Abstract
This review provides an update on recent findings from basic, translational, and clinical studies on the molecular mechanisms of mitochondrial dysfunction and apoptosis of hepatocytes in multiple liver diseases, including but not limited to alcohol-associated liver disease (ALD), metabolic dysfunction-associated steatotic liver disease (MASLD), and drug-induced liver injury (DILI). While the ethanol-inducible cytochrome P450-2E1 (CYP2E1) is mainly responsible for oxidizing binge alcohol via the microsomal ethanol oxidizing system, it is also responsible for metabolizing many xenobiotics, including pollutants, chemicals, drugs, and specific diets abundant in n-6 fatty acids, into toxic metabolites in many organs, including the liver, causing pathological insults through organelles such as mitochondria and endoplasmic reticula. Oxidative imbalances (oxidative stress) in mitochondria promote the covalent modifications of lipids, proteins, and nucleic acids through enzymatic and non-enzymatic mechanisms. Excessive changes stimulate various post-translational modifications (PTMs) of mitochondrial proteins, transcription factors, and histones. Increased PTMs of mitochondrial proteins inactivate many enzymes involved in the reduction of oxidative species, fatty acid metabolism, and mitophagy pathways, leading to mitochondrial dysfunction, energy depletion, and apoptosis. Unique from other organelles, mitochondria control many signaling cascades involved in bioenergetics (fat metabolism), inflammation, and apoptosis/necrosis of hepatocytes. When mitochondrial homeostasis is shifted, these pathways become altered or shut down, likely contributing to the death of hepatocytes with activation of inflammation and hepatic stellate cells, causing liver fibrosis and cirrhosis. This review will encapsulate how mitochondrial dysfunction contributes to hepatocyte apoptosis in several types of liver diseases in order to provide recommendations for targeted therapeutics.
Keywords: Alcohol-associated liver disease, Metabolic dysfunction-associated steatotic liver disease, Drug-induced liver injury, Viral hepatitis, Oxidative stress, CYP2E1, ALDH2, Post-translational modifications, Mitochondrial dysfunction, Apoptosis, Intestinal barrier dysfunction, Microbiota, Endotoxemia, Gut–liver axis
Introduction
Liver diseases are named based on their etiology. The hepatotoxicity of some drugs causes drug-induced liver diseases (DILI) and alcohol-associated liver disease (ALD) is a harmful consequence that may occur from excessive and chronic alcohol consumption. Liver diseases can also occur from overnutrition and obesity [metabolic dysfunction-associated steatotic liver disease (MASLD)], toxic agents [toxicant-associated fatty liver disease (TAFLD)], and hepatitis viruses (viral hepatitis). However, liver diseases, regardless of the cause, share many pathophysiological features like oxidative stress, post-translational modifications (PTMs), fat accumulation, metabolic signaling alterations, mitochondrial dysfunction, gut barrier dysfunction, inflammation, and hepatocyte apoptosis. Overall, we have reviewed the cellular and molecular pathologies of these liver diseases to derive potential preventions or therapeutic targets against liver diseases [1].
Oxidative stress in liver diseases
Oxidative stress is an imbalance between pro-oxidants and antioxidants. Usually, the cell can remove reactive oxygen species (ROS) and reactive nitrogen species (RNS) through antioxidant molecules such as reduced glutathione (GSH) and primary defense enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione-dependent peroxidases (GPx) [1]. So why has oxidative stress been observed in studies on DILI [2–5], TAFLD [6–8], ALD [9–12], and MASLD [13–23]? When organelles such as mitochondria, endoplasmic reticula (ER), and peroxisomes are damaged and dysfunctional, more ROS are produced [1, 18] in a vicious cycle [14, 24]. Excessive oxidative stress may cause DNA oxidation [25], lipid peroxidation, oxidative protein modifications [26], impaired fat metabolism [6, 20, 26–28], systemic inflammation [26, 29, 30], and tissue damage [31–33], all of which contribute to the progression of liver diseases [17, 34, 35].
Hepatocytes respond to oxidative stress in diverse ways. Hepatic stellate cells (HSCs), when activated by ROS and damage-associated molecular patterns (DAMPs) from injured or necrotic hepatocytes, will produce extracellular matrix components to construct fibrotic tissue [36]. On the other hand, Kupffer cells, upon activation by endotoxins (including lipopolysaccharide, LPS) or superoxide anions (⋅O2−), will then produce more ROS through stimulation of NADPH-dependent oxidases (NOXs), and the redox-sensitive transcription factor nuclear factor-κB (NF-κB)-mediated pro-inflammatory storm of cytokines, chemokines, and cell adhesion molecules (CAMs) [36]. Consequently, in response to oxidative stress, hepatocytes stimulate necrotic and apoptotic pathways, leading to impaired liver function, worsened paracrine inflammation, and fibrogenesis [36].
Post-translational protein modifications in liver diseases
PTMs regulate the localization, stability, and final activity of virtually all proteins in the context of liver diseases [37, 38]. The proteins involved in promoting various PTMs exist in several subcellular organelles, including the cytoplasm [39], ER [40, 41], mitochondria [42], and nucleus [43]; PTMs may also be observed in the proteomes of the liver [37, 44–47], gut [7], and other peripheral tissues [29]. PTMs found in liver diseases include protein acetylation [37, 42, 48], nitration [10, 26, 37, 49–53], S-nitrosylation [49, 54, 55], oxidation [37], phosphorylation [26, 56, 57], succinylation [58], ADP-ribosylation [44], ubiquitination [37, 59], SUMOylation [60–65], carbonylation [66–68], S-palmitoylation [45–47, 69], glycosylation [7, 37, 70, 71], protein adducts of aldehyde (i.e., acetaldehydes) [9, 72], lipid peroxidation products (LPOs), and advanced glycation end products (AGEs) [6, 66, 74–79]. Several studies detail that specific PTMs correlate with exposures to excessive alcohol [80, 81], CCl4 [7, 56], acetaminophen (APAP) [53, 82], 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) [39], or fructose [51]. In these cases, PTMs accumulate in cells and contribute significantly to fat accumulation [54], hepatocyte apoptosis, inflammation, fibrosis [51], and cirrhosis [37], by altering signaling pathways affiliated with liver disease progression [43, 66, 67] and by targeting proteins for ubiquitin-mediated proteolysis [59]. Overall, PTMs are one of the various ROS-mediated changes that occur on cellular and translational levels [29] to, directly and indirectly, contribute to alcohol-mediated hepatic injuries [12, 83, 84]. The contributing roles of specific PTMs or simple consequences in the disease process can be elucidated by carefully studying their time-dependent events. The functional activities of a specific PTM on a few selected proteins should also be found in disease models to figure out their roles further. Based on the concept and approaches, precisely characterizing the roles of specifically targeted PTMs in designated subcellular organelles or tissues can provide valuable information to understand better the molecular mechanisms of liver diseases or even genetic- and aging-related disorders. For example, in various alcohol exposure models, an elevated intestinal activity or expression of the ethanol-inducible cytochrome P450-2E1 (CYP2E1) contributes to increased ROS/RNS [12, 85], promoting PTMs (e.g., nitration [26, 51, 52, 59, 68, 86]), which structurally alters the intestinal environment creating inflammation, gut tight junction (TJ) and adherens junction (AJ) protein degradation, apoptosis of enterocytes in the intestines [11, 86, 87], systemic endotoxemia, and the progression of ALD [88, 89].
Mitochondrial dysfunction in liver diseases
Mitochondria are critical sites of bioenergetics, fat oxidation, intermediary metabolism, apoptosis, mitophagy, and redox homeostasis [90–93]. They are one of the primary sources of oxidative stress involved in fatty liver diseases [94]; α-ketoglutarate dehydrogenase and pyruvate dehydrogenase are involved in redox reactions to generate NADH and FADH2 in the tricarboxylic acid (TCA) cycle [95], then in the electron transport chain (ETC), complexes I, II, and III perform redox reactions to generate ATP [18]. Thus, to balance out the ROS/RNS generated from these redox reactions, many mitochondrial antioxidants and enzymes exist.
Mitochondrial antioxidant proteins in the first line of defense against ROS/RNS fall into three categories, including non-enzymatic antioxidants (i.e., GSH), direct enzymatic antioxidants (i.e., SOD2 and GPx), and indirect enzymatic antioxidants [i.e., glutathione reductases (GR), peroxiredoxins, and thioredoxins (Trx)] [96, 97]. The transcription of many of these antioxidants is either regulated by peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) [98] or by nuclear factor erythroid 2-related factor (Nrf2) separating from its regulatory binding protein Kelch-like ECH-associated protein (Keap1) [99, 100]. Typically, in the second line of defense, mitochondria trigger mitophagy to restore redox homeostasis and retain the function of other mitochondria and organelles for cellular activities [101]. However, these natural lines of defense in mitochondria can be altered after excessive consumption of or exposure to alcohol (ethanol) [85, 94], drugs [66, 102, 103], viruses [104], and some nutrients, including fructose [105]. Specifically, these exposures can alter mitochondrial redox homeostasis and oxidatively damage DNA [106, 107], RNA [106], lipids, and proteins, through PTMs [8, 26, 48, 70, 108, 109], causing inactivation of their functions and signaling pathways, leading to mitochondrial dysfunction [26, 53, 68, 83], death of hepatocytes, and liver damage [17, 110] or age-related conditions [91, 111–113]. Not only can many types of PTMs accumulate in mitochondria [85], but they can also downregulate the expression of or inactivate mitochondrial deacetylases like sirtuin 3 (SIRT3) [114–117], sirtuin 4 (SIRT4) [118–121], and sirtuin 5 (SIRT5) [8, 48,108, 109, 117, 122, 123] in the context of ALD [124, 125], MASLD [51, 126, 127], and other conditions [128]. Nucleus-localized sirtuin 1 (SIRT1) migrates to mitochondria to exert its effects [125] and is another prevalent target in the context of ALD [125, 129–133] and MASLD [22, 117, 133]. Other than sirtuin pathways, signaling pathways of PGC-1α [131, 134], AMPK [135, 136], as well as Bax and Bcl2 [132, 137], are altered to cause mitochondrial dysfunction and apoptosis of hepatocytes in liver diseases [131, 132, 134–137]. However, these proteins represent important targets for prevention and therapy [75, 85, 138–140]. Overall, mitochondrial conditions likely provide a more comprehensive picture of the molecular pathology of liver diseases [75, 127, 141, 142].
Intestinal barrier dysfunction in liver diseases
The gut, like many organs, has multiple barriers; the immunological barrier is composed of gut-associated lymphoid tissue, the physical barrier consists of epithelial TJ/AJ proteins and microbiota, and the chemical barrier is composed of antimicrobial proteins, IgA, and a mucus layer [143]. Gut dysbiosis is a term to describe changes in the relative abundance of beneficial and pathogenic bacteria, where excessive Gram-negative bacteria may stick to and cause perforations in the gut barrier wall (intestinal permeability changes) and “leak” various toxic metabolites [e.g., bile acids, trimethylamine (TMA), and LPS, a cell wall component of Gram-negative bacteria] into systemic circulation [144]. The atypical transmission of pathogenic gut bacteria and toxic metabolites (endotoxemia) acts to permeate the barriers of the liver through the portal vein, enterohepatic circulation, or bile acid secretions [145–147]. Gut dysbiosis is inextricably linked to the exacerbation and progression of various liver diseases [148–153]. Altered microbiota compositions have been found in several clinical cases of ALD [154, 155], cirrhosis [156–159], MASLD [160, 161], viral hepatitis including Hepatitis B viral infections (HBV) [162–165] and Hepatitis C viral infections (HCV) [166–168], as well as hepatocellular carcinoma (HCC) [158, 169]. Furthermore, mechanistic animal studies on hepatic endotoxemia have shown it to be positively correlated with intestinal barrier dysfunction; elevated PTMs that disrupt TJ/AJ protein networks allow intestinal contents such as pathogenic bacteria and LPS to leak out into systemic circulation, characteristically manifesting in hepatic nitro-oxidative stress [170–172]. Endotoxemia is also affiliated with mitochondrial dysfunction [25] and systemic inflammation [172] in rodent models of ALD [11, 126, 151, 173] and MASLD [20, 51, 68, 151, 174–178]. Sometimes, the etiology of ALD and MASLD may even overlap; several reports detail several taxa that produce endogenous ethanol, as observed in experimental models [51, 179], adult patients with MASLD [160], and even young children with metabolic dysfunction-associated steatohepatitis (MASH) [180]. Some cases of ALD [126] and MASLD [181–183] may even affect other peripheral organs via the intestinal route.
