Abstract
Photorespiration, an essential component of plant metabolism, was upregulated under abiotic stress conditions, such as high light or drought. One of the signals for such upregulation was the rise in reactive oxygen species (ROS). Photorespiration was expected to mitigate oxidative stress by reducing ROS levels. However, it was unclear if ROS levels would increase when photorespiration was lowered. Our goal was to examine the redox status in leaves when photorespiratory metabolism was restricted under low O2 (medium flushed with N2 gas) or by adding aminooxyacetic acid (AOA), a photorespiratory inhibitor. We examined the impact of low O2 and AOA in leaves of Arabidopsis thaliana under dark, moderate, or high light. Downregulation of typical photorespiratory enzymes, including catalase (CAT), glycolate oxidase (GO), and phosphoglycolate phosphatase (PGLP) under low O2 or with AOA confirmed the lowering of photorespiratory metabolism. A marked increase in ROS levels (superoxide and H2O2) indicated the induction of oxidative stress. Thus, our results demonstrated for the first time that restricted photorespiratory conditions increased the extent of oxidative stress. We propose that photorespiration is essential to sustain normal ROS levels and optimize metabolism in cellular compartments of Arabidopsis leaves.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01388-4.
Keywords: Reactive oxygen species, High light, Photorespiration, Aminooxyacetic acid, Low oxygen, Anoxia
Introduction
Abiotic or biotic stress often modulates metabolic components, including photorespiration, by raising reactive oxygen species (ROS) levels. Exposure to such stress can harm the photosynthetic machinery over time, especially photosystem II, leading to photoinhibition (Gururani et al. 2015; Szymańska et al. 2017; Gunell et al. 2023). Although it is a significant source of ROS by itself, photorespiration could help to restrict ROS levels (Voss et al. 2013; Sunil et al. 2019). Photorespiration utilized ATP/NADPH/reduced ferredoxin, thus forming a sink for excess energy. Strong photorespiratory flux helped avoid excessive reduction and photoinhibition of the chloroplastic electron transport chain (Saji et al. 2017; Huang et al. 2019). Thus, it was clear that photorespiratory metabolism was upregulated in response to elevated ROS levels under oxidative or photo-oxidative stress (Sunil et al. 2019; Bapatla et al. 2021). Though it implied that any restriction of photorespiration should increase the cellular ROS levels, there were no studies to prove or disprove such a possibility. We, therefore, attempted to examine the consequences of restricted photorespiration in ROS levels of leaves. In contrast to the extensive literature on the minimization of ROS/oxidative stress by photorespiration, very few attempts were made to assess the consequences of reduced photorespiration on the redox status of plant leaves.
Several methods were employed to restrict photorespiration. These included photorespiratory mutants, high CO2, low O2, and photorespiratory inhibitors. Each strategy has its advantages as well as drawbacks. Exposure to elevated CO2 required a sealed growing chamber with CO2 levels of at least 3000 µL/L (Queval et al. 2007). The oxygen content of the medium could be reduced by nitrogen purging, low-pressure boiling, and sonication (Butler et al. 1994). Purging the solution for 20 to 30 min with N2 gas was the quickest and most efficient technique to reduce the amount of O2 in the solution (Riazunnisa et al. 2006; Ershova et al. 2011; Vergara et al. 2012).
Photorespiration was limited by inhibitors that interfered with the enzymes or transporters in particular organelles. Examples are isonicotinyl hydrazide (INH), glycine hydroxamate (GHA), aminoacetonitrile (AAN), aminooxyacetic acid (AOA), α-hydroxy-2-pyridinemethansulfonic acid (HPMS), and sodium fluoride (NaF). Among these, AAN, GHA, and INH interfered with glycine conversion to serine (Riazunnisa et al. 2006; Kleczkowski et al. 1987; Kang et al. 2018). NaF inhibited PGLP activity, whereas HPMS interfered with the glycolate oxidase (Hewitt et al. 1990). AOA blocked glycine oxidation to inhibit the photorespiratory pathway (Kleczkowski et al. 1987; Han et al. 2018).
Photorespiratory mutants lacking essential genes were another approach for studying photorespiration (Timm and Bauwe 2013; Eisenhut et al. 2019). Most of these photorespiratory mutants could not grow in regular air, except hpr1 (Wang et al. 2022). As a result, the photorespiratory mutants needed to be raised at high CO2. When these plants were moved from high CO2 to normal air, they manifested stress symptoms, including chlorotic and bleached leaves (Timm et al. 2012).
After evaluating available techniques reported in the literature, we employed lowered O2 and treatment with a photorespiratory inhibitor. There were no attempts to examine the status of photorespiration in lowered O2/anoxia. A few reports showed the effect of photorespiratory inhibitors on photosynthesis, but none of them checked the ROS levels under such suppressed photorespiratory conditions (Riazunnisa et al. 2006; Kang et al. 2018). Ours is the first attempt to assess the redox status of leaves when the photorespiratory metabolism was interrupted by AOA or low O2. We are also the first to demonstrate photorespiration’s inverse relationship with leaf ROS levels. The status of key photorespiratory enzymes and ROS levels were evaluated in Arabidopsis leaves on treatment with low O2 and AOA and exposing leaves to dark, ML, and HL. The photorespiratory enzymes in different organelles, such as CAT, GO, and PGLP, were downregulated. A marked increase in ROS levels (superoxide and H2O2) was noticed, reflecting the induction of severe oxidative stress. Our results emphasize that photorespiration helps to minimize ROS levels, while restricted photorespiration leads to an increase in ROS and oxidative stress.
