicenter prospective observational study. Int J Ophthalmol. 2021:14(10):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.•.Hori Y, Oka K, Inai M. Efficacy and safety of the long-acting diquafosol ophthalmic solution DE-089C in patients with dry eye: a randomized, double-masked, placebo-controlled phase 3 study. Adv Ther. 2022;39(8):3654–67 [DOI] [PMC free article] [PubMed] [Google Scholar]; A randomized clinical trial in Japan demonstrating safety and efficacy of DE-089C, a long-acting diquafosol ophthalmic solution, compared to placebo in reducing fluorescein corneal and LG conjunctival staining over a 4-week period.
- 35.••.Huang R, Su C, Fang L, Lu J, Chen J, Ding Y. Dry eye syndrome: comprehensive etiologies and recent clinical trials. Int Ophthalmol. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]; A review outlining the etiologies of DED and upcoming and ongoing clinical trials for DED therapies.
- 36.Miura M, Inomata T, Nojiri S, Sung J, Nagao M, Shimazaki J, et al. Clinical efficacy of diquafosol sodium 3% versus hyaluronic acid 0.1% in patients with dry eye disease after cataract surgery: a protocol for a single-centre, randomised controlled trial BMJ Open. 2022;12(1):e052488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Shin J, Lee JE. A randomised, prospective study of the effects of 3% diquafosol on ocular surface following cataract surgery. Sci Rep. 2021;11(1):9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Jin R, Li Y, Yoon HS, Yoon HJ, Yoon KC. Effects of eye drops containing a mixture of 3% diquafosol sodium and tocopherol acetate (vitamin E) on the ocular surface of murine dry eye. Cutan Ocul Toxicol. 2021;40(4):350–8. [DOI] [PubMed] [Google Scholar]
- 39.Ogami T, Asano H, Hiraoka T, Yamada Y, Oshika T. The effect of diquafosol ophthalmic solution on clinical parameters and visual function in soft contact lens-related dry eye. Adv Ther. 2021;38(11):5534–47. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Zhang H, Qin G, Wu Y, Song Y, Yang L, et al. Impact of diquafosol ophthalmic solution on tear film and dry eye symptom in type 2 diabetic dry eye: a pilot study. J Ocul Pharmacol Ther. 2022:38(2):133–40. [DOI] [PubMed] [Google Scholar]
- 41.Dota A, Sakamoto A, Nagano T, Murakami T, Matsugi T. Effect of diquafosol ophthalmic solution on airflow-induced ocular surface disorder in diabetic rats. Clin Ophthalmol. 2020:14:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagi-Yaguchi Y, Kojima T, Higa K, Dogru M, Ibrahim OM, Shimizu T, et al. The effects of 3% diquafosol sodium eye drops on tear function and the ocular surface of Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice treated with antiglaucoma eye medications. Diagnostics (Basel). 2020:10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi Y, Munesue M, Shimazaki J, Takamura E, Yokoi N, Watanabe H, et al. Long-term safety and effectiveness of diquafosol for the treatment of dry eye in a real-world setting: a prospective observational study. Adv Ther. 2020:37(2):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji YW, Kim HM, Ryu SY, Oh JW, Yeo A, Choi CY, et al. Changes in human tear proteome following topical treatment of dry eye disease: cyclosporine a versus diquafosol tetrasodium. Invest Ophthalmol Vis Sci. 2019;60(15):5035–44. [DOI] [PubMed] [Google Scholar]
- 45.Kang DH, Lee YW, Hwang KY, Koh KM, Kwon YA, Kim BY, et al. Changes of tear film lipid layer thickness by 3% diquafosol ophthalmic solutions in patients with dry eye syndrome. Int J Ophthalmol. 2019;12(10):1555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun I, Choi S, Lee GY, Choi YJ, Lee HK, Kim EK, et al. Effects of preservative-free 3% diquafosol in patients with pre-existing dry eye disease after cataract surgery: a randomized clinical trial. Sci Rep. 2019:9(1):12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuoka S, Arita R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: a randomized clinical study. Sci Rep. 2019;9(1):9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam K, Kim HJ, Yoo A. Efficacy and safety of topical 3% diquafosol ophthalmic solution for the treatment of multifactorial dry eye disease: meta-analysis of randomized clinical trials. Ophthalmic Res. 2019;61(4):188–98. [DOI] [PubMed] [Google Scholar]