The causal roles of oxidative stress, PTMs, mitochondrial dysfunction, and intestinal barrier dysfunction in promoting several chronic and acute liver diseases
Alcohol-associated liver disease
Alcohol-mediated oxidative stress and gut barrier dysfunction
Alcohol is first absorbed in the small intestines, then transported to the liver and metabolized mainly by oxidative and non-oxidative metabolism [184–186]. The majority of alcohol is oxidized in the cytosol by aldehyde dehydrogenase (ADH), in peroxisomes by CAT, and in microsomes and mitochondria by CYP2E1 via the microsomal ethanol-oxidizing system (MEOS) before toxic acetaldehyde is further detoxified by mitochondrial aldehyde dehydrogenase 2 (ALDH2) [187, 188]. Liver diseases have been widely studied in the context of these enzymes that either oxidatively metabolize ethanol into toxic metabolites, or detoxify alcohol and are made less effective through genetic polymorphisms. It is known that oxidative damage from NAPDH-dependent CYP2E1 metabolism [189] leads to toxicological damage in ALD [190], causing events such as increased ROS production [190], antioxidant downregulation, mitochondrial dysfunction [191, 192], suppressed fatty acid oxidation, oxidative DNA damage, and protein adduct formation via lipid peroxidation [i.e., acrolein, malondialdehyde (MDA), and 4-hydroxynonenal (4-HNE)] in the liver [193]. In addition, another study showed a crucial role of CYP2E1 in alcohol-mediated oxidative DNA damage in the liver [194].
However, there is evidence that alcohol absorption along with oxidative and non-oxidative metabolism, occurs in the gut [187, 195]. Studies have found the expression of ADH 4 isoform [196], CYP2E1 [87], and ALDH2 in the intestines [87], as well as inactivated ALDH2 [55, 83] and upregulated CYP2E1 [87], acetaldehyde, and LPS [55, 83, 87, 196] in models of ALD. These changes indicate signs of oxidative ethanol metabolism that results in alcohol-induced oxidative stress and intestinal barrier dysfunction. Recently, it has been shown that protective ALDH2 is inactivated through PTMs such as oxidation [55] and S-nitrosylation in ALD [55, 83], nitration in APAP-mediated DILI [53, 59], phosphorylation in CCl4-mediated TAFLD [56, 197], oxidation in MDMA-induced TAFLD [198], and lipid peroxidation products in ALD [199]. Some believe that acetaldehyde independently causes alcohol-associated organ damage; one study displayed that it independently disrupted intestinal TJ and AJ protein networks, leading to endotoxemia and liver injury [9]. Similar effects of acetaldehyde have been shown in models of chronic alcohol exposure in Aldh2-KO mice, resulting in ALD [200]. Other studies conducted with binge alcohol-exposed Aldh2-KO mice suggest that acetaldehyde stimulates intestinal barrier dysfunction, leading to acute liver injury [10].
When ALDH2 activity is depleted in Aldh2-KO mice [201], pro-oxidant CYP2E1 has been found to be upregulated in the intestines upon alcohol exposure [87, 201, 202]. Intestinal CYP2E1-mediated oxidative stress can also sensitize the liver to toxicity through endotoxemia and gut-derived TNF-α through the CYP2E1-thioredoxin-ASK1-JNK1 pathway [203]. The effects of intestinal CYP2E1 on gut barrier dysfunction and endotoxemia are worsened with concomitant LPS administration [11]. Hepatic CYP2E1 is also another significant contributor to alcohol-induced oxidative stress and signaling pathway alterations [9], as shown in multiple studies examining the effects of inhibiting or knocking out the Cyp2e1 gene for [204–206]. For example, polyenyl phosphatidylcholine (PPC) effectively suppressed alcohol-mediated oxidative stress and then was found to be inhibiting CYP2E1 [40, 207]. In another study, transgenic over-expression of CYP2E1 in mice exacerbated the pathogenesis of ALD [206, 208] and MASLD [209, 210]. Perhaps reversing the expression and activities of CYP2E1 and ALDH2 may serve a purpose in protecting against alcohol-induced hepato-intestinal oxidative stress and gut barrier dysfunction. For example, a translational study showed that ALDH2 suppression was protected by physiologically relevant levels of omega-3 polyunsaturated fatty acids [103, 211], and in a recent phase II clinical trial, treatment with clomethiazole (CMZ), an inhibitor of CYP2E1, mitigated the biomarkers of alcohol-induced oxidative stress and ALD progression [212].
Alcohol-mediated oxidative stress and mitochondrial dysfunction in the fatty liver
Alcohol-mediated steatosis (or fat accumulation) can be induced by activating de novo fat synthesis, blocking fat degradation pathways, or increasing the transport of lipids from other tissues [190]. On a molecular level, this may also occur through mitochondrial dysfunction associated with decreased fat degradation due to PTM-mediated suppression of the enzymes in β-oxidation [103, 211], upregulated immune cell infiltrations, protein adducts, lipid peroxidatifon, and DNA damage [190].Alcohol-mediated ROS and RNS can also downregulate β-oxidation activity by inhibiting peroxisome proliferator-activated receptor-α (PPAR-α) and a lipid catabolism regulator named AMP-activated protein kinase (AMPK) [9, 213] while upregulating sterol regulatory binding protein-1 (SREBP-1) to increase hepatic fatty acid and cholesterol biosynthesis [9, 213]. Furthermore, chronic and binge alcohol models show increased lipid transport to the liver, contributing to fat accumulation in hepatocytes [214, 215]. A study from Ceni et al. theorizes that in cases of ALD, steatosis may happen epigenetically by targeting forkhead box (FoxO3a) and SIRT1, which serve as intermediaries between autophagy and transcriptional lipid metabolism regulation [9].
The vicious cycle of oxidative stress and inflammation in promoting fibrosis
Oxidative stress and inflammation reciprocally communicate in a positive feedback loop to stimulate fibrosis. The accumulation of ROS and LPOs promotes hepatocyte apoptosis/necrosis, which activates hepatic stellate cells (HSCs) [190]. In an attempt to heal this “wound,” HSCs may promote fibrosis and cirrhosis in an inflammatory signaling response [190]. The ROS-mediated activation of HSCs can be seen through the accumulation of α-smooth muscle actin (α-SMA), vimentin (VIM), and collagen in the extracellular matrices of hepatocytes [9, 191, 192]. Fibrosis may also be stimulated by upregulating the MDA/4-HNE pathway; this pathway will, in turn, upregulate pro-fibrogenic matrix metalloproteinase-2 (MMP2) and downregulate matrix metalloproteinase-1 (MMP1), two enzymes responsible for remodeling the hepatic extracellular matrix [9, 191, 192, 216]. Another method of alcohol-induced ROS-mediated liver fibrosis happens when acetaldehyde and other reactive aldehydes upregulate the expression of fibrogenic transforming-growth-factor-β (TGF-β). This stimulates the production of collagen and α-SMA and decreases interferon-γ signaling in HSCs to cause fibrosis [9]. Other PTMs, such as acetylation and methylation, have been known to contribute to the progression of fibrosis in ALD [72, 195, 217, 218]. This accumulated inflammation is worsened by increased intestinal barrier dysfunction [144, 219]. The likely-resulting endotoxemia from intestinal barrier dysfunction stimulates toll-like receptor 4 (TLR4) in Kupffer cells to produce NADPH-oxidase (NOX)-dependent ROS [213] and activate HSCs, leading to fibrosis [144, 219]. Additionally, the efforts of Nrf2 and its downstream antioxidant enzymes and proteins may be exhausted or suppressed, leading to lowered antioxidant levels, in the progression of liver injury to hepatitis and fibrosis [220].
Metabolic dysfunction-associated steatotic liver disease
MASLD is closely affiliated with obesity [221, 222], type-2 diabetes mellitus (T2DM) [221, 222], hypertension [221], and metabolic syndrome [221]. MASLD may be further specified as TAFLD. However, it should be noted that the overall mechanism of MASLD shares many commonalities with that of ALD. In MASLD and ALD, dysregulated lipid metabolism contributes to lipotoxicity and peroxidation [223], leading to ER stress, mitochondrial dysfunction, and hepatocyte damage. These alterations activate HSCs, leading to inflammation and fibrogenesis [193, 223–225]. Liver insults begin with the phase of steatosis; mitochondrial respiration increases to meet the increased need for energy. As a result, ROS production increases, activating antioxidant responses [95, 226, 227]. Lipid accumulation will develop due to the excess of free fatty acids (FFAs) [223], compromising mitophagy responses through the activation of JNK-dependent apoptosis [95, 226, 227]. In the next stage of MASH, inflammation and oxidative stress occur in a vicious, positive feedback loop [228], triggering apoptosis of hepatocytes [227], and potentially compromising cellular respiration, mitophagy, and antioxidant pathways. Fibrosis is initiated when increased inflammation, oxidative stress, and hepatocyte apoptosis cumulatively stimulate Kupffer cells and HSCs, as well as neutrophil infiltration, to repair the “wounds” [95, 229].
Despite this common pathology of ALD and MASLD, the Multiple-Hit Hypothesis remains a phenomenon more studied in the context of MASH/MASLD. The Multiple-Hit Hypothesis details increased fat accumulation to be the “first hit” [230]. According to Day and James, oxidative stress follows steatosis as the “second hit” [230]. MASLD has many manifestations of metabolic syndrome, from as little as lipid droplets to total systemic inflammation seen in MASH, with the possibility of progression to fibrosis and HCC. Other factors, such as inflammation, altered hepatocyte apoptosis signaling, and activation of HSCs, allow milder cases of MASLD to progress to more severe cases, including MASH [14]. More reports mention the Three-Hit Hypothesis, involving dysregulated lipid metabolism, mitochondrial dysfunction with decreased fat degradation, and oxidative stress that happens in cycles in the progression of MASLD cases [231, 232]. Additional theories on the Multiple-Hit Hypothesis of MASLD detail the role of lipid and sugar metabolism, gut barrier dysfunction, and systemic inflammation as common factors in metabolic syndrome [233]
MASLD/MASH, caused by non-alcohol substances, such as fructose/sucrose and Western-style high-fat diets (HFDs) (containing high ratios of pro-inflammatory omega-6 fatty acids to anti-inflammatory omega-3 fatty acids), is a hepatic manifestation of metabolic syndrome [234]. Similar to ALD, increased de novo fat synthesis and fat transport from adipose tissue with decreased mitochondrial fat degradation are usually observed in MASLD [235]. Increased oxidative stress and nitrative stress also significantly contribute to the progression of MASLD/MASH. Oxidative stress may happen partly through the involvement of translocation and activation of mitochondrial NOX4 [235], CYP2E1-generated ROS [68, 210, 236], HFD-mediated insulin resistance [22], and various PTMs of mitochondrial proteins, causing mitochondrial dysfunction, decreased fat degradation, and elevated hepatocyte death [49].
The effect of fat metabolism dysregulation on oxidative stress
Elevated FFAs can be more hepatotoxic than TG accumulation since FFAs can cause JNK-mediated hepatocyte apoptosis [227] and the production of a cytokine storm in the progression of MASLD and MASH [226]. In addition, an increased FFA pool may lead to oxidative stress in cells, altering apoptosis and causing NF-κB-related inflammatory pathways that induce cytokine production and activate HSCs [235]. The specific mechanism of FFA and ROS-related apoptosis of hepatocytes involves the regulation of the mitochondria by Bcl-2 and Bax. For instance, when NOX4 and CYP2E1 oxidize substrates like long-chain FFAs, uncoupled electrons leak from the mitochondrial ETC [68]. When FFAs are oxidized in peroxisomes and the ER, oxidative stress accumulates and activates the Bax/Bcl-2 complex through FoxOa3 and JNK [237]. Bax, when released from Bcl-2, will induce a mitochondrial permeability transition (MPT) in response to this oxidative stress [238], which will trigger cytochrome c to be released from mitochondria, activating caspase-mediated apoptosis [235]. Alternatively, activated JNK can stimulate the phosphorylation of Bax, leading to its translocation to mitochondria to cause mitochondrial permeability changes and hepatocyte apoptosis [239].
Besides the accumulation of FFAs and LPOs, cholesterol (dys)regulation significantly affects the onset and progression of MASLD. One study suggests that the cholesterol-to-bile acid ratio is vital to supporting the homeostatic redox environment of HSCs [240]. One report showed that cholesterol could be a selective inducer of oxidative stress and mitigate fibrosis by inducing HSC apoptosis [240]. A recent genetic study suggested that having a good cholesterol index (i.e., more high-density lipoprotein (HDL) cholesterol and less low-density lipoprotein (LDL) cholesterol) could significantly prevent FLD because HDLs allow LDLs and other fats to be filtered through the liver and excreted rather than accumulating in blood vessels and tissues [241].
The effect of insulinemia on fat metabolism and inflammation
Insulin resistance/insulinemia, genetics, and metabolic syndrome account for most cases of MASLD [231, 235]. Insulinemia has been shown to serve as a cause of FFA accumulation [242]. Mainly, most FFAs in hepatocytes are found in the FFA pool in the liver and transported after lipolysis from other tissues [242]. Insulin typically signals lipolysis of triglycerides (TGs) into FFAs. However, in cases of insulinemia, adipose cells are in constant lipolysis, causing excess FFAs to travel to the liver and lead to fat accumulation [18, 235].