Materials and methods
Plant growth
Seeds of Arabidopsis thaliana wild type (Columbia) (from Arabidopsis Biological Resource Centre, Ohio State University, Columbus, Ohio) were sown in a mixture of vermiculite, perlite, and soilrite (1:1:1) in plastic disposable pots and kept at 4˚C in the dark for 48 h. The seedings were transferred to the pots, and the plants were grown. After 30 days of germination, individual plants were transferred into the disposable pots containing soil mixture and raised under a photoperiod of 8 h light/16 h dark and a temperature of 20–22˚C. The nutrient solution was applied twice a week, as suggested by Somerville (1982). Seven to eight-week-old plants provided the leaves for the experiments.
For treatment at low O2, the Petri dishes were sealed with parafilm, and the incubation medium (2 mM potassium phosphate buffer pH 6.5, 1 mM KCl, and 1 mM CaCl2) was purged with N2 gas for 30 min. In the case of AOA, leaves were incubated in dark or moderate light (ML, 150 µmol m−2 s−1) or high light (HL, 600 µmol m−2 s−1) for 3 h. At the end of treatment, leaves were frozen, stored in liquid N2, and to be used later.
Chemicals/Antibodies/Primers
AOA and premix-BCIP/NBT solutions were from Sigma Aldrich (USA). Antibodies and primers of photorespiratory enzymes were from Agrisera AB (Sweden) and Allied Scientific Products (India), respectively. Others were of analytical grade from India.
Levels of ROS (H2O2 and superoxide)
Nitroblue tetrazolium chloride (NBT) and 3,3’-diaminobenzidine (DAB) were used to monitor the accumulation of superoxide or H2O2, respectively. Superoxide or H2O2 levels were quantified as described (Kwon et al. 2013; Bapatla et al. 2021). Standard curves generated with known amounts of H2O2/NBT were used for calculations.
Assays of photorespiratory enzymes
The extraction from leaves (100 mg) and enzyme assays were as described (Bapatla et al. 2021). The principles were: GO – glycolate oxidation to glyoxylate (Yamaguchi and Nishimura 2000); catalase - consumption of H2O2 monitored at A240nm (Patterson et al. 1984) and PGLP – hydrolysis of phosphoglycolate releasing Pi (Somerville and Ogren 1979).
Glycolate oxidase (GO) activity
100 mg of leaves were homogenized in extraction buffer containing 100 mM HEPES-KOH (pH 7.2), 1 mM EDTA, and 10 mM 2-mercaptoethanol. After centrifugation at 10,000 g for 10 min at 4˚C, the supernatant was collected and used for enzyme assay. The reaction mixture contains 100 mM triethanolamine (pH 7.8), 0.75 mM oxidized GSSH, 4 mM phenylhydrazine, 3 mM EDTA, 2.3 mM sodium glycolate, and supernatant equivalent to 12.5 µg chlorophyll. The reaction was monitored for the increase in absorbance for five minutes at A324 nm. The phenylhydrazine extinction coefficient (16.8 mM−1 cm−1) was used to calculate the enzyme activity (Yamaguchi and Nishimura 2000).
Catalase (CAT) activity
100 mg of leaves were homogenized in an extraction buffer containing 50 mM phosphate buffer (pH 7.0). After centrifugation at 10,000 g for 10 min at 4˚C, the supernatant was collected and used for enzyme assay. The reaction mixture contains 50 mM phosphate buffer (pH 7.0), 20 mM H2O2, and a supernatant equivalent to 25 µg chlorophyll. The reaction was monitored for the decrease in absorbance for three minutes at A240 nm. The molar extinction coefficient of H2O2 (43.6 M−1 cm−1) was used to calculate the enzyme activity (Patterson et al. 1984).
Phosphoglycolate phosphatase (PGLP) activity
100 mg of leaves were homogenized in extraction buffer containing 10 mM HEPES (pH 7.0) at 4˚C. After centrifugation at 20,000 g for 10 min at 4˚C, the supernatant was collected and used for enzyme assay. The reaction mixture contains 40 mM sodium cacodylate (pH 6.3), 5 mM HEPES, 0.5 mM EDTA, 5 mM ZnSO4 and a supernatant equivalent to 12.5 µg chlorophyll. The reaction was started by adding 2 mM phosphoglycolate. After five minutes at 25˚C, the reaction was stopped by adding acid molybdate reagent [1:6 mixture of 10% (w/v) ascorbate and 0.42% (w/v) ammonium molybdenum in 1 N sulfuric acid]. The samples were incubated at 45˚C for 20 min, and the released phosphate was measured at A820 nm. A standard curve with known phosphate concentrations was used to determine the enzyme activity (Somerville and Ogren 1979).
Chlorophyll and protein estimation
Chlorophyll was determined by extraction into 80% (v/v) acetone (Arnon 1949). Protein was estimated by the Bradford assay. A standard curve with a known concentration of BSA was used to calculate the protein concentration (Bradford 1976).
Protein and transcript levels
The protein levels of photorespiratory enzymes were studied by Western blotting. The quantification of protein band intensities was done by Image J software. Ponceau-stained gels of Rubisco large subunit were used for normalization (Bapatla et al. 2021).
The extraction of RNA, cDNA synthesis, and sqRT-PCR using primer sequences (Supplementary Table 1) were described earlier (Bapatla et al. 2021). We optimized the number of cycles required for amplification of photorespiratory genes (CAT1, CAT2, GOX1, GOX2, and PGLP1) and actin-8 genes. We did not get much amplification of photorespiratory genes and actin 8 on 32, 34, or 36 cycles. However, the amplification was good at 40 cycles. Image J was used to quantify the transcript level, normalized with actin 8.
Replication and significance
The presented data were averages ± SE from at least three experiments conducted on different days. ANOVA was used to derive P values. A P value of less than 0.05 was considered significant.