- 49.Park CH, Lee HK, Kim MK, Kim EC, Kim JY, Kim TI, et al. Comparison of 0.05% cyclosporine and 3% diquafosol solution for dry eye patients: a randomized, blinded, multicenter clinical trial. BMC Ophthalmol. 2019:19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Benitez-Del-Castillo JM, Moreno-Montanes J, Jimenez-Alfaro I, Munoz-Negrete FJ, Turman K, Palumaa K, et al. Safety and efficacy clinical trials for SYL1001, a novel short interfering RNA for the treatment of dry eye disease. Invest Ophthalmol Vis Sci. 2016;57(14):6447–54. [DOI] [PubMed] [Google Scholar]
- 51.Moreno-Montanes J, Bleau AM, Jimenez AI. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin Investig Drugs. 2018;27(4):421–6. [DOI] [PubMed] [Google Scholar]
- 52.Crawford KS, Schuh C, Schuh J, Hsu H. Effects of ALY688 on atropine-induced dry eye in rabbits. Invest Ophthal Vis Sci. 2019;60(9):305. [Google Scholar]
- 53.Shikama Y, Kurosawa M, Furukawa M, Ishimaru N, Matsushita K. Involvement of adiponectin in age-related increases in tear production in mice. Aging (Albany NY). 2019;11(19):8329–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Woo JM, Chung SW, Kwon MY, Choi JS, Oh HJ, et al. Therapeutic effect of topical adiponectin in a mouse model of desiccating stress-induced dry eye. Invest Ophthalmol Vis Sci. 2013;54(1):155–62. [DOI] [PubMed] [Google Scholar]
- 55.Kim CE, Kleinman HK, Sosne G, Ousler GW, Kim K, Kang S, et al. RGN-259 (thymosin beta4) improves clinically important dry eye efficacies in comparison with prescription drugs in a dry eye model. Sci Rep. 2018;8(1):10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sosne G, Dunn SP, Kim C. Thymosin beta4 significantly improves signs and symptoms of severe dry eye in a phase 2 randomized trial. Cornea. 2015;34(5):491–6. [DOI] [PubMed] [Google Scholar]
- 57.Sosne G, Ousler GW. Thymosin beta 4 ophthalmic solution for dry eye: a randomized, placebo-controlled. Phase II clinical trial conducted using the controlled adverse environment (CAE) model. Clin Ophthalmol. 2015:9:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.•.Baiula M, Spampinato S. Experimental pharmacotherapy for dry eye disease: a review. J Exp Pharmacol. 2021;13:345–58 [DOI] [PMC free article] [PubMed] [Google Scholar]; A review that details novel approaches to the treatment of DED currently in preclinical and clinical development.
- 59.Chen GL, Lei M, Zhou LP, Zeng B, Zou F. Borneol is a TRPM8 agonist that increases ocular surface wetness. PLoS One. 2016;11(7):e0158868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corcoran P, Hollander DA, Ousler GW 3rd, Angjeli E, Rimmer D, Lane K, et al. Dynamic sensitivity of corneal TRPM8 receptors to menthol instillation in dry eye versus normal subjects. J Ocul Pharmacol Ther. 2017;33(9):686–92. [DOI] [PubMed] [Google Scholar]
- 61.Yang JM, Li F, Liu Q, Ruedi M, Wei ET, Lentsman M, et al. A novel TRPM8 agonist relieves dry eye discomfort. BMC Ophthalmol. 2017;17(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang JM, Wei ET, Kim SJ, Yoon KC. TRPM8 channels and dry eye. Pharmaceuticals (Basel). 2018;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.•.Yoon HJ, Kim J, Yang JM, Wei ET, Kim SJ, Yoon KC. Topical TRPM8 agonist for relieving neuropathic ocular pain in patients with dry eye: A pilot study. J Clin Med. 2021;10(2) [DOI] [PMC free article] [PubMed] [Google Scholar]; A human clinical pilot study demonstrating the efficacy of C3, a TRPM8 agonist, on relieving neuropathic ocular pain in DED patients.
- 64.•.Jeon D, Jun I, Lee HK, Park J,Kim BR, Ryu K, et al. Novel CFTR activator Cact-3 ameliorates ocular surface dysfunctions in scopolamine-induced dry eye mice. Int J Mol Sci. 2022;23(9) [DOI] [PMC free article] [PubMed] [Google Scholar]; An original study using high-throughput screening of small molecules for CFTR activation and demonstrating robust activity of Cact-3, a novel CFTR activator.
- 65.•.Lee HK, Park J, Kim BR, Jun I, Kim TI, Namkung W. Isorhamnetin ameliorates dry eye disease via cftr activation in mice. Int J Mol Sci. 2021;22(8) [DOI] [PMC free article] [PubMed] [Google Scholar]; An original study using high-throughput screening of natural products for CFTR activation and demonstrating robust activity of isorhamnetin, a novel CFTR activator.
- 66.Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun. 2016:7:12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.•.Hirabayashi T, Shibato J, Kimura A, Yamashita M, Takenoya F, Shioda S. Potential therapeutic role of pituitary adenylate cyclase-activating polypeptide for dry eye disease. Int J Mol Sci. 2022;23(2) [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of causes and symptoms of DED that includes an overview of PACAP as a therapeutic candidate for DED.