Other studies have detailed an interaction between the NF-κB pathway and insulin resistance to MASLD [19]. Many therapeutic effects against MASLD related to oxidative stress and lipid peroxidation have been explored through this pathway, including normalizing mitochondrial function with proper mitochondrial β-oxidation and ATP synthesis [19]. Inhibiting the redox-sensitive transcription factor NF-κB is also an essential therapy targeting inflammation in MASLD to prevent the progression to worse disease stages such as MASH [19].
The effect of oxidative stress on fat metabolism and inflammation
Recent reviews suggest that increased oxidative stress may cause de novo lipogenesis through upregulation of SREBP-1 and mitochondrial dysfunction in MASLD [24, 228]. In MASH, ROS primarily come from mitochondrial electron leakage, pro-oxidative enzyme activation (i.e., CYP2E1, NOX4), iron accumulation and Fenton reaction metabolism [18, 243], and antioxidant depletion [235]. While it is well documented that CYP2E1 contributes to oxidative stress pathways in ALD and DILI, many reports have also demonstrated the critical role of CYP2E1 in MASLD through the production of ROS and LPOs; this may represent the second hit in the progression of steatosis to MASH [26, 41, 68, 178, 244]. Thus, this oxidative and lipotoxic stress must be balanced out with the help of various antioxidants.
The Nrf2/ARE pathway is a crucial prevention for MASLD because it counteracts oxidative stress and corrects lipid metabolism [19]. Under physiological states, Nrf2, a transcription factor, is usually bound to Keap1. Under oxidative stress conditions, ROS oxidizes Keap1, which is degraded by ubiquitin-dependent degradation and releases Nrf2. When Nrf2 is released, it travels to the nucleus to bind antioxidant response elements (ARE). Activation of the Nrf2/ARE pathway upregulates the transcription of several antioxidant enzymes, including HO-1, NADPH-dependent quinone reductase, and GSH synthesis enzymes like GR and glutamate-cysteine ligase modifier subunit (GCLM) [5].
Many antioxidants have been known to target MASLD, including vitamins E and C in MASLD patients, caffeine and coffee polyphenols in murine models of Western-style HFDs [14]. Other studies detail the use of metformin and Hesperetin in rat hepatocytes and HepG2 cells, and caffeine in zebrafish [14]. Mitochondria-targeting synthetic and naturally-occurring antioxidants like melatonin have an immense potential to treat or prevent MASLD [17].
Role of intestinal barrier dysfunction in MASLD
Earlier reports showed that intestinal barrier dysfunction plays a causal role in MASLD [160, 245–248]. In our opinion, intestinal barrier dysfunction in MASLD is caused by increased oxidative stress, which can cause apoptosis of gut epithelial cells (enterocytes), and PTMs of intestinal TJ/AJ proteins that lead to their decrements via ubiquitin-dependent proteolytic degradation [86]. WT mice exposed to a Western-style HFD (containing cholesterol to represent a fast food diet) showed elevated serum LPS within 2 weeks of feeding, indicating gut barrier dysfunction; insulin resistance, hepatic inflammation, and fibrosis followed at 10 and 22 weeks of feeding, suggesting a causal role of gut barrier dysfunction in the progression of liver disease [244]. [CYP2E1 levels may have been induced by endogenous ethanol production by gut microbiota [180, 249]. Although the cell death mechanisms of gut enterocytes in these rodent models of MASLD/MASH were not described, mitochondrial dysfunction may have happened due to elevated oxidative PTMs and Bax-mediated apoptosis. Thus, CYP2E1 may be an essential target to mitigate gut barrier dysfunction in MASLD/MASH [41, 232]. However, NADPH-oxidase may not be as important as CYP2E1 in the development of intestinal barrier dysfunction in MASH, as done in one study using a methionine and choline-deficient diet (MCD) [250].
Toxicant-associated fatty liver disease
Carbon tetrachloride
Carbon tetrachloride (CCl4) has been widely used as a hepatotoxic agent in experimental models. Since 1924, scientists have understood that CCl4 causes acute hepatotoxicity, fatty liver, and liver fibrosis, depending on the dosage and treatment duration. In the last hundred years, scientists have understood this mechanism to include lipid peroxidation, hepatotoxicity, and liver damage through CYP2E1-mediated metabolism into toxic trichloromethyl and trichloromethyl peroxyl radicals; these toxic metabolites cause oxidative damage in the mitochondria and ER [78]. CCl4-mediated hepatotoxicity is exacerbated by a Western-style HFD [251] and alcohol consumption [252], which both happen to elevate CYP2E1 levels. Previous animal studies also found that Cyp2e1-KO mice were relatively resistant to CCl4-mediated hepatotoxicity [189, 253].
Despite many reports, the molecular mechanisms of CCl4-mediated hepatotoxicity and acute liver injury could be further elucidated. A recent report detailed the time-dependent events of PTMs and hepatotoxicity in WT versus Cyp2e1-KO mice [56]. The results showed that JNK-mediated phosphorylation of many mitochondrial proteins occurred 1–8 h in WT mice after CCl4 treatment [56]. At the same time, acute hepatotoxicity, assessed by serum ALT activity, LPO levels, and H&E-stained liver histology, was observed 24 hours after IP injection of a single toxic dose (50 mg/kg) of CCl4 [56]. In this model, activated p-JNK translocated to mitochondria at 2 h and phosphorylated many mitochondrial proteins, such as ALDH2, ubiquinone-dependent NADH dehydrogenase (Complex I), and α-ketoglutarate dehydrogenase, decreasing their activities [56]. These changes in protein phosphorylation, decreased activities, and liver injury were markedly prevented when CCl4-exposed WT mice were co-treated with a highly selective JNK inhibitor (i.e., SU3327 or BI-78D3) and mitochondria-targeted Mito-TEMPO [56]. This model also demonstrated that Cyp2e1-KO mice were protected from CCl4-mediated cellular changes, JNK-mediated phosphorylation, mitochondrial dysfunction, and liver injury [56]. Thus, CYP2E1-mediated metabolic activation of CCl4 was shown to play a significant role in ROS production, and JNK-mediated PTMs in promoting mitochondrial dysfunction and acute liver injury. In fact, increased oxidative stress stimulated the activation of JNK, which translocated to mitochondria and phosphorylated many target proteins ( decreasing their activities), leading to hepatotoxicity at a later time point. These results support the causal role of PTMs in promoting mitochondrial dysfunction and the characteristic hepatotoxicity of TAFLD.
Thioacetamide
Thioacetamide (TAA) was developed as an anti-fungal agent. However, TAA exposure has caused acute liver injury, cirrhosis, and HCC, in experimental models [254–257] and humans [257], depending on the dosage and TAA exposure duration [255, 258]. TAA-mediated hepatotoxicity and other tissue damage, including renal and cardiac toxicity, are believed to be induced through CYP2E1-mediated TAA metabolism in mammals. In fact, Cyp2e1-KO mice were protected from TAA-mediated hepatotoxicity and HCC [259]. A recent report showed that TAA-mediated hepatocyte pyroptosis in mice can be attenuated by administration of an anaerobic bacterial species named Parabacteroides distasonis by modulating intestinal bile acid metabolism [260]. In this report, decreased levels of Parabacteroides distasonis were observed in people with hepatic fibrosis. Administration of this bacteria inhibited bile salt hydrolase and suppressed intestinal expression of Farnesoid X receptor (FXR) and its signaling [261]. It also reduced hepatic levels of a component of bile acid named taurochenodeoxycholic acid (TCDCA), which typically induces mitochondrial permeability transitions and caspase-11-dependent pyroptosis; therefore, reduction of TCDCA mitigated TAA-mediated liver fibrosis in mice [261]. Additionally, co-administration of the natural compound celastrol increased the relative abundance of Parabacteroides distasonis, promoting bile acid excretion and hepatic fibrosis attenuation. Celastrol was also shown to increase SIRT1 expression and FXR signaling to improve cholestatic liver disease [261]. These results suggest the beneficial effects of Parabacteroides distasonis and celastrol against liver disease and suggest crosstalk between the gut microbiota and liver disease. Based on these results, more studies detailing gut–liver interactions are needed to improve liver disease prognosis.
Drug-induced liver injuries
Drug-induced liver injuries (DILI) account for 50% of all acute liver diseases. Of this 50%, 37% of DILI are associated with acetaminophen (APAP, paracetamol) and the other 13% are caused by isoniazid (isonicotinic acid hydrazide, INAH), TAA, erythromycin, diclofenac, and others [262]. DILI cases fall into two major categories: intrinsic/direct hepatotoxicity or idiosyncratic hepatotoxicity, with a third emerging category being indirect mechanisms of hepatotoxicity (that may include gut dysbiosis) [263]. The most common cause of intrinsic DILI is APAP overdose mechanistically through mitochondrial dysfunction and hepatocyte damage [4], and the severity of idiosyncratic DILI varies based on the geographical region of prevalence [264] and environmental factors such as alcohol consumption and other pathological conditions, including obesity, insulin resistance, and (pre)diabetes. For example, the leading cause (45.4% according to the American DILI Network) of idiosyncratic DILI cases in the US and UK were due to antibiotic use, followed by herbal and dietary supplements, whereas, in Korea, herbal and dietary supplements were the cause of 70% of idiosyncratic DILI cases [142, 264]. The National Institutes of Health (NIH) supplies a database named LiverTox (http://livertox.nih.gov) that one meta-analysis [265] grouped into categories based on types of hepatotoxicity and should provide more detailed information on idiosyncratic cases. However, this review will focus on a few models illustrating the mechanisms of intrinsic cases of DILI.
Over-the-counter pain medicines: acetaminophen
APAP, the active ingredient found in Tylenol, Panamax, Excedrin, and Panadol, is generally prescribed and available as an over-the-counter medicine to treat pain, fever, and inflammation by reducing the production of prostaglandins. However, APAP overdose is the leading cause of acute liver diseases in the UK and USA [262, 266] and is responsible for 50% of acute DILI in the USA [267]. A multitude of investigations have elucidated the critical role of oxidative stress, mitochondrial dysfunction, and hepatocyte death in the mechanism of APAP overdose-induced liver diseases [110, 267–270]. In normal doses, APAP toxicity is not observed because its toxic metabolites are neutralized by GSH. However, after fasting (which decreases GSH), large amounts of APAP rapidly deplete cellular GSH, leading to acute liver injury [110, 268, 269]. In one mechanism, APAP becomes hepatotoxic after its metabolism by CYP2E1 and other P450 isoforms, and produces a reactive metabolite named N-acetyl-p-benzoquinone imine (NAPQI) [110, 271, 272]. This reactive metabolite produces conjugation adducts for many cellular proteins, including those involved in the mitochondrial ETC and others [110, 268, 269]. These protein-adducts, in turn, create mitochondrial nitro-oxidative stress, likely promoting PTMs of mitochondrial proteins [26, 41, 68, 178, 244], leading to impaired mitochondrial function and energy production. However, the roles of NAPQI-related covalent protein adducts have been challenged with evidence of similar patterns of protein-adducts observed in studies with a non-hepatotoxic structural analog named 3’-hydroxyacetanilide [26, 41, 68, 178, 244, 273]. In one study, pretreatment with gadolinium chloride, a suppressor of Kupffer cells, significantly prevented APAP-mediated liver injury but not NAPQI-protein adducts, suggesting a noncritical role of NAPQI-protein adducts in APAP-related hepatotoxicity [274]. Thus, scientists have explored the role of various PTMs in APAP-mediated acute hepatotoxicity. APAP can trigger a mitogen-activated protein kinase (MAPK) cascade [269], ultimately activating c-Jun N-terminal kinase (JNK) phosphorylation [267, 268, 271]. Phosphorylated JNK travels into the mitochondria to further phosphorylate many mitochondrial proteins, including the mitochondrial ETC, causing even more ROS leakage and oxidative stress by binding to SH3 homology associated BTK binding protein (Sab) [110, 275]. This excessive buildup of ROS causes a mitochondrial permeability change and the release of mitochondrial proteins that induce DNA damage and activate hepatocyte apoptosis [269, 276]. Ultimately, this process also impairs the autophagosome, leading to defective mitophagy [110] and receptor interacting protein (RIP) kinase-mediated necrosis of the cell [110, 268, 269, 277–279].