Results
Changes in typical photorespiratory enzymes: activities and protein levels
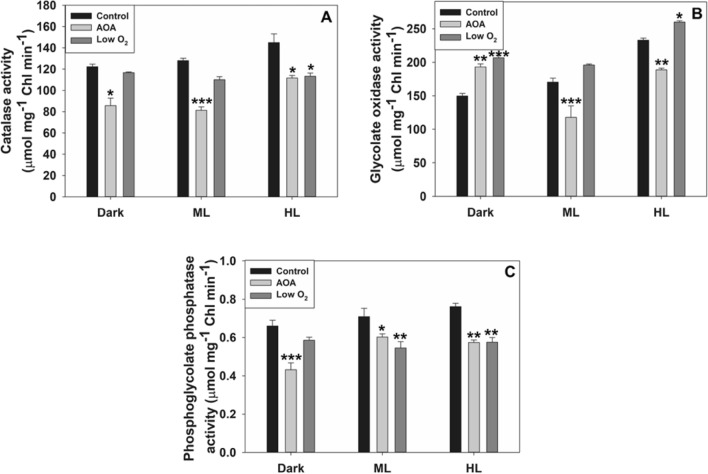
Among the enzymes studied, PGLP was chloroplastic, while GO and CAT were in peroxisomes. The activities of CAT and PGLP were lowered with AOA (photorespiratory inhibitor) treatment or low O2, compared to control. The activity of GO was either marginally enhanced or decreased under low O2, or by AOA (Fig. 1).
Fig. 1.
The activities of key photorespiratory enzymes, peroxisomal CAT/ glycolate oxidase (GO) A/B, chloroplastic phosphoglycolate phosphatase (PGLP) C, in leaves on treatment with aminooxyacetic acid (AOA) or low O2 for 3 h under dark, moderate light (ML, 150 µmol m−2 s−1), or high light (HL, 600 µmol m−2 s−1). Data were averages ± SE of three independent experiments. Wherever relevant, the significance of AOA or low O2 effects compared to control are indicated. *P < 0.05; **P < 0.01, ***P < 0.001
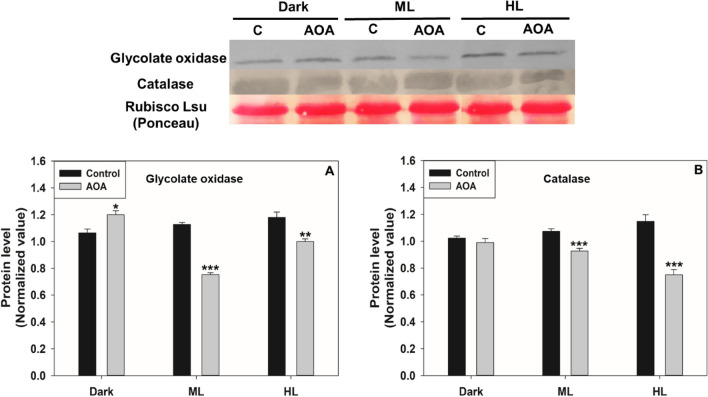
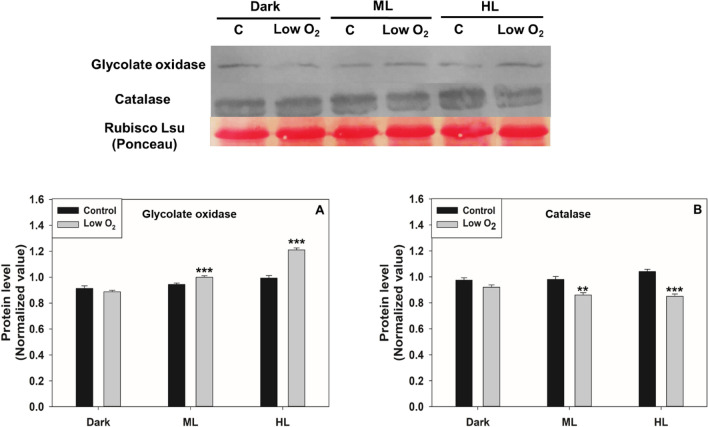
To validate the enzyme assay data, we checked the protein levels of GO and CAT under restricted photorespiration. The protein level of PGLP was not checked due to the non-availability of antibodies. Treatment with AOA decreased the GO and CAT proteins, particularly under ML and HL, compared to that of the respective control (Fig. 2). Similarly, the protein levels of CAT were decreased in ML on exposure to low O2. However, the GO protein levels were marginally increased under HL (Fig. 3).
Fig. 2.
The protein levels of GO and CAT on exposure to AOA. Ponceau staining was done to show equal loading (Top Panel). The proteins levels were normalized using Rubisco large subunit and quantified by Image J (Bottom Panel). Other details, including the significance were as in Fig. 1 and Materials and Methods
Fig. 3.