- 68.Ma Y, Zhao S, Wang X, Shen S, Ma M, Xu W, et al. A new recombinant PACAP-derived peptide efficiently promotes corneal wound repairing and lacrimal secretion. Invest Ophthalmol Vis Sci. 2015;56(8):4336–49. [DOI] [PubMed] [Google Scholar]
- 69.Nakamachi T. Novel tear secretion system - the effect and the mechanism of PACAP on tear secretion. Nihon Yakurigaku Zasshi. 2018;151(6):232–8. [DOI] [PubMed] [Google Scholar]
- 70.Shioda S, Takenoya F, Hirabayashi T, Wada N, Seki T, Nonaka N, et al. Effects of PACAP on dry eye symptoms, and possible use for therapeutic application. J Mol Neurosci. 2019;68(3):420–6. [DOI] [PubMed] [Google Scholar]
- 71.Callejo G, Castellanos A, Castany M, Gual A, Luna C, Acosta MC, et al. Acid-sensing ion channels detect moderate acidifications to induce ocular pain. Pain. 2015;156(3):483–95. [DOI] [PubMed] [Google Scholar]
- 72.Levin MH, Verkman AS. CFTR-regulated chloride transport at the ocular surface in living mice measured by potential differences. Invest Ophthalmol Vis Sci. 2005;46(4):1428–34. [DOI] [PubMed] [Google Scholar]
- 73.Li H, Sheppard DN, Hug MJ. Transepithelial electrical measurements with the Ussing chamber. J Cyst Fibros. 2004;3(Suppl 2):123–6. [DOI] [PubMed] [Google Scholar]
- 74.Candia OA. Forskolin-induced HCO3- current across apical membrane of the frog corneal epithelium. Am J Physiol. 1990;259(2 Pt 1):C215–23. [DOI] [PubMed] [Google Scholar]
- 75.Candia OA. The flux ratio of the Na-Cl cotransport mechanism in the frog corneal epithelium. Curr Eye Res. 1985;4(4):333–8. [DOI] [PubMed] [Google Scholar]
- 76.Segev A, Garcia-Oscos F, Kourrich S. Whole-cell patch-clamp recordings in brain slices. J Vis Exp. 2016;112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jouhanneau JS, Poulet JFA. Multiple two-photon targeted whole-cell patch-clamp recordings from monosynaptically connected neurons in vivo. Front Synaptic Neurosci. 2019;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oak AA, Chhetri PD, Rivera AA, Verkman AS, Cil O. Repurposing calcium-sensing receptor agonist cinacalcet for treatment of CFTR-mediated secretory diarrheas. JCI. Insight 2021;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magaki S, Hojat SA, Wei B, So A, Yong WH. An Introduction to the Performance of Immunohistochemistry. Methods Mol Biol. 2019;1897:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47(10):4309–15. [DOI] [PubMed] [Google Scholar]
- 81.Tomlinson A, McCann LC, Pearce EI. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea. 2010;29(9):1036–41. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–30. [DOI] [PubMed] [Google Scholar]
- 83.Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35(7):553–64. [DOI] [PubMed] [Google Scholar]
- 84.Craig JP, Downie LE. 5 - tears and contact lenses. In: Phillips AJ, Speedwell L, editors. Contact lenses. Sixth ed. London: Elsevier; 2019. p. 97–116. [Google Scholar]
- 85.••.Nichols KK, Mousavi M. Chapter 2 - clinical assessments of dry eye disease: tear film and ocular surface health. In: Galor A, editor. Dry eye disease: Elsevier; 2023. p. 15–23. [Google Scholar]; A textbook chapter detailing various techniques used to diagnose DED and clinically assess the health of the ocular surface.
- 86.Pellegrini M, Bernabei F, Moscardelli F, Vagge A, Scotto R, Bovone C, et al. Assessment of corneal fluorescein staining in different dry eye subtypes using digital image analysis. Transl Vis Sci Technol. 2019;8(6):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruiz-Ederra J, Levin MH, Verkman AS. In situ fluorescence measurement of tear film [Na+], [K+], [Cl−], and pH in mice shows marked hypertonicity in aquaporin-5 deficiency. Invest Ophthalmol Vis Sci. 2009;50(5):2132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verkman AS, Ruiz-Ederra J, Levin M. Functions of aquaporins in the eye. Progress in retinal and eye research. 2008;27:420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abughanam G, Maria OM, Tran SD. Studying Sjogren’s syndrome in mice: What is the best available model? J Oral Biol Craniofac Res. 2021;11(2):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175(3):1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr Eye Res. 2014;39(8):759–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101 (5):982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: an old tale with a new twist. Arch Immunol Ther Exp (Warsz). 2009;57(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