In addition to JNK-mediated protein phosphorylation, the essential roles of nitrated proteins in mitochondria and cytosol were reported [53, 59]. In these studies, time-dependent events of protein nitration and necrotic cell death were compared after a single toxic dose (350–400 mg/kg) of APAP was administered to WT and Cyp2e1-KO mice. Nitrated cytosolic and mitochondrial proteins were observed around 2–4 h, and hepatocyte necrosis and elevated serum ALT levels were noticed 24 hours after APAP exposure in WT mice. Mitochondrial ALDH2, ATP synthetase, GPx, 3-ketoacyl-CoA thiolase (KAT), SOD2, and cytosolic SOD1 were nitrated, and their activities were suppressed at 2–4 hours, suggesting a causal role of protein nitration in promoting mitochondrial dysfunction, leading to apoptosis or necrosis of hepatocytes. Additionally, the non-toxic analog 3’-hydroxyacetanilide did not cause nitration and liver injury in WT mice. In contrast, protein nitration and hepatotoxicity were not seen in the corresponding Cyp2e1-KO mice, supporting the vital roles of CYP2E1 and nitration in APAP-mediated DILI [53, 59]. Recent studies suggest that boosting antioxidants in the mitochondria through mitoquinone [280] and others [270] may serve as effective treatments for APAP-induced DILI.
It is also known that APAP toxicity is enhanced by co-existing conditions such as obesity and MASLD [281] and is often potentiated by alcohol intake [282–285]. APAP toxicity is also observed in people with alcohol use disorder (AUD), possibly due to upregulated CYP2E1 activity [282, 286, 287] or a response to fasting [288] (which is known to decrease GSH levels and increase CYP2E1 [202]). Thus, co-administration of excessive alcohol and therapeutic doses of APAP may put individuals at risk of severe liver injury [289].
Misused substances: cocaine, amphetamines, and MDMA
It has been well-established that many misused substances such as pain-relieving drugs, mood-enhancing drugs, and recreational psychostimulants such as cocaine, amphetamines, and 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy, Molly) are known to cause significant toxicity to the liver as well as many other organs [290]. Cocaine toxicity is mediated by its oxidative metabolism by P450 isozymes, including CYP2B, CYP3a, and CYP2E1. Reactive metabolites of cocaine (i.e., norcocaine and norcocaine nitric oxide) and increased ROS produced during P450-mediated catalysis are known to increase oxidative stress, leading to mitochondrial dysfunction, hepatocyte death, and liver injury [77, 291]. Hepatotoxicity due to cocaine has been significantly worsened in the presence of other agents such as endotoxin [292] and alcohol; co-administration of cocaine and ethanol produces potently toxic cocaethylene [293] and suppresses mitochondrial ALDH2 activity through PTMs [294]. Although the detailed cell death signaling mechanisms are unknown, one recent report showed that cocaine toxicity was attenuated in p53-knockout (p53-KO) mice, suggesting the involvement of the p53-mediated apoptosis pathway [295]. A recent study showed that cocaine caused mitochondrial dysfunction and acute liver injury in WT mice, and hepatotoxicity was prominently observed in Gpx-1-KO mice but protected in Gpx1-overexpressing transgenic mice [66]. Based on the importance of PTMs in mitochondrial dysfunction and hepatocyte cell death in DILI, ALD, and MASLD models, the contributing roles of various PTMs in cocaine-mediated mitochondrial dysfunction and hepatotoxicity are expected, although this needs to be verified by future research.
Overdoses of amphetamine-type psychostimulants like amphetamines and MDMA can cause hyperthermia, tissue injury, acute liver failure, and death, depending on their dosage and host conditions (e.g., hepatic GSH levels). MDMA toxicity is thought to occur through the P450-mediated production of its reactive metabolites, which can activate lysosomal functionand increase mitochondrial swelling and dysfunction [103, 296]. Other risk factors, such as hyperthermia,elevated neurotransmitter effluxes, increased LPOs, oxidized biogenic amines, decreased GSH, and dysregulated host environments, have also been suggested [103]. Although the detailed mechanisms of tissue injury are poorly understood, it is widely accepted that increased oxidative stress and nitrative stress play a key role in promoting MDMA-mediated hepatotoxicity. Targeted proteomics approaches revealed that many mitochondrial and cytosolic proteins were oxidatively modified upon exposure to MDMA, and some of them, like mitochondrial ALDH2, 3-ketoacyl-CoA, and ATP synthetase, were inactivated [198]. These reports support the role of oxidatively modified cellular proteins in promoting mitochondrial dysfunction and ER stress, contributing to cell death of hepatocytes and liver injury [49, 296, 297]. Other types of PTMs, such as nitration, JNK-mediated phosphorylation, and acetylation, of proteins, can also take part in the pathology of MDMA-mediated tissue injury, although this area solicits further investigation.
Anti-cancer agents: cisplatin
Many chemotherapies have been found to cause hepatotoxicity as well as other tissues. Antitumor antibiotics (e.g., dactinomycin, doxorubicin, and mitomycin) and alkylating agents (e.g., Busulfan, Melphalan, and Cyclophosphamide) have been shown to elevate serum ALT and AST activities in liver function tests [298]. Platinum-based agents (e.g., Oxaliplatin, Cisplatin, and Carboplatin) have also been shown to elevate serum levels of liver transaminases to cause steatohepatitis [298]. Cisplatin is a platinum-based chemotherapy drug that was approved by the FDA in 1978 despite its harsh side effects of inducing organ damage (including the liver and kidneys) [299], through oxidative metabolism via CYP2E1 and CYP4A11 [300]. Since then, the effects of cisplatin have been studied and reviewed; cisplatin induces oxidative stress [301–303], inflammation [299, 301, 304], mitochondrial dysfunction [304], apoptosis [305], and DNA damage [299, 304, 306]. Cisplatin causes oxidative stress by increasing MDA and decreasing GSH, GPx, CAT, and SOD [307]. Cisplatin also stimulates apoptotic signaling pathways involving TNF-α, Bax and Bcl-2, cytochrome c, and caspase-3, and stimulates IL-6 related inflammatory pathways [307].
There are also theories on how cisplatin-induced hepatotoxicity happens through the gut–liver axis. One study mentioned that cisplatin-induced liver toxicity is accelerated by inflammation and oxidative stress in the gut through the increased abundance of pathological bacteria like Escherichia, Parabacteroides, and Ruminococcus, all of which are Gram-negative bacteria [301]. In this study, antibiotic treatment improved liver histology, promoted Nrf2 activation, increased the levels of GSH, and inhibited the JNK- and p38-related cell death signaling pathways [301, 308], demonstrating its significance as an effective treatment for cisplatin-mediated hepatotoxicity.
Anti-tuberculosis agent: isoniazid
It is known that an anti-tuberculosis agent, isoniazid (isonicotinic acid hydrazide, INAH), can cause hepatitis through CYP2E1-mediated metabolism [309]. INAH or INAH metabolites, such as hydrazine, can cause mitochondrial injury, mitochondrial oxidative stress, and impaired metabolic homeostasis [310]. Consequently, these reactive metabolites and ROS will likely promote oxidative PTMs, lipid peroxidation, and cell death pathways. In addition, these reactive metabolites can bind to proteins, lipids, or nucleic acids and inhibit the enzymes in the mitochondrial ETC, resulting in oxidative stress, mitochondrial dysfunction, and hepatotoxicity [310, 311].
Like cisplatin, INAH alters CAT activity, GPx activity, and GSH content while increasing ROS and MDA levels. It also decreases the expression of microRNA-122 and PPAR-α and increases AP1 and JNK phosphorylation [312]. It also upregulates the mRNA expression of ER stress-related factors, including glucose-related protein 78 (Grp78), activating-transcription-factor-6 (ATF6), protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), x-box binding protein 1 (XBP1s), and C/EBP homologous protein (CHOP) [312]. Furthermore, it upregulates apoptotic signaling pathways, including Bax, cytochrome c release from mitochondria, and activation of caspases 3, 8, and 9. Lastly, it suppresses the Nrf2 signaling pathway, including Nrf2 and its downstream targets of heme oxygenase-1 (HO1), NAD(P)H quinone dehydrogenase 1 (NQO1), GCLM, and glutamate-cysteine ligase catalytic subunit (GCLC) [312].
Anti-depressants and anti-psychotics
Recent reports have shown that many anti-depressant medications clinically used are known to cause side effects of liver toxicity and weight gain [313, 314]. The mechanisms of these undesirable effects are poorly understood. Most of these side effects are idiosyncratic hepatotoxicity, and their symptoms appear as early as 5 days and last up to 3 years. Some severe cases are linked to users’ deaths [313], possibly due to severe drug interactions with other agents or pre-existing conditions like metabolic syndrome, obesity, and diabetes [315]. In contrast, people with MASLD may be prone to developing anxiety and depression [316]. Thus, this newly emerging area with anti-depressants related liver injury needs more studies.
Much of depression, schizophrenia, bipolar disorders, and psychotic disorders are associated with oxidative stress in the brain through the gut–brain axis [317, 318]. However, it is also known that the metabolisms of anti-depressants, including monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), first-generation anti-psychotics (FGAs), and second-generation anti-psychotics (SGAs), cause oxidative stress which may affect mitochondrial functions [314]. The mechanism by which anti-depressant and anti-psychotic medications induce oxidative stress in hepatocytes begins with their metabolism through cytochrome P450 isoforms, causing reduced GSH to convert to oxidized glutathione disulfide (GSSG), as well as producing ROS and reactive metabolites that covalently bind proteins, lipids, and nucleic acids [314]. These changes affect multiple mitochondrial metabolism pathways, including the proton gradient in oxidative phosphorylation and the proportion of superoxide anion metabolites leaking from the ETC [314]. These ROS activate apoptotic pathways involving Bax and Bcl-2, cytochrome c release from mitochondria, and activation of caspase-3 cleavage, as well as inducing cell damage and necrosis [314]. They can also decrease the activities of GST, GPx, and CAT [314]. A recent report also showed that a tricyclic anti-depressant named clomipramine caused mitochondrial dysfunction by decreasing ATP production [319]. It is important to note that all anti-depressants and anti-psychotics collectively follow this umbrella mechanism, although there are some subtle differences in individual mechanisms of creating hepatotoxic oxidative stress [314]. Oxidative stress is also produced from inflammatory pathways induced by chronic usage of anti-depressants and anti-psychotics; DAMP signals are likely to stimulate the apoptotic and necrotic pathways [314]. They also activate Kupffer cells through TLRs and inflammatory signaling pathway, including NF-κB, tumor necrosis factor-α (TNF-α), ROS, nitric oxide (NO), and chemokine and cytokine storms [314]. Chemokine and cytokine storms can also recruit circulating lymphocytes, eosinophils, and neutrophils, infiltrating the liver to start further inflammation and hepatotoxicity [314].
Viral hepatitis
It is also known that infection with hepatitis B (HBV) and C virus (HCV) can cause mitochondrial dysfunction, hepatic inflammation, and chronic liver disease, in people and cell lines, through the hepatitis virus core and other proteins associated with HBV/HCV [16, 104]. For instance, over-expression of the HCV core protein has been shown to induce oxidative stress and mitochondrial depolarization, leading to cell death [104]. These events were Ca2+-dependent and could be prevented by Ca2+ chelation. In addition, chronic infection with HCV increased oxidative DNA damage with high levels of MDA and 4-HNE, which correlated with the degree of liver inflammation and fibrosis [320]. The activity of the mitochondrial complex I enzyme (NADH-ubiquinone oxidoreductase) was suppressed in the genomic HCV replicon cells. This suppression decreased mitochondrial GSH and increased ROS production; these changes were restored by decreasing HCV replication with Fluvastatin [321]. Another study showed that the HCV core protein and CYP2E1, which produces ROS, work additively to decrease mitochondrial GSH and sensitize hepatocytes to ROS-mediated cell death [322]. Furthermore, a retrospective study with HCV-infected patients before and after antiviral treatment revealed that higher levels of serum LPS and intestinal fatty acid binding proteins, markers of intestinal permeability, were observed in patients with fibrosis/cirrhosis than those of patients without fibrosis and healthy volunteers [149] in both HBV [162–165] and HCV infections [166–168]. These studies indicate the importance of intestinal barrier dysfunction in accelerating hepatitis virus-induced liver disease outcomes through the gut–liver axis.
Hepatocellular carcinoma (HCC)
ALD and MASLD have been known to ultimately progress to HCC; 4.4 per 100,000 people with MASLD were diagnosed with HCC globally from 1989 to 2015 [221]. HCC is highly associated with metabolic risk factors and alcohol consumption [323], and is exacerbated by underlying cirrhosis [324]. Humans with the ALDH2*2 gene variants are more susceptible to alcohol-induced HCC [325] and esophageal cancers [73, 326]. ALDH2*2 protein variants are also individually-correlated with HCC [327, 328] and are identified as potential biomarkers for HCC [329, 330]. Some believe that accumulated acetaldehyde, LPOs, and ROS are carcinogenic and contribute to HCC through aging; this may allow fibrosis to progress to HCC by modulating various PTMs that increase DNA adducts of liver cells [9, 331]. Alcohol-mediated DNA oxidation, resulting from the polymorphisms of the ALDH2 gene and activation of CYP2E1, may also partake in the development of HCC and other cancers [9, 194, 331–333]. Overall, excessive alcohol intake induces CYP2E1 and/or inactivates mitochondrial ALDH2; both can lead to oxidative DNA damage, apoptosis, suppressed cell proliferation, and altered inflammatory pathways [9, 331], contributing to the development of tumors. Thus, targeting CYP2E1 or activating ALDH2 with safe chemicals, including naturally occurring dietary supplements [334], is a promising strategy to prevent alcohol-induced HCC. Recent reports showed that CMZ, a specific inhibitor of CYP2E1, was shown to prevent or improve ALD in humans [335] and experimental models of fibrosis and HCC [192]. Oxidative stress and dysbiosis are critical factors contributing to the progression of ALD to HCC [169, 331, 336, 337], and thus serve as important targets to prevent the onset of alcohol-related HCC [336, 338].