The protein levels of GO and CAT on exposure to low O2. Ponceau staining was done to show equal loading (Top Panel). The proteins levels were normalized using Rubisco large subunit and quantified by Image J (Bottom Panel). Other details, including the significance were as in Fig. 1 and Materials and Methods
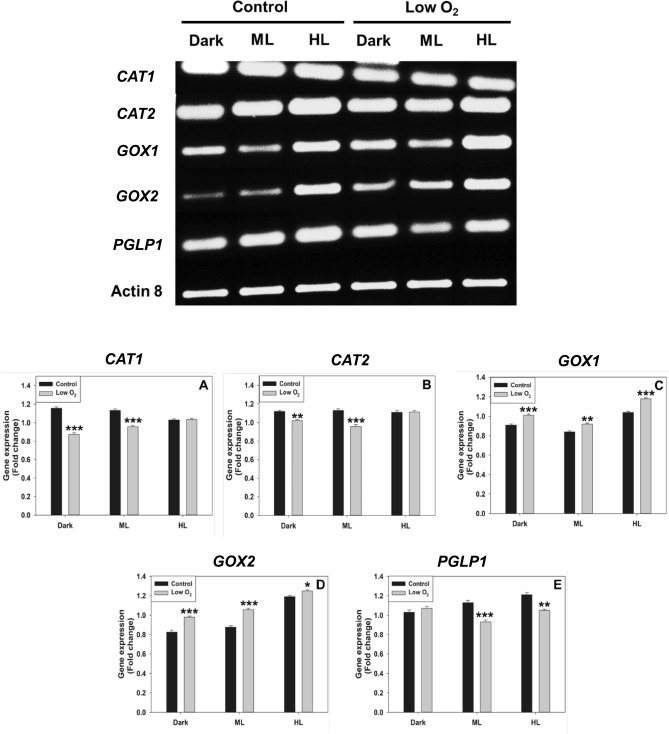
Transcripts of photorespiratory enzymes
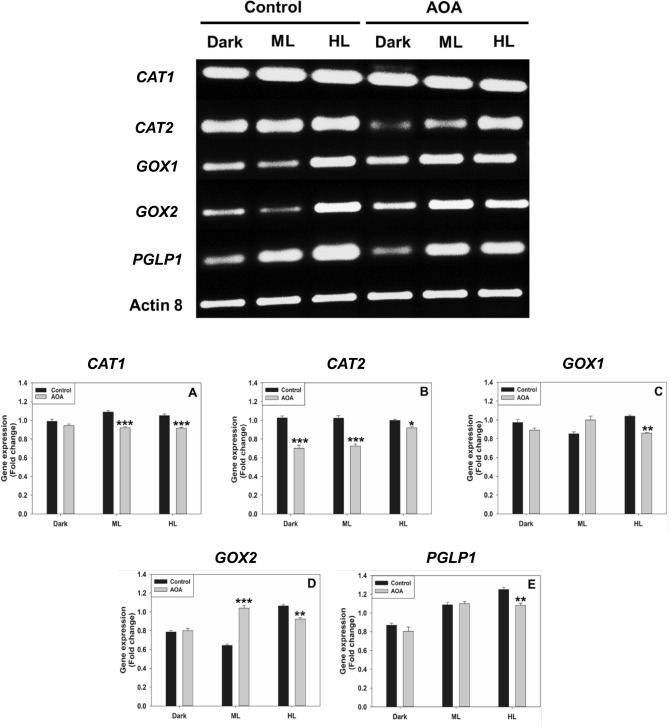
To further validate the enzyme and protein data, we have checked the transcript levels of CAT1, CAT2, GOX1, GOX2, and PGLP1 under restricted photorespiratory conditions. The transcripts of CAT2, GOX1, and GOX2 decreased when treated with AOA compared to the control. The downregulation of these transcripts was pronounced under HL. Similarly, the transcripts of CAT1 and PGLP1 were also downregulated by AOA under HL (Fig. 4). When treated with low O2, the transcripts of CAT1, CAT2, and PGLP1 were downregulated under ML and HL conditions. In contrast, transcripts of GOX1/GOX2 were upregulated (Fig. 5). The fold-change of the transcripts was calculated after normalization with reference to actin 8.
Fig. 4.
The transcript levels of CAT1, CAT2, GOX1, GOX2, and PGLP1 on exposure to AOA under dark, moderate, or high light (Top Panel). The fold-change was calculated after normalization with reference to actin 8 (Bottom Panel). Other details, including the significance were as in Fig. 1 and Materials and Methods
Fig. 5.
The transcript levels of CAT1, CAT2, GOX1, GOX2, and PGLP1 on exposure to low O2 under dark, moderate, or high light (Top Panel). The fold-change was calculated after normalization with reference to Actin 8 (Bottom Panel). Other details, including the significance were as in Fig. 1 and Materials and Methods
ROS accumulation in leaves
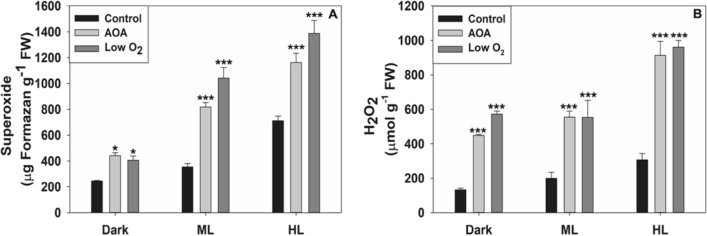
Superoxide and H2O2 levels increased markedly in ML and HL, as indicated by NBT or DAB staining (Fig. 6). Superoxide content of leaves rose by 2 to 3-fold with AOA or low O2 (Fig. 7A). Similar to this, DAB staining revealed that the H2O2 level in Arabidopsis leaves was also enhanced by 2 to 3-fold when treated with AOA or low O2 under HL (Fig. 7B). The levels of superoxide were far higher than those of H2O2 in Arabidopsis leaves.
Fig. 6.
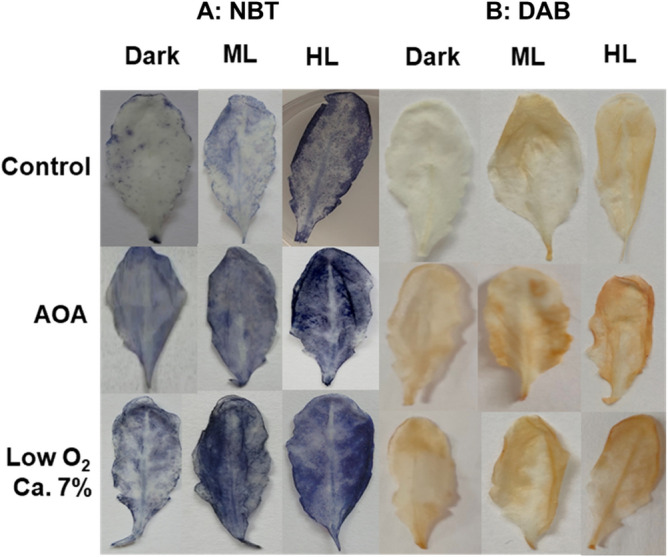
The accumulation of superoxide and H2O2 in Arabidopsis leaves, visualized by NBT and DAB staining. The leaves were treated with AOA or low O2 and exposed to moderate light (ML) or high light (HL). Other details were as in Fig. 1 and Materials and Methods
Fig. 7.