Challenges and opportunities
Challenges
One challenge is that many overlapping similarities exist between ALD and other liver diseases [339], but treatments for each liver disease can differ. A practical guideline for treating ALD patients described the clinical observation that ALD frequently occurred with other liver diseases, including MASLD and HCV [340]. Specific studies have been designed to distinguish the characteristics of ALD and MASLD. However, their histological features and pathological mechanisms are very similar [341]; this is because many metabolic risk factors for MASLD/MASH overlap with those of ALD [342] (and significantly increase the risk of severe liver disease [343]) and patients need to be treated for both types of liver disease.
Another challenge is the additive or synergistic interactions between alcohol intake and many other risk factors for liver diseases. For instance, Åberg et al. showed that mild and moderate consumers of alcohol with obesity, waist circumference, and diabetes may have increased levels of liver disease; in contrast, heavy drinkers with diabetes, large waist circumferences, and high BMIs had elevated risks of severe liver disease [343]. Other studies have detailed that BMI and moderate alcohol intake increase the risk of liver disease [344, 345]. Raynard et al. showed that BMI and blood glucose are independent risk factors for alcohol-associated fibrosis (cirrhosis) [342]. However, more studies are needed to differentiate the risk factors for ALD and MASLD. Due to the synergistic or potentiation effects of concurrent exposures to alcohol, HFDs, smoking, recreational and pharmaceutical drugs, individual mechanisms of liver injury and disease listed in previous sections may not be observed in isolation.
Likewise, a third consideration is that each of these exposures promotes unique patterns of ROS/RNS, inflammation, dysbiosis, and PTMs, and most of these features take place simultaneously. Overlapping pathological risk factors of liver diseases could further increase the difficulty of prognosis and therapeutic benefits unless those multiple interventions target many pathways simultaneously.
A fourth consideration is that the pharmacokinetics, specifically the absorption, distribution, metabolism, excretion, and toxicity (ADME-Tox) properties of hepatotoxic compounds and targeted interventions vary. These complexities present a significant challenge in the context of DILI. However, the Liver Tox Knowledge Base (LTKB) may provide more insight into adverse drug reactions in DILI research [255] and allow medical professionals to distinguish DILI caused by agents with different latencies, symptoms, mechanisms, and responses [346]. An editorial suggests that digitalizing these assessments differentiating DILI from ALD and MASLD and detecting specific agents of hepatotoxicity with a Revise Electronic Causality Assessment Method (RECAM) may improve the likelihood of treating emergency cases of DILI [346, 347]. Others mention the possibility of artificial intelligence (AI) for DILI predictions [348], but additional research is needed in the emerging field of AI.
The final challenge in liver disease research is establishing the correct diagnosis to differentiate between various liver diseases. Serum enzymes that are elevated in ALD may not necessarily be elevated in DILI diagnoses. Additionally, the current diagnosis of many liver diseases, such as MASLD and MASH, involves invasive access to biomarkers, often requiring biopsy surgeries for targeted interventions [349]. Some studies suggest screening for MASLD using less-invasive procedures (i.e., ultrasounds) [350], but deciding on a screening method may prove complex given the varied cost of diagnosis and treatment under different health insurance plans.
Opportunities
Fortunately, many preventions and treatments are being investigated for various liver diseases. Other than what has already been mentioned, interventions for MASLD include but are not limited to four main specific targets. The first intervention class targets hepatic fat accumulation by modulating PPARs (i.e., pemafibrate and elafibranor), targeting FXR signaling (i.e., obeticholic acid and celastrol), inhibiting de novo lipogenesis (i.e., aramchol and ACC inhibitors), and utilizing fibroblast growth factor analogues [347]. The second intervention class aims to alleviate oxidative stress, inflammation, and apoptosis, including ASK1 and caspase inhibitors (i.e., Emricasan) [347]. The third intervention class targets the intestinal microbiome and metabolic endotoxemia through IMMe124, TLR antagonists, and antibiotics (i.e., solithromycin). The fourth intervention class works to mitigate hepatic fibrosis through antifibrotic agents [i.e., cenicriviroc, (a cysteine-cysteine-motif chemokine receptor-2,5 antagonist) and gelectin-3 antagonists] [347].
Current interventions for ALD include abstinence, nutritional support, glucocorticosteroids, Pentoxifylline, anti-TNF therapy, antioxidants, liver transplantations, probiotics, antibiotics, S-adenosyl methionine, betaine, endocannabinoid antagonists, osteopontin inhibitors, and stem cell therapy, as reviewed [351]. To facilitate the development of safe and effective therapeutic drugs for treating ALD patients, the National Institute on Alcoholism and Alcohol Abuse (NIAAA, NIH) supports a multi-center Consortia for drug evaluation studies in double-blind randomized clinical trials. Since the start of the multi-center consortia in the early 2010s, many reports on clinical trials have been published. In addition, the executive summary of many published reports and other information related to various clinical trials by the multi-center Consortia, including DASH (Defeat Alcoholic Steatohepatitis) and TREAT (Translational Research and Evolving Alcoholic Hepatitis Treatment), have been compiled and available in Alcoholic Hepatitis Network (AlcHepNet). Currently, three agents, anakinra as an inhibitor of the IL-1 receptor [352], prednisone [353, 354], zinc sulfate [355], and coffee [356] are being evaluated for their efficacies in phase II clinical studies for alcohol-associated hepatitis. We expect more reports on the clinical trial outcomes to be published by the Consortia in the future. However, the most crucial factor for long-term survival and improvement of ALD patients is abstinence without relapse of AUD. Thus, many scientists proposed that ALD patients be treated with integrated care by preventing AUD [357] and obtaining nutritional support [358–361].
MASLD and ALD prevention may also begin with identifying bacterial signatures that distinguish the two and differentially diagnosing each through fecal samples [362]. Many other natural compounds such as silymarin, resveratrol, curcumin, and berberine have effectively prevented the progression of MASLD and MASH [363, 364]. Some of these naturally occurring compounds function as antioxidants and inhibitors of CYP2E1 [365–367] to prevent ALD, MASLD, and DILI. In addition, many naturally occurring antioxidants, including resveratrol, quercetin, and curcumin, are known to activate sirtuins, Nrf2, and PGC1α to improve liver disease outcomes [85, 138, 139, 363, 364, 368]. Based on the previous information regarding the prevalence of sirtuins 3, 4, and 5 in mitochondria and protectors against liver diseases and general age-related diseases, sirtuin activators including naturally occurring polyphenols would exhibit great therapeutic benefits [86, 126, 369]. Additionally, given the plethora of studies suggesting that SIRT1 is a therapeutic target in ALD, SIRT1 is another opportunity for precision medicine and targeted interventions [370]. Lastly, ghrelin has been shown to improve oxidative stress, apoptosis, and inflammation in MASLD development [371].
DILI may be prevented with adequate nutrition and abstinence. It may also be prevented by predicting its onset and type; this may be done by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372]. Current interventions for DILI include abstinence, N-acetylcysteine, corticosteroids, ursodeoxycholic acid, silymarin, glycyrrhizin, bile acid washouts, and emergency liver transplants [373].
Modulation of mitochondrial dysfunction, PTMs, and oxidative stress for liver diseases
Overall, mitochondrial dysfunction is associated with decreased function of energy supply, redox homeostasis, fat oxidation, cellular metabolism, and cell survival signals [374]. Webb et al. detailed that targeting mitochondrial dysfunction to prevent liver disease progressions includes altering metabolic signaling cascades to increase energetic efficiency, mitigating ROS/RNS, and restoring mitophagy and other systems to maintain homeostasis within mitochondria [91]. There are many ways to treat mitochondrial dysfunction liver diseases, including the consumption of a less caloric diet, anti-diabetic drugs (i.e., elafibranor, liraglutide, metformin, thiazolidinediones, MSDC 0602K), bile acid regulators (i.e., obeticholic acid, ursodeoxycholic acid), and antioxidants that act on nuclear receptors or mitochondrial metabolism (i.e., vitamin E, tempol, resveratrol) [91, 375]. Other mitochondria-targeted antioxidants, including mitoquinone [280], MitoE [376], mitoquinol mesylate (Mito-MES) [377], mitochondria-targeted ubiquinone [52], and quercetin [378] were reported to affect mitophagy and improve liver conditions. Furthermore, silymarin, corilagin, anthocyanins, dihydromyricetin, berberine, hydroxytyrosol, cysteamine, pentoxifylline, avocado oil, and pegbelfermin, mitotherapy, as well as ACC inhibitors, genistein, and aramchol were reported to improve mitochondrial function in MASLD [237]. Naturally occurring terpenoid polyphenols, including capsaicin [140], are excellent liver disease preventions that target mitochondrial dysfunction [379, 380] and CYP2E1-induced oxidative stress [381]. N-acetylcysteine [141], naturally occurring terpenoid polyphenols, and phenolic acids [368] were reported to minimize oxidative stress and hepatotoxicity by targeting antioxidant enzymes [299]. Melatonin is another promising agent that improves mitochondrial abnormalities in APAP-induced DILI [382, 383], chemotherapies [384], and other liver injuries [385].
Targeting intestinal barrier dysfunction for liver diseases
Gut dysbiosis is responsible for the exacerbation and progression of various liver diseases [386] through the promotion of toxic metabolites [150]; in fact, targeting gut dysbiosis has the potential to mitigate the onset and progression of liver diseases [150, 387]. Given this information, it is necessary to create intestinal eubiosis or symbiosis to mitigate liver disease development and progression. Many gut bacteria are helpful in their ability to turn toxic compounds into non-toxic compounds; for instance, B. xylanisolvens can metabolize nicotine in smokers with MASLD, preventing the progression to MASH [387].
Many reports reviewed different mechanisms by which the gut can be targeted to prevent liver disease or metabolic diseases caused by intestinal barrier disruption and endotoxemia. Considering the critical connection of the gut microbiota and liver, fecal microbiota transplants (FMT), or administering probiotics of Lactobacillus rhamnosus GG [388] and Akkermansia muciniphila [389] may be used to treat ALD [390] or metabolic disease in general [391]. In addition to probiotics, other gut-targeted treatments include antibiotics, prebiotics, and “postbiotics”. These treatments have various targets, acting on the microbial barrier, physical barrier, or chemical barrier of the intestines [152, 392]. Modulation of the microbial composition of the intestines would decrease the production and release of LPS into serum [393], regulate FXR signaling [394], limit or lower bacterial metabolites such as trimethylamine (TMA) and trimethylamine-N-oxide [395, 396], and prevent oxidative stress, inflammation, and gut barrier dysfunction [393] (Table 1).
Table 1.