Superoxide and H2O2 levels were quantified from NBT and DAB-stained leaves on exposure to AOA and low O2. Other details including the significance were as in Fig. 1 and Materials and Methods
Discussion
Upregulation of photorespiration under abiotic or biotic stress was well established. It was envisaged that such upregulation could adapt to the oxidative stress challenge (Voss et al. 2013; Sunil et al. 2019). However, whether photorespiration can modulate leaf redox in return was unclear. The present article is the first attempt to assess the redox status of leaves when the photorespiratory metabolism is interrupted.
Evidence of suppression of selected photorespiratory enzyme components
Two approaches to limit photorespiration were employed, namely low O2 and incubation with AOA. Low O2 levels were caused by purging with N2 gas that slowed the activity of RuBP oxygenase. On the other hand, AOA blocked the glycine oxidation to ammonia, a critical step in the photorespiratory pathway. We focussed on CAT/GO in peroxisomes and PGLP in chloroplasts. Marked suppression of enzyme components related to GO, CAT, and PGLP confirmed that the photorespiratory metabolism was restricted under low O2 (Figs. 1, 3 and 5). Similarly, the use of AOA too ensured the down-regulation of photorespiration (Figs. 1, 2 and 4).
The existing literature on the pattern of GO activity under low O2 or hypoxia was ambiguous. There was a marked upregulation of GO gene expression in Arabidopsis under hypoxia (Engqvist et al. 2015). When roots were flooded, creating anoxia, there was a decrease in the GO activity in barley and common bean plants (Yordanova et al. 2003; Posso et al. 2018). Since these studies on the responses to low O2 (anoxia) focussed on roots, they may not all be relevant, as we studied leaves. Studies made with rice and Hibiscus hamabo seedlings observed a slight transient increase in GO activity, followed by a decrease (Igamberdiev et al. 1991; Wu et al. 2013). Our results demonstrated a downregulation of not only CAT but also PGLP under low O2, confirming that the use of low O2 was effective in lowering photorespiratory metabolism in Arabidopsis leaves.
Similarly, there were attempts to restrict photorespiration using suitable inhibitors. For e.g., AOA and PPT treatment decreased photosynthesis and stomatal conductance/ transpiration while decreasing the CAT and Rubisco activity in Zea mays, Amaranthus palmeri, and Chlamydomonas reinhardtii (González-Moro et al. 1993, 1997; Coetzer and Al-Khatib 2001; Goyal 2002). Glycidate, an inhibitor of glycolate synthesis, restricted photorespiration and increased the photosynthetic rate in tobacco (Zelitch 1974). Our observations with AOA complement the earlier work that an appropriate compound can restrict photorespiration.
ROS accumulation under restricted photorespiration
Whether the incubation was in the dark or in the light, the levels of ROS (H2O2 and superoxide) increased considerably when photorespiration was restricted by low O2 or AOA (Figs. 6 and 7). The majority of the reports typically indicated an increase in ROS under hypoxia in several plants: pea, soybean, Arabidopsis thaliana, rice, and tobacco (Ershova et al. 2011; Kamal and Komatsu 2015; Paradiso et al. 2016; Liu et al. 2017; Yamauchi et al. 2017; Jayawardhane et al. 2020; Liu et al. 2023).
One of the reasons for elevated ROS under flood and hypoxia was the decrease in the CAT activity, as in lentil, rice, and lettuce (Tang et al. 2015; Bharadwaj et al. 2023; Liu et al. 2023). Glufosinate treatment in Amaranthus palmeri led to the accumulation of superoxide and H2O2 (ROS) and downregulated the photosynthetic rate (Takano et al. 2019, 2020). Photorespiratory mutants, like hpr1, accumulated ROS, which was detrimental to metabolism and growth, under high light (Wang et al. 2022).
Under ambient air, photorespiration occurs, but it is reduced under high CO2 conditions (Wujeska-Klause et al. 2019; Celebi-Ergin et al. 2022). Photorespiration could be minimal once photosynthesis is saturated at high CO2 (Lenka et al. 2019; Marçal et al. 2021). However, the advantages of elevated CO2 depend on the nitrogen and phosphate status of leaves (Tcherkez et al. 2020). The benefits would not be available if elevated CO2 were not well-supplemented with nitrogen and phosphorus. In an analogy to our approach, a question arises about the consequences of exposing plants to high CO2. Since RuBP oxygenase is still functional even under high CO2, photorespiratory metabolism continues to be necessary to ensure the conversion of 2-PG to 3-PGA with the help of enzymes located in peroxisomes, mitochondria and chloroplasts (Timm and Bauwe 2013; Timm and Hagemann 2020).
There are contrasting claims about the effect of elevated CO2 on oxidative stress. Oxidative stress was increased at high CO2, as indicated by increased protein carbonylation and ROS accumulation in Arabidopsis, soybean and pea (Qiu et al. 2008; Ershova et al. 2011). In contrast, increased CO2 reduced oxidative damage in plants, particularly under abiotic stress conditions, such as heat, salt, and drought (Pérez-López et al. 2009; Mishra and Agrawal 2014; Zinta et al. 2014). We suggest that under elevated CO2 if sufficient nitrogen is unavailable, and if plants are not under abiotic stress, photorespiration is likely to be restricted. As a result, the cellular ROS levels would increase.
Based on our findings, we emphasize that ROS generation increased under various situations of restricted photorespiratory metabolism. The increased ROS would harm physiological functions like photosynthesis and respiration (Mittler 2002, 2017). In return, photorespiration helped to keep optimal ROS levels in leaves.