Opportunities to treat liver diseases through various targets
| Designed to target | ALD | MASLD | DILI |
|---|---|---|---|
| Mitochondrial dysfunction |
Abstinence [351] Nutritional support: Vitamins like folate, vitamin B6, vitamin B12, vitamin A, and thiamine [351] Minerals (like selenium, zinc, copper, and magnesium [351] Nicotinamide riboside [85] Mitochondria-targeted ubiquinone [52] |
Silymarin, corilagin, anthocyanins, dihydromyricetin, berberine, hydroxytyrosol, cysteamine, pentoxifylline, avocado oil, and pegbelfermin, mitotherapy, as well as ACC inhibitors, genistein, and aramchol [237] Ursodeoxycholic acid [373] Less caloric diet, anti-diabetic drugs (i.e., elafibranor, liraglutide, metformin, thiazolidinediones, and MSDC 0602 K) [91, 375] MitoE [376] Mitoquinol mesylate (Mito-MES) [377] Capsaicin for septic acute liver injury [140] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] Melatonin for chemotherapy hepatotoxicity [382] [384] Mitoquinone for acetaminophen hepatotoxicity [280] |
| Oxidative stress |
Abstinence [351] Nutritional support: Vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals (like selenium, zinc, copper, and magnesium [351] Antioxidants [351] Nicotinamide riboside (targets SIRT1)[139] Melatonin and N-acetylcysteine [385] Mitochondria-targeted ubiquinone [52] |
ASK1 and caspase inhibitors (i.e., emricasan) [347] Silymarin, resveratrol, curcumin, and berberine [363, 364] Ghrelin [371] Berberine [363] Melatonin and N-acetylcysteine [385] Various flavonoids for CYP2E1-induced oxidative stress [381] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] N-acetylcysteine [141, 373, 397] Melatonin for chemotherapy hepatotoxicity [384] Melatonin with N-acetylcysteine for many types of DILI [385] Plant extracts and oil rich in flavonoids, terpenoids, polyphenols, and phenolic acids for cisplatin-mediated hepatoxicity [299] |
| PTMs |
Abstinence [351] Nutritional support: Vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals (like selenium, zinc, copper, and magnesium [351] Naturally occurring polyphenols to target SIRT3, SIRT4, and SIRT5 [11, 58, 86, 126, 369] SIRT1 targets [370] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] |
|
| Apoptosis |
Abstinence [351] Nutritional support: vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals like selenium, zinc, copper, and magnesium [351] |
ASK1 and caspase inhibitors (i.e., Emricasan) [347] Ghrelin [371] Quercetin [378] Capsaicin for septic acute liver injury [140] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] |
| Dysbiosis/intestinal barrier dysfunction/endotoxemia |
Abstinence [351] Nutritional support: vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals (like selenium, zinc, copper, and magnesium [351] Probiotics and antibiotics [351] Fecal microbiota transplants (FMT), or administering probiotics of Lactobacillus rhamnosus GG [388] and Akkermansia muciniphila [389, 390] Nicotinamide riboside [138] |
IMMe124 [347] TLR antagonists [347] Antibiotics (i.e., solithromycin) [347] Fecal samples to identify bacterial signature differences from ALD [362] B. xylanisolvens can metabolize nicotine in smokers with MASLD, preventing progression into MASH [387] Resveratrol [363] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] |
| Inflammation |
Abstinence [351] Nutritional support: vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals like selenium, zinc, copper, and magnesium [351] Glucocorticosteroids [351] Pentoxifylline [351] Anti-TNF therapy [351] Betaine [351] Osteopontin inhibition [351] Stem cell therapy [351] Nicotinamide riboside (targets SIRT1) [139] |
ASK1 and caspase inhibitors (i.e., emricasan) [347] Ghrelin [371] Silymarin [363] Resveratrol, curcumin, and berberine [363, 364] Quercetin [378] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] Corticosteroids [373] Silymarin [373] Glycyrrhizin [373] |
| Fat accumulation |
Abstinence [351] Nutritional support: vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals like selenium, zinc, copper, and magnesium [351] Betaine [351] Mitochondria-targeted ubiquinone [52] |
Modulating PPARs (i.e., pemafibrate and elafibranor) [347] Targeting FXR signaling (i.e., obeticholic acid and celastrol) [347] Inhibiting de novo lipogenesis (i.e., aramchol and ACC inhibitors) [347] Utilizing fibroblast growth factor analogues [347] Bile acid regulators (i.e., obeticholic acid, ursodeoxycholic acid) [373] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] Bile acid washouts [373] |
| Fibrosis |
Abstinence [351] Nutritional support: vitamins like folate, vitamin B6, vitamin B12, vitamin A, thiamine [351] Minerals like selenium, zinc, copper, and magnesium [351] Pentoxifylline [351] Liver transplantation [351] Betaine [351] Stem cell therapy [351] |
Antifibrotic agents (i.e., cenicriviroc, (a cysteine-cysteine-motif chemokine receptor-2,5 antagonist)) [347] Gelectin-3 antagonists] [347] Less caloric diet, anti-diabetic drugs (i.e., elafibranor, liraglutide, metformin, thiazolidinediones, MSDC 0602 K) [91, 375] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] Silymarin [373] |
| Hepatitis | Anakinra (IL-1 inhibitor) [352], prednisone [353, 354], zinc sulfate [355], and coffee [356] are being evaluated for their efficacies in phase II clinical studies [352–356] |
Adequate nutrition and abstinence [372] Predicting its onset and type by measuring hepatic transporter inhibition, mitochondrial toxicity, reactive metabolite formation, hepatocyte cytotoxicity, as well as the dose and physiochemical properties of the drug ingested [372] |
|
| Liver failure | Emergency liver transplants [373] |
Concluding remarks
This article briefly reviewed the causes and manifestations of various liver diseases, including ALD, MASLD/MASH, TAFLD, and DILI. We have precisely described the roles of oxidative stress and examples of PTMs (e.g., oxidation, S-nitrosylation, JNK-mediated phosphorylation, nitration, acetylation, and adduct formation) of mitochondrial proteins and transcription factors in promoting fat synthesis, mitochondrial dysfunction and apoptosis of hepatocytes, leading to individual liver diseases caused by different etiological agents. We have also explained the causal roles of CYP2E1 and NOXs as initial sources of ROS through the metabolism of many substrates, including alcohol (ethanol), long-chain FFAs, APAP, INAH, cisplatin, CCl4, cocaine, MDMA, antidepressants, and TAA. In addition, we have mentioned the protective role of mitochondrial ALDH2 against oxidative stress-mediated mitochondrial dysfunction and hepatotoxicity, apoptosis/necrosis of hepatocytes and gut enterocytes, the production of DAMPs, the activation of Kupffer cells and HSCs, altered inflammation, and fibrosis, leading to cirrhosis and HCC. We have also discussed the role of gut dysbiosis in promoting intestinal barrier dysfunction, endotoxemia, and liver disease through the gut–liver axis. Furthermore, we proposed four challenges regarding the diagnosis and prognosis of several liver diseases. Based on the mechanistic insights and challenges, we have suggested basic and translational research opportunities against liver diseases by listing the benefits of many agents, including naturally occurring antioxidants and synthetic compounds. We hope our review can contribute to developing new and effective preventive or therapeutic agents against individual liver diseases as well as organ damage in other tissues.
Acknowledgements
Figures 1 and 2 were created with a software from Biorender.com.
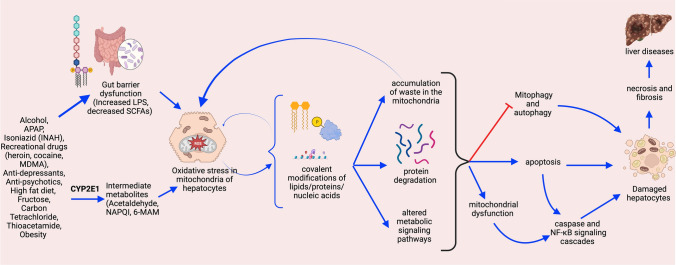
Fig. 1.
The functional outcomes of oxidative stress in hepatocytes. Oxidative stress in hepatocyte mitochondria may lead to mitochondrial dysfunction by promoting covalent modifications of lipids, proteins, and nucleic acids. These covalent modifications target some proteins for degradation and accumulate oxidized macromolecules in the mitochondria. These events may inactivate mitophagy and autophagy or cause mitochondrial dysfunction, leading to fat accumulation, caspase-mediated apoptosis, and NF-κB-mediated inflammation. These events collectively activate Kupffer and stellate cells in the liver, leading to further damage and dysfunction of liver physiology
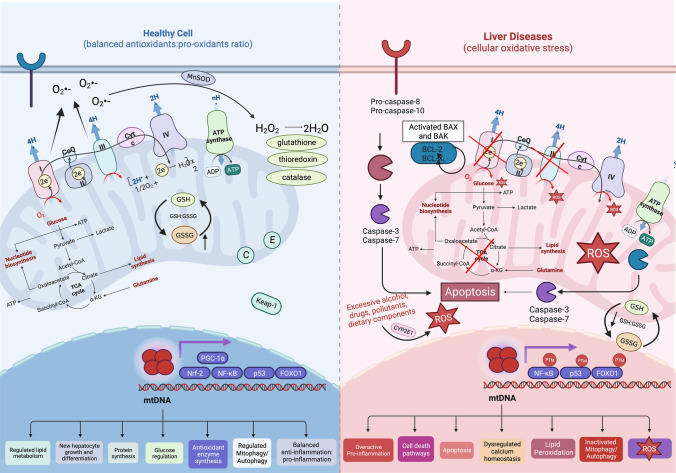
Fig. 2.
Mitochondrial function changes from healthy hepatocytes to liver diseases. Normally, mitochondria can clear out mild oxidative stress generated by oxidative phosphorylation and other cellular activities through enzymatic and non-enzymatic ways. They can transcribe new antioxidant enzymes to balance oxidative stress and the cycle of inflammation, and to regulate of metabolic pathways and mitochondrial biogenesis. However, mitochondrial dysfunction is a staple of ALD, MASLD, and other liver diseases due to its contributions to elevated oxidative stress, inflammation, dysfunctional metabolic pathways, and promotion of cellular death pathways
Abbreviations
- ADH
Alcohol dehydrogenase
- AGE-adduct
Advanced glycation end product adduct
- ALD
Alcohol-associated liver disease
- AJ
Adherens junction
- AMPK
AMP-activated protein kinase
- ALDH2
Mitochondrial aldehyde dehydrogenase 2
- APAP
Acetaminophen
- ARE
Antioxidant response element
- AUD
Alcohol use disorder
- CAT
Catalase
- CYP2E1
Ethanol-inducible cytochrome P450-2E1
- DAMP
Danger-associated molecular pattern
- DILI
Drug-induced liver injury
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- FFA
Free fatty acid
- FLD
Fatty liver disease
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Reduced glutathione
- GSSG
Oxidized glutathione
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HFD
Western-style high-fat diet
- 4-HNE
4-hydroxynonenal
- HSC
Hepatic stellate cells
- INAH
Isoniazid
- JNK
C-Jun N-terminal protein kinase
- Keap1
Kelch-like ECH-associated protein
- KO
Knockout
- LPO
Lipid peroxidation product
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MASH
Metabolic dysfunction-associated steatohepatitis
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- MDA
Malondialdehyde
- MDMA
3,4-methylenedioxy methamphetamine
- MEOS
Microsomal ethanol-oxidizing system
- NF-κB
Nuclear factor-κB
- NO
Nitric oxide
- NOX
NADPH-oxidase
- Nrf2
Nuclear factor erythroid 2-related factor
- PARP
Poly ADP-ribose polymerase
- PGC-1α
Peroxisomal proliferator-activated receptor-γ coactivator-1α
- PPAR-α
Peroxisomal proliferator-activated receptor-α
- PTMs
Post-translational modifications
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- α-SMA
α-Smooth muscle actin
- SOD
Superoxide dismutase
- SREBP-1
Sterol regulatory element-binding protein-1
- TAA
Thioacetamide
- TAFLD
Toxicant-associated fatty liver disease
- TG
Triglyceride
- TGF-β
Transforming growth factor-β
- TJ
Tight junction
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- SIRT1
NAD+-dependent deacetylase Sirtuin 1
- VIM
Vimentin
Author contributions
Manuscript conceptualization: KRL, BJS. Literature search and article acquisition: KRL, BJS. Writing drafts and revision: KRL, BJS. Figure drawing and table construction: KRL. Proofreading of the drafts and critical comments: KRL, BJS, WR. Final manuscript review and editing: KRL, WR, BJS. Post-submission review and editing: KRL, BJS, WR.
Funding
This research was supported by the Intramural Research Fund (to BJS) from the National Institute on Alcohol Abuse and Alcoholism.
Availability of data and material
Not applicable.
Declarations
Conflict of interest
All authors read this review article and have declared no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read the current version of this article and agreed to publish it.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Karli R. LeFort, Email: Karli.LeFort@nih.gov
Byoung-Joon Song, Email: bj.song@nih.gov.
References
- 1.Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther. 2012;342(3):608–618. doi: 10.1124/jpet.112.192120. [DOI] [PubMed] [Google Scholar]
- 2.Rada P, et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxid Redox Signal. 2018;28(13):1187–1208. doi: 10.1089/ars.2017.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu LY, et al. Involvement of histone hypoacetylation in INH-induced rat liver injury. Toxicol Res (Camb) 2018;7(1):41–47. doi: 10.1039/C7TX00166E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosedale M, Watkins PB. Drug-induced liver injury: advances in mechanistic understanding that will inform risk management. Clin Pharmacol Ther. 2017;101(4):469–480. doi: 10.1002/cpt.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiemstra S, et al. Dynamic modeling of Nrf2 pathway activation in liver cells after toxicant exposure. Sci Rep. 2022;12(1):7336. doi: 10.1038/s41598-022-10857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, et al. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77(15):1902–1915. doi: 10.1016/j.lfs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan SK, et al. Intestinal mucosal alterations in rats with carbon tetrachloride-induced cirrhosis: changes in glycosylation and luminal bacteria. Hepatology. 2006;43(4):837–846. doi: 10.1002/hep.21097. [DOI] [PubMed] [Google Scholar]
- 8.Hartley DP, Kroll DJ, Petersen DR. Prooxidant-initiated lipid peroxidation in isolated rat hepatocytes: detection of 4-hydroxynonenal- and malondialdehyde-protein adducts. Chem Res Toxicol. 1997;10(8):895–905. doi: 10.1021/tx960181b. [DOI] [PubMed] [Google Scholar]
- 9.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20(47):17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rungratanawanich W, et al. ALDH2 deficiency increases susceptibility to binge alcohol-induced gut leakiness, endotoxemia, and acute liver injury in mice through the gut–liver axis. Redox Biol. 2023;59:102577. doi: 10.1016/j.redox.2022.102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelmegeed MA, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang YM, Cho YE, Hwang S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver disease. Int J Mol Sci. 2022;23(2):774. doi: 10.3390/ijms23020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SK, et al. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch. 2019;471(6):829–843. doi: 10.1007/s00424-018-02250-3. [DOI] [PubMed] [Google Scholar]
- 14.Ore A, Akinloye OA. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina (Kaunas) 2019;55(2):26. doi: 10.3390/medicina55020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascè A, et al. NADPH oxidases connecting fatty liver disease, insulin resistance and type 2 diabetes: current knowledge and therapeutic outlook. Antioxidants (Basel) 2022;11(6):1131. doi: 10.3390/antiox11061131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155(3):629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Park JS, Roh YS. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch Pharm Res. 2019;42(11):935–946. doi: 10.1007/s12272-019-01178-1. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, et al. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Arroyave-Ospina JC, et al. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants (Basel) 2021;10(2):174. doi: 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng W, et al. Protective effects of sesamol against liver oxidative stress and inflammation in high-fat diet-induced hepatic steatosis. Nutrients. 2021;13(12):4484. doi: 10.3390/nu13124484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yildirim A, et al. The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br J Nutr. 2019;122(8):841–855. doi: 10.1017/S0007114519001466. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Abdelmegeed MA, Song BJ. Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. J Nutr Biochem. 2016;38:70–80. doi: 10.1016/j.jnutbio.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clare K, Dillon JF, Brennan PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10(5):939–946. doi: 10.14218/JCTH.2022.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podszun MC, et al. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. 2020;37:101710. doi: 10.1016/j.redox.2020.101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med. 2003;167(4):570–579. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- 26.Song BJ, et al. Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol. 2014;3:109–123. doi: 10.1016/j.redox.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai J, Cederbaum AI. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem. 2006;281(8):5128–5136. doi: 10.1074/jbc.M510484200. [DOI] [PubMed] [Google Scholar]
- 28.Laethem RM, et al. Formation of 19(S)-, 19(R)-, and 18(R)-hydroxyeicosatetraenoic acids by alcohol-inducible cytochrome P450 2E1. J Biol Chem. 1993;268(17):12912–12918. doi: 10.1016/S0021-9258(18)31472-8. [DOI] [PubMed] [Google Scholar]
- 29.Pisoschi AM, et al. Oxidative stress mitigation by antioxidants—an overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 30.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 31.Johnson J, et al. Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch Biochem Biophys. 2021;702:108698. doi: 10.1016/j.abb.2020.108698. [DOI] [PubMed] [Google Scholar]
- 32.Supinski GS, Schroder EA, Callahan LA. Mitochondria and critical illness. Chest. 2020;157(2):310–322. doi: 10.1016/j.chest.2019.08.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogvadze V, Zhivotovsky B. Analysis of mitochondrial dysfunction during cell death. Methods Mol Biol. 2021;2276:215–225. doi: 10.1007/978-1-0716-1266-8_16. [DOI] [PubMed] [Google Scholar]
- 34.Jakubczyk K, et al. Reactive oxygen species—sources, functions, oxidative damage. Pol Merkur Lekarski. 2020;48(284):124–127. [PubMed] [Google Scholar]
- 35.Alkadi H. A review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20(1):16–26. doi: 10.2174/1871526518666180628124323. [DOI] [PubMed] [Google Scholar]
- 36.Luangmonkong T, et al. Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71–102. doi: 10.1007/112_2018_10. [DOI] [PubMed] [Google Scholar]
- 37.Gligorijević N, Minić S, Nedić O. Structural changes of proteins in liver cirrhosis and consequential changes in their function. World J Gastroenterol. 2022;28(29):3780–3792. doi: 10.3748/wjg.v28.i29.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upreti VV, et al. Increased oxidative-modifications of cytosolic proteins in 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-exposed rat liver. Proteomics. 2011;11(2):202–211. doi: 10.1002/pmic.201000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev. 2004;36(3–4):511–529. doi: 10.1081/DMR-200033441. [DOI] [PubMed] [Google Scholar]
- 41.Abdelmegeed MA, et al. Role of CYP2E1 in mitochondrial dysfunction and hepatic injury by alcohol and non-alcoholic substances. Curr Mol Pharmacol. 2017;10(3):207–225. doi: 10.2174/1874467208666150817111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali HR, et al. Quantifying competition among mitochondrial protein acylation events induced by ethanol metabolism. J Proteome Res. 2019;18(4):1513–1531. doi: 10.1021/acs.jproteome.8b00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein S, et al. Impaired SUMOylation of nuclear receptor LRH-1 promotes nonalcoholic fatty liver disease. J Clin Invest. 2017;127(2):583–592. doi: 10.1172/JCI85499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin J, et al. Nutritional stress exacerbates hepatic steatosis induced by deletion of the histidine nucleotide-binding (Hint2) mitochondrial protein. Am J Physiol Gastrointest Liver Physiol. 2016;310(7):G497–509. doi: 10.1152/ajpgi.00178.2015. [DOI] [PubMed] [Google Scholar]
- 45.Shen LF, et al. Role of S-palmitoylation by ZDHHC13 in mitochondrial function and metabolism in liver. Sci Rep. 2017;7(1):2182. doi: 10.1038/s41598-017-02159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, et al. S-palmitoylation of PCSK9 induces sorafenib resistance in liver cancer by activating the PI3K/AKT pathway. Cell Rep. 2022;40(7):111194. doi: 10.1016/j.celrep.2022.111194. [DOI] [PubMed] [Google Scholar]
- 47.Ziemlińska E, et al. Palm oil-rich diet affects murine liver proteome and S-palmitoylome. Int J Mol Sci. 2021;22(23):13094. doi: 10.3390/ijms222313094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, et al. CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy. 2022;18(8):1879–1897. doi: 10.1080/15548627.2021.2007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song BJ, et al. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuhendler AJ, et al. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol. 2014;32(4):373–380. doi: 10.1038/nbt.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho YE, et al. Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450–2E1-mediated oxidative and nitrative stress. Hepatology. 2021;73(6):2180–2195. doi: 10.1002/hep.30652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chacko BK, et al. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54(1):153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdelmegeed MA, et al. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moon KH, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135(4):1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon KH, Abdelmegeed MA, Song BJ. Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett. 2007;581(21):3967–3972. doi: 10.1016/j.febslet.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jang S, et al. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015;6:552–564. doi: 10.1016/j.redox.2015.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lua YH, et al. Ethanol-induced CYP2E1 expression is reduced by lauric acid via PI3K pathway in HepG2 cells. Trop Life Sci Res. 2020;31(3):63–75. doi: 10.21315/tlsr2020.31.3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen XF et al (2018) SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep 19(5):e45124 [DOI] [PMC free article] [PubMed]
- 59.Abdelmegeed MA, et al. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79(1):57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomasi ML, et al. S-adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers. Hepatology. 2012;56(3):982–993. doi: 10.1002/hep.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J, et al. Mesencephalic astrocyte-derived neurotrophic factor inhibits liver cancer through small ubiquitin-related modifier (SUMO)ylation-related suppression of NF-κB/snail signaling pathway and epithelial-mesenchymal transition. Hepatology. 2020;71(4):1262–1278. doi: 10.1002/hep.30917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, et al. SIRT1 regulates N(6)-methyladenosine RNA modification in hepatocarcinogenesis by inducing RANBP2-dependent FTO SUMOylation. Hepatology. 2020;72(6):2029–2050. doi: 10.1002/hep.31222. [DOI] [PubMed] [Google Scholar]
- 63.Zeng M, et al. Sumoylation in liver disease. Clin Chim Acta. 2020;510:347–353. doi: 10.1016/j.cca.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 64.Yuan H, et al. The role of protein SUMOylation in human hepatocellular carcinoma: a potential target of new drug discovery and development. Cancers (Basel) 2021;13(22):5700. doi: 10.3390/cancers13225700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Q, et al. GTPBP4 promotes hepatocellular carcinoma progression and metastasis via the PKM2 dependent glucose metabolism. Redox Biol. 2022;56:102458. doi: 10.1016/j.redox.2022.102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mai HN, et al. Protective potential of glutathione peroxidase-1 gene against cocaine-induced acute hepatotoxic consequences in mice. J Appl Toxicol. 2018;38(12):1502–1520. doi: 10.1002/jat.3666. [DOI] [PubMed] [Google Scholar]
- 67.Aranda M, et al. In vivo hepatic oxidative stress because of carbon tetrachloride toxicity: protection by melatonin and pinoline. J Pineal Res. 2010;49(1):78–85. doi: 10.1111/j.1600-079X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 68.Abdelmegeed MA, et al. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57(4):860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu Z, Zhong L. New insights into the posttranslational regulation of human cytosolic thioredoxin by S-palmitoylation. Biochem Biophys Res Commun. 2015;460(4):949–956. doi: 10.1016/j.bbrc.2015.03.132. [DOI] [PubMed] [Google Scholar]
- 70.Zhu G, et al. Proteomics of post-translational modifications in colorectal cancer: discovery of new biomarkers. Biochim Biophys Acta Rev Cancer. 2022;1877(4):188735. doi: 10.1016/j.bbcan.2022.188735. [DOI] [PubMed] [Google Scholar]
- 71.Rodríguez JM, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adhikari R et al (2023) Alcohol-induced tubulin post-translational modifications directly alter hepatic protein trafficking. Hepatol Commun 7(4):e0103 [DOI] [PMC free article] [PubMed]
- 73.Yukawa Y, et al. Combination of ADH1B*2/ALDH2*2 polymorphisms alters acetaldehyde-derived DNA damage in the blood of Japanese alcoholics. Cancer Sci. 2012;103(9):1651–1655. doi: 10.1111/j.1349-7006.2012.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niemelä O, et al. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res. 1998;22(9):2118–2124. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhao X, et al. Quercetin protects ethanol-induced hepatocyte pyroptosis via scavenging mitochondrial ROS and promoting PGC-1α-regulated mitochondrial homeostasis in L02 cells. Oxid Med Cell Longev. 2022;2022:4591134. doi: 10.1155/2022/4591134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Seitz HK, Wang XD. Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res. 2010;34(3):567–573. doi: 10.1111/j.1530-0277.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vitcheva V. Cocaine toxicity and hepatic oxidative stress. Curr Med Chem. 2012;19(33):5677–5682. doi: 10.2174/092986712803988929. [DOI] [PubMed] [Google Scholar]
- 78.Unsal V, Cicek M, Sabancilar İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environ Health. 2021;36(2):279–295. doi: 10.1515/reveh-2020-0048. [DOI] [PubMed] [Google Scholar]
- 79.Sodhi CP, et al. Study of oxidative stress in isoniazid-induced hepatic injury in young rats with and without protein-energy malnutrition. J Biochem Toxicol. 1996;11(3):139–146. doi: 10.1002/(SICI)1522-7146(1996)11:3<139::AID-JBT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 80.Song BJ, et al. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. 2011;74(12):2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh SK, et al. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4(11):3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 82.Adelusi OB, et al. The role of iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice. Toxicol Appl Pharmacol. 2022;445:116043. doi: 10.1016/j.taap.2022.116043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moon KH, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44(5):1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 84.Wang F, et al. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem Biol Interact. 2017;275:171–177. doi: 10.1016/j.cbi.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89–98. doi: 10.1016/j.redox.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho YE, et al. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J Hepatol. 2018;69(1):142–153. doi: 10.1016/j.jhep.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forsyth CB, Voigt RM, Keshavarzian A. Intestinal CYP2E1: a mediator of alcohol-induced gut leakiness. Redox Biol. 2014;3:40–46. doi: 10.1016/j.redox.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seitz HK, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 89.Keshavarzian A, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50(3):538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cloonan SM, et al. Mitochondrial dysfunction in lung ageing and disease. Eur Respir Rev. 2020;29(157):200165. doi: 10.1183/16000617.0165-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webb M, Sideris DP, Biddle M. Modulation of mitochondrial dysfunction for treatment of disease. Bioorg Med Chem Lett. 2019;29(11):1270–1277. doi: 10.1016/j.bmcl.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 92.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saleh J, et al. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siggins RW, et al. Mitochondrial dysfunction: at the nexus between alcohol-associated immunometabolic dysregulation and tissue injury. Int J Mol Sci. 2023;24(10):8650. doi: 10.3390/ijms24108650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shum M, et al. Mitochondrial oxidative function in NAFLD: friend or foe? Mol Metab. 2021;50:101134. doi: 10.1016/j.molmet.2020.101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Modanloo M, Shokrzadeh M. Analyzing mitochondrial dysfunction, oxidative stress, and apoptosis: potential role of l-carnitine. Iran J Kidney Dis. 2019;13(2):74–86. [PubMed] [Google Scholar]