An inverse relationship between photorespiratory components and the leaf ROS levels
The process of photorespiration had an inverse relationship with the redox status of leaves. Abiotic stress, e.g., drought or high light, elevated the photorespiratory enzyme activities (like GO, CAT, or HPR) in plants (Yuan et al. 2016; Cui et al. 2016; Bapatla et al. 2021). Photorespiration protected photosynthesis from stress-induced oxidative damage (Voss et al. 2013; Sunil et al. 2019). The present study emphasized that photorespiration and increased ROS/ oxidative stress in leaves exhibited an inverse relationship. Thus, photorespiration and cellular ROS levels were well coordinated in leaves.
Conclusion
The ROS (both superoxide and H2O2) levels were elevated under restricted photorespiratory conditions, confirming the hypothesis that photorespiration could minimize the levels of ROS and reduce oxidative stress. Photorespiration could complement the antioxidant enzyme systems to sustain low ROS levels in leaves. Further experiments are necessary to understand if photorespiration could be complemented with other components such as cyclic electron flow (CEF) or alternative oxidase (AOX) pathway during plant adaptation to abiotic stress.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Supported by a SERB Grant No. CRG/2022/003753 and IoE Research Chair Professor Contingency Grant (to ASR). DS was a CSIR - senior research fellow. PB was a recipient of UoH-BBL and SG was a UGC - senior research fellow.
Author contributions
ASR planned the investigation. DS conducted several experiments, while PB and SG helped in some. ASR and DS drafted the manuscript. All the authors helped to edit and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapatla RB, Saini D, Aswani V, Rajsheel P, Sunil B, Timm S, Raghavendra AS. Modulation of photorespiratory enzymes by oxidative and photo-oxidative stress induced by menadione in leaves of pea (Pisum sativum) Plants. 2021;10(5):987. doi: 10.3390/plants10050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj B, Mishegyan A, Nagalingam S, Guenther A, Joshee N, Sherman SH, Basu C. Physiological and genetic responses of lentil (Lens culinaris) under flood stress. Plant Stress. 2023;7:100130. doi: 10.1016/j.stress.2023.100130. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butler IB, Schoonen MA, Rickard DT. Removal of dissolved oxygen from water: a comparison of four common techniques. Talanta. 1994;41(2):211–215. doi: 10.1016/0039-9140(94)80110-X. [DOI] [PubMed] [Google Scholar]
- Celebi-Ergin B, Zimmerman RC, Hill VJ. Photorespiration in eelgrass (Zostera marina L.): a photoprotection mechanism for survival in a CO2-limited world. Front Plant Sci. 2022;13:1025416. doi: 10.3389/fpls.2022.1025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer E, Al-Khatib K. Photosynthetic inhibition and ammonium accumulation in Palmer Amaranth after glufosinate application. Weed Sci. 2001;49(4):454–459. doi: 10.1614/0043-1745(2001)049[0454:PIAAAI]2.0.CO;2. [DOI] [Google Scholar]
- Cui LL, Lu YS, Li Y, Yang C, Peng XX. Overexpression of glycolate oxidase confers improved photosynthesis under high light and high temperature in rice. Front Plant Sci. 2016;7:1165. doi: 10.3389/fpls.2016.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Roell MS, Weber AP. Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol. 2019;223(4):1762–1769. doi: 10.1111/nph.15872. [DOI] [PubMed] [Google Scholar]
- Engqvist MK, Schmitz J, Gertzmann A, Florian A, Jaspert N, Arif M, Balazadeh S, Mueller-Roeber B, Fernie AR, Maurino VG. GLYCOLATE OXIDASE3, a glycolate oxidase homolog of yeast L-lactate cytochrome c oxidoreductase, supports L-lactate oxidation in roots of Arabidopsis. Plant Physiol. 2015;169(2):1042–1061. doi: 10.1104/pp.15.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershova AN, Popova NV, Berdnikova OS. Production of reactive oxygen species and antioxidant enzymes of pea and soybean plants under hypoxia and high CO2 concentration in medium. Russ J Plant Physiol. 2011;58:982–990. doi: 10.1134/S1021443711050074. [DOI] [Google Scholar]
- González-Moro B, Lacuesta M, Becerril JM, Gonzalez-Murua C, Muñoz-Rueda A. Glycolate accumulation causes a decrease of photosynthesis by inhibiting RUBISCO activity in maize. J Plant Physiol. 1997;150(4):388–394. doi: 10.1016/S0176-1617(97)80087-9. [DOI] [Google Scholar]
- González-Moro M, Lacuesta M, Royuela M, Muñoz-Rueda A, González-Murua C. Comparative study of the inhibition of photosynthesis caused by aminooxyacetic acid and phosphinothricin in Zea mays. J Plant Physiol. 1993;142(2):161–166. doi: 10.1016/S0176-1617(11)80957-0. [DOI] [Google Scholar]
- Goyal A. Glycolate metabolism in algal chloroplasts: inhibition by salicylhydroxamic acid (SHAM) Physiol Plant. 2002;116(2):264–270. doi: 10.1034/j.1399-3054.2002.1160217.x. [DOI] [PubMed] [Google Scholar]
- Gunell S, Lempiäinen T, Rintamäki E, Aro EM, Tikkanen M. Enhanced function of non-photoinhibited photosystem II complexes upon PSII photoinhibition. Biochim Biophys Acta (BBA) Bioenerg. 2023;1864(3):148978. doi: 10.1016/j.bbabio.2023.148978. [DOI] [PubMed] [Google Scholar]
- Gururani MA, Venkatesh J, Tran LSP. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant. 2015;8(9):1304–1320. doi: 10.1016/j.molp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Han MA, Hong SJ, Kim ZH, Cho BK, Lee H, Choi HK, Lee CG. Enhanced production of fatty acids via redirection of carbon flux in marine microalga Tetraselmis Sp. J Microbiol Biotechnol. 2018;28(2):267–274. doi: 10.4014/jmb.1702.02064. [DOI] [PubMed] [Google Scholar]
- Hewitt CN, Monson RK, Fall R. Isoprene emissions from the grass Arundo donax L. are not linked to photorespiration. Plant Sci. 1990;66(2):139–144. doi: 10.1016/0168-9452(90)90197-V. [DOI] [Google Scholar]
- Huang W, Yang YJ, Wang JH, Hu H. Photorespiration is the major alternative electron sink under high light in alpine evergreen sclerophyllous Rhododendron species. Plant Sci. 2019;289:110275. doi: 10.1016/j.plantsci.2019.110275. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Ivanov BF, Nichugovskaya VD, Shevchenko LV. Intermediates of peroxisomal metabolism under conditions of oxygen deficit and carbon dioxide excess. Soviet Plant Physiol. 1991;38(5):673–679. [Google Scholar]
- Jayawardhane J, Cochrane DW, Vyas P, Bykova NV, Vanlerberghe GC, Igamberdiev AU. U Roles for plant mitochondrial alternative oxidase under normoxia, hypoxia, and reoxygenation conditions. Front Plant Sci. 2020;11:566. doi: 10.3389/fpls.2020.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AHM, Komatsu S. Involvement of reactive oxygen species and mitochondrial proteins in biophoton emission in roots of soybean plants under flooding stress. J Proteome Res. 2015;14(5):2219–2236. doi: 10.1021/acs.jproteome.5b00007. [DOI] [PubMed] [Google Scholar]
- Kang T, Wu HD, Lu BY, Luo XJ, Gong CM, Bai J. Low concentrations of glycine inhibit photorespiration and enhance the net rate of photosynthesis in Caragana korshinskii. Photosynthetica. 2018;56(2):512–519. doi: 10.1007/s11099-017-0688-1. [DOI] [Google Scholar]
- Kleczkowski LA, Randall DD, Blevins DG. Inhibition of spinach leaf NADPH (NADH)-glyoxylate reductase by acetohydroxamate, aminooxyacetate, and glycidate. Plant Physiol. 1987;84(3):619–623. doi: 10.1104/pp.84.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Verma D, Jin S, Singh ND, Daniell H. Release of proteins from intact chloroplasts induced by reactive oxygen species during biotic and abiotic stress. PLoS ONE. 2013;8(6):e67106. doi: 10.1371/journal.pone.0067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka NK, Lenka S, Singh KK, Kumar A, Aher SB, Yashona DS, Dey P, Agrawal PK, Patra AK. Effect of elevated carbon dioxide on growth, nutrient partitioning, and uptake of major nutrients by soybean under varied nitrogen application levels. J Plant Nutr Soil Sci. 2019;182(4):509–514. doi: 10.1002/jpln.201800488. [DOI] [Google Scholar]
- Liu B, Sun L, Ma L, Hao FS. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 2017;36(6):947–957. doi: 10.1007/s00299-017-2128-x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang J, Zhang T, Li M, Yan H, Liu Q, Wei Y, Ji X, Zhao Q. Exogenous melatonin positively regulates rice root growth through promoting the antioxidant system and mediating the auxin signaling under root-zone hypoxia stress. Agronomy. 2023;13(2):386. doi: 10.3390/agronomy13020386. [DOI] [Google Scholar]
- Marçal DM, Avila RT, Quiroga-Rojas LF, de Souza RP, Junior CCG, Ponte LR, Barbosa ML, Oliveira LA, Martins SCV, Ramalho JDC, DaMatta FM. Elevated CO2 benefits coffee growth and photosynthetic performance regardless of light availability. Plant Physiol Biochem. 2021;158:524–535. doi: 10.1016/j.plaphy.2020.11.042. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Agrawal SB. Cultivar Specific response of CO2 fertilization on Two Tropical Mung Bean (Vigna radiata L.) cultivars: ROS Generation, antioxidant status, physiology, growth, yield and seed quality. J Agron Crop Sci. 2014;200(4):273–289. doi: 10.1111/jac.12057. [DOI] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Paradiso A, Caretto S, Leone A, Bove A, Nisi R, De Gara L. ROS production and scavenging under anoxia and re-oxygenation in Arabidopsis cells: a balance between redox signaling and impairment. Front Plant Sci. 2016;7:1803. doi: 10.3389/fpls.2016.01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BD, Payne LA, Chen YZ, Graham D. An inhibitor of catalase induced by cold in chilling-sensitive plants. Plant Physiol. 1984;76(4):1014–1018. doi: 10.1104/pp.76.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Muñoz‐Rueda A, Navari‐Izzo F, Mena‐Petite A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant. 2009;135(1):29–42. doi: 10.1111/j.1399-3054.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- Posso DA, Borella J, Reissig GN, Bacarin MA. Root flooding-induced changes in the dynamic dissipation of the photosynthetic energy of common bean plants. Acta Physiol Plant. 2018;40(12):212. doi: 10.1007/s11738-018-2790-9. [DOI] [Google Scholar]
- Qiu QS, Huber JL, Booker FL, Jain V, Leakey AD, Fiscus EL, Yau PM, Ort DR, Huber SC. Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated CO2. Photosynth Res. 2008;97:155–166. doi: 10.1007/s11120-008-9310-5. [DOI] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Maslow MM, Breusegem FV, Noctor G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength‐dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2‐induced cell death. Plant J. 2007;52(4):640–657. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- Riazunnisa K, Padmavathi L, Bauwe H, Raghavendra AS. Markedly low requirement of added CO2 for photosynthesis by mesophyll protoplasts of pea (Pisum sativum): possible roles of photorespiratory CO2 and carbonic anhydrase. Physiol Plant. 2006;128(4):763–772. doi: 10.1111/j.1399-3054.2006.00803.x. [DOI] [Google Scholar]
- Saji S, Bathula S, Kubo A, Tamaoki M, Aono M, Sano T, Tobe K, Timm S, Bauwe H, Nakajima N, Saji H. Ozone-sensitive Arabidopsis mutants with deficiencies in photorespiratory enzymes. Plant Cell Physiol. 2017;58(5):914–924. doi: 10.1093/pcp/pcx027. [DOI] [PubMed] [Google Scholar]
- Somerville CR. Isolation of photorespiration mutants in Arabidopsis thaliana. In: Hallick M, Chua RB, editors. Methods Chloroplast Mol Biol Edelman. Amsterdam: Elsevier Biomed Press; 1982. pp. 129–138. [Google Scholar]
- Somerville CR, Ogren WL. A phosphoglycolate phosphatase-deficient mutant of Arabidopsis. Nature. 1979;280(5725):833–836. doi: 10.1038/280833a0. [DOI] [Google Scholar]
- Sunil B, Saini D, Bapatla RB, Aswani V, Raghavendra AS. Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth Res. 2019;139(1):67–79. doi: 10.1007/s11120-018-0577-x. [DOI] [PubMed] [Google Scholar]
- Szymańska R, Ślesak I, Orzechowska A, Kruk J. Physiological and biochemical responses to high light and temperature stress in plants. Environ Exp Bot. 2017;139:165–177. doi: 10.1016/j.envexpbot.2017.05.002. [DOI] [Google Scholar]
- Takano HK, Beffa R, Preston C, Westra P, Dayan FE. Reactive oxygen species trigger the fast action of glufosinate. Planta. 2019;249:1837–1849. doi: 10.1007/s00425-019-03124-3. [DOI] [PubMed] [Google Scholar]
- Takano HK, Beffa R, Preston C, Westra P, Dayan FE. A novel insight into the mode of action of glufosinate: how reactive oxygen species are formed. Photosynth Res. 2020;144(3):361–372. doi: 10.1007/s11120-020-00749-4. [DOI] [PubMed] [Google Scholar]
- Tang Y, Gao F, Guo S, Li F. The morphology, physiology and nutritional quality of lettuce grown under hypobaria and hypoxia. Acta Astronaut. 2015;112:29–36. doi: 10.1016/j.actaastro.2015.03.005. [DOI] [Google Scholar]
- Tcherkez G, Ben Mariem S, Larraya L, García-Mina JM, Zamarreño AM, Paradela A, Cui J, Badeck FW, Meza D, Rizza F, Bunce J, Han X, Tausz-Posch S, Cattivelli L, Fangmeier A, Aranjuelo I. Elevated CO2 has concurrent effects on leaf and grain metabolism but minimal effects on yield in wheat. J Exp Bot. 2020;71(19):5990–6003. doi: 10.1093/jxb/eraa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm S, Bauwe H. The variety of photorespiratory phenotypes–employing the current status for future research directions on photorespiration. Plant Biol. 2013;15(4):737–747. doi: 10.1111/j.1438-8677.2012.00691.x. [DOI] [PubMed] [Google Scholar]
- Timm S, Hagemann M. Photorespiration—how is it regulated and how does it regulate overall plant metabolism? J Exp Bot. 2020;71(14):3955–3965. doi: 10.1093/jxb/eraa183. [DOI] [PubMed] [Google Scholar]
- Timm S, Mielewczik M, Florian A, Frankenbach S, Dreissen A, Hocken N, Fernie AR, Walter A, Bauwe H. High-to-low CO2 acclimation reveals plasticity of the photorespiratory pathway and indicates regulatory links to cellular metabolism of Arabidopsis. PLoS ONE. 2012;8:e42809. doi: 10.1371/journal.pone.0042809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara R, Parada F, Rubio S, Pérez FJ. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot. 2012;63(11):4123–4131. doi: 10.1093/jxb/ers094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss I, Sunil B, Scheibe R, Raghavendra AS. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013;15(4):713–722. doi: 10.1111/j.1438-8677.2012.00710.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Wang Y, Li H, Wen Z, Hou X. HPR1 is required for high light intensity induced photorespiration in Arabidopsis thaliana. Int J Mol Sci. 2022;23(8):4444. doi: 10.3390/ijms23084444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Gu S, Zhou H, Wang GG, Cheng X, Yu M. Photosynthetic and physiological responses of native and exotic tidal woody seedlings to simulated tidal immersion. Estuar Coastal Shelf Sci. 2013;135:280–284. doi: 10.1016/j.ecss.2013.10.028. [DOI] [Google Scholar]
- Wujeska-Klause A, Crous KY, Ghannoum O, Ellsworth DS. Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob Change Biol. 2019;25(4):1282–1295. doi: 10.1111/gcb.14555. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nishimura M. Reduction to below threshold levels of glycolate oxidase activities in transgenic Tobacco enhances photoinhibition during irradiation. Plant Cell Physiol. 2000;41(12):1397–1406. doi: 10.1093/pcp/pcd074. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Yoshioka M, Fukazawa A, Mori H, Nishizawa NK, Tsutsumi N, Yoshioka H, Nakazono M. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell. 2017;29(4):775–790. doi: 10.1105/tpc.16.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova RY, Alexieva VS, Popova LP. Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants1. Russ J Plant Physiol. 2003;50(2):163–167. doi: 10.1023/A:1022908811211. [DOI] [Google Scholar]
- Yuan H, Cheung CM, Poolman MG, Hilbers PA, van Riel NA. A genome-scale metabolic network reconstruction of tomato (Solanum lycopersicum L.) and its application to photorespiratory metabolism. Plant J. 2016;85(2):289–304. doi: 10.1111/tpj.13075. [DOI] [PubMed] [Google Scholar]
- Zelitch I. The effect of glycidate, an inhibitor of glycolate synthesis, on photorespiration and net photosynthesis. Arch Biochem Biophys. 1974;163(1):367–377. doi: 10.1016/0003-9861(74)90488-3. [DOI] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens IA, Beemster GTS, Asard H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob Change Biol. 2014;20(12):3670–3685. doi: 10.1111/gcb.12626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.