- 97.Marí M, et al. Mitochondrial glutathione: recent insights and role in disease. Antioxidants (Basel) 2020;9(10):909. doi: 10.3390/antiox9100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rius-Pérez S, et al. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020;2020:1452696. doi: 10.1155/2020/1452696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21(13):4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fan P, et al. Molecular regulation mechanisms and interactions between reactive oxygen species and mitophagy. DNA Cell Biol. 2019;38(1):10–22. doi: 10.1089/dna.2018.4348. [DOI] [PubMed] [Google Scholar]
- 102.Ahadpour M, et al. Mitochondrial oxidative stress and dysfunction induced by isoniazid: study on isolated rat liver and brain mitochondria. Drug Chem Toxicol. 2016;39(2):224–232. doi: 10.3109/01480545.2015.1092039. [DOI] [PubMed] [Google Scholar]
- 103.Song BJ, et al. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol. 2010;11(5):434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang T, et al. Role of hepatitis C virus core protein in viral-induced mitochondrial dysfunction. J Viral Hepat. 2010;17(11):784–793. doi: 10.1111/j.1365-2893.2009.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizwan H, et al. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020;241:117148. doi: 10.1016/j.lfs.2019.117148. [DOI] [PubMed] [Google Scholar]
- 106.Dombi E, Mortiboys H, Poulton J. Modulating mitophagy in mitochondrial disease. Curr Med Chem. 2018;25(40):5597–5612. doi: 10.2174/0929867324666170616101741. [DOI] [PubMed] [Google Scholar]
- 107.Protasoni M, Zeviani M. Mitochondrial structure and bioenergetics in normal and disease conditions. Int J Mol Sci. 2021;22(2):586. doi: 10.3390/ijms22020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Y, et al. Regulation of SIRT1 and its roles in inflammation. Front Immunol. 2022;13:831168. doi: 10.3389/fimmu.2022.831168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagappan A, et al. Cryptotanshinone from the Salvia miltiorrhiza Bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways. Int J Mol Sci. 2019;21(1):265. doi: 10.3390/ijms21010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moles A, et al. Mitochondrial-lysosomal axis in acetaminophen hepatotoxicity. Front Pharmacol. 2018;9:453. doi: 10.3389/fphar.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Natarajan V, et al. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics. 2020;20(5–6):e1800404. doi: 10.1002/pmic.201800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nesci S, Lenaz G. Impaired mitochondrial bioenergetics under pathological conditions. Life (Basel) 2022;12(2):205. doi: 10.3390/life12020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998;1366(1–2):53–67. doi: 10.1016/S0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 114.Junli Z, et al. The role and mechanism of CREBH regulating SIRT3 in metabolic associated fatty liver disease. Life Sci. 2022;306:120838. doi: 10.1016/j.lfs.2022.120838. [DOI] [PubMed] [Google Scholar]
- 115.Li R, et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun R, et al. Sirtuin 3-mediated deacetylation of acyl-CoA synthetase family member 3 by protocatechuic acid attenuates non-alcoholic fatty liver disease. Br J Pharmacol. 2020;177(18):4166–4180. doi: 10.1111/bph.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu T, et al. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann Clin Lab Sci. 2014;44(4):410–418. [PubMed] [Google Scholar]
- 118.Kundu A, et al. EX-527 prevents the progression of high-fat diet-induced hepatic steatosis and fibrosis by upregulating SIRT4 in Zucker rats. Cells. 2020;9(5):1101. doi: 10.3390/cells9051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, et al. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Exp Clin Cancer Res. 2019;38(1):469. doi: 10.1186/s13046-019-1456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang YS, et al. Sirtuin 4 depletion promotes hepatocellular carcinoma tumorigenesis through regulating adenosine-monophosphate-activated protein kinase alpha/mammalian target of rapamycin axis in mice. Hepatology. 2019;69(4):1614–1631. doi: 10.1002/hep.30421. [DOI] [PubMed] [Google Scholar]
- 121.Yin X, et al. Targeting glutamine metabolism in hepatic stellate cells alleviates liver fibrosis. Cell Death Dis. 2022;13(11):955. doi: 10.1038/s41419-022-05409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo D, et al. Vimentin acetylation is involved in SIRT5-mediated hepatocellular carcinoma migration. Am J Cancer Res. 2018;8(12):2453–2466. [PMC free article] [PubMed] [Google Scholar]
- 123.van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med. 2017;23(4):320–331. doi: 10.1016/j.molmed.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lieber CS, et al. Alcohol alters hepatic FoxO1, p53, and mitochondrial SIRT5 deacetylation function. Biochem Biophys Res Commun. 2008;373(2):246–252. doi: 10.1016/j.bbrc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 125.You M, et al. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobil Surg Nutr. 2015;4(2):88–100. doi: 10.3978/j.issn.2304-3881.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choi Y, Abdelmegeed MA, Song BJ. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: role of gut–liver-adipose tissue axis. J Nutr Biochem. 2018;55:12–25. doi: 10.1016/j.jnutbio.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao P, Liu Y. Terpenoids: natural compounds for non-alcoholic fatty liver disease (NAFLD) therapy. Molecules. 2022;28(1):272. doi: 10.3390/molecules28010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Osborne B, et al. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med. 2016;100:164–174. doi: 10.1016/j.freeradbiomed.2016.04.197. [DOI] [PubMed] [Google Scholar]
- 129.Goikoetxea-Usandizaga N et al (2023) The outcome of boosting mitochondrial activity in alcohol-associated liver disease is organ-dependent. Hepatology 78(3):878-895 [DOI] [PMC free article] [PubMed]
- 130.Lee SE, et al. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. J Pineal Res. 2020;68(3):e12638. doi: 10.1111/jpi.12638. [DOI] [PubMed] [Google Scholar]
- 131.Oliva J, et al. Sirt1 is involved in energy metabolism: the role of chronic ethanol feeding and resveratrol. Exp Mol Pathol. 2008;85(3):155–159. doi: 10.1016/j.yexmp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu J, et al. The combination of blueberry juice and probiotics reduces apoptosis of alcoholic fatty liver of mice by affecting SIRT1 pathway. Drug Des Dev Ther. 2016;10:1649–1661. doi: 10.2147/DDDT.S102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017;13(7):852–867. doi: 10.7150/ijbs.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Westerbacka J, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56(11):2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 135.Zhao P, et al. An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 2020;367(6478):652–660. doi: 10.1126/science.aay0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang R, et al. Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation and fibrosis. Phytomedicine. 2021;92:153739. doi: 10.1016/j.phymed.2021.153739. [DOI] [PubMed] [Google Scholar]
- 137.Xiao Q, et al. Orientin reverses acetaminophen-induced acute liver failure by inhibiting oxidative stress and mitochondrial dysfunction. J Pharmacol Sci. 2022;149(1):11–19. doi: 10.1016/j.jphs.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 138.Li W, et al. NAD supplement alleviates intestinal barrier injury induced by ethanol via protecting epithelial mitochondrial function. Nutrients. 2022;15(1):174. doi: 10.3390/nu15010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang H, Park YK, Lee JY. Nicotinamide riboside, an NAD(+) precursor, attenuates inflammation and oxidative stress by activating sirtuin 1 in alcohol-stimulated macrophages. Lab Invest. 2021;101(9):1225–1237. doi: 10.1038/s41374-021-00599-1. [DOI] [PubMed] [Google Scholar]
- 140.Ghorbanpour A, et al. Capsaicin protects against septic acute liver injury by attenuation of apoptosis and mitochondrial dysfunction. Heliyon. 2023;9(3):e14205. doi: 10.1016/j.heliyon.2023.e14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yan M, et al. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–283. doi: 10.1016/j.redox.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katarey D, Verma S. Drug-induced liver injury. Clin Med (Lond) 2016;16(Suppl 6):s104–s109. doi: 10.7861/clinmedicine.16-6-s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tranah TH, et al. Targeting the gut–liver-immune axis to treat cirrhosis. Gut. 2021;70(5):982–994. doi: 10.1136/gutjnl-2020-320786. [DOI] [PubMed] [Google Scholar]
- 144.Kalyan M, et al. Role of endogenous lipopolysaccharides in neurological disorders. Cells. 2022;11(24):4038. doi: 10.3390/cells11244038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Konturek PC et al (2018) Gut–liver axis: how do gut bacteria influence the liver? Med Sci (Basel) 6(3):79 [DOI] [PMC free article] [PubMed]
- 146.Chávez-Talavera O, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679–1694.e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 147.Tripathi A, et al. The gut–liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Y, et al. Within-host evolution of a gut pathobiont facilitates liver translocation. Nature. 2022;607(7919):563–570. doi: 10.1038/s41586-022-04949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sandler NG, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141(4):1220–1230. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ji Y, et al. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD) Nutrients. 2019;11(8):1712. doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Borrelli A, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Albillos A, de Gottardi A, Rescigno M. The gut–liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 153.Fukui H. Leaky gut and gut–liver axis in liver cirrhosis: clinical studies update. Gut Liver. 2021;15(5):666–676. doi: 10.5009/gnl20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cassard AM, Ciocan D. Microbiota, a key player in alcoholic liver disease. Clin Mol Hepatol. 2018;24(2):100–107. doi: 10.3350/cmh.2017.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dubinkina VB, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5(1):141. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 157.Bajaj JS, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H, et al. Identification reproducible microbiota biomarkers for the diagnosis of cirrhosis and hepatocellular carcinoma. AMB Express. 2023;13(1):35. doi: 10.1186/s13568-023-01539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qin N, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 160.Zhu L, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 161.Calabrese FM, et al. A low glycemic index mediterranean diet combined with aerobic physical activity rearranges the gut microbiota signature in NAFLD patients. Nutrients. 2022;14(9):1773. doi: 10.3390/nu14091773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang J, et al. Gut microbial dysbiosis is associated with altered hepatic functions and serum metabolites in chronic hepatitis B patients. Front Microbiol. 2017;8:2222. doi: 10.3389/fmicb.2017.02222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang R, et al. The immunologic role of gut microbiota in patients with chronic HBV infection. J Immunol Res. 2018;2018:2361963. doi: 10.1155/2018/2361963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kang Y, Cai Y. Gut microbiota and hepatitis-B-virus-induced chronic liver disease: implications for faecal microbiota transplantation therapy. J Hosp Infect. 2017;96(4):342–348. doi: 10.1016/j.jhin.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 165.Ren YD, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65(5):1765–1768. doi: 10.1002/hep.29008. [DOI] [PubMed] [Google Scholar]
- 166.Aly AM, et al. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016;8(1):42. doi: 10.1186/s13099-016-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Preveden T, et al. Gut microbiota changes and chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2017;11(9):813–819. doi: 10.1080/17474124.2017.1343663. [DOI] [PubMed] [Google Scholar]
- 168.Heidrich B, et al. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int. 2018;38(1):50–58. doi: 10.1111/liv.13485. [DOI] [PubMed] [Google Scholar]
- 169.Grąt M, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant Proc. 2016;48(5):1687–1691. doi: 10.1016/j.transproceed.2016.01.077. [DOI] [PubMed] [Google Scholar]
- 170.Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–170. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.(2012) Structure, function and diversity of the healthy human microbiome. Nature. 486(7402):207–214 [DOI] [PMC free article] [PubMed]
- 172.Wang HM, et al. Inhibition of LPS-induced oxidative damages and potential anti-inflammatory effects of phyllanthus emblica extract via down-regulating NF-κB, COX-2, and iNOS in RAW 264.7 cells. Antioxidants (Basel) 2019;8(8):270. doi: 10.3390/antiox8080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim DH, et al. Ellagic Acid prevents binge alcohol-induced leaky gut and liver injury through inhibiting gut dysbiosis and oxidative stress. Antioxidants (Basel) 2021;10(9):1386. doi: 10.3390/antiox10091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Teunis C, Nieuwdorp M, Hanssen N. Interactions between tryptophan metabolism, the gut microbiome and the immune system as potential drivers of non-alcoholic fatty liver disease (NAFLD) and metabolic diseases. Metabolites. 2022;12(6):514. doi: 10.3390/metabo12060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sarkar S, et al. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):8742. doi: 10.1038/s41598-019-45009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peiseler M et al (2022) Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease—novel insights into cellular communication circuits. J Hepatol [DOI] [PubMed]
- 177.Delli Bovi AP, et al. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front Med (Lausanne) 2021;8:595371. doi: 10.3389/fmed.2021.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zong H, et al. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2012;302(5):E532–E539. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 180.Engstler AJ, et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65(9):1564–1571. doi: 10.1136/gutjnl-2014-308379. [DOI] [PubMed] [Google Scholar]
- 181.Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72(4):785–801. doi: 10.1016/j.jhep.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 182.Adams LA, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 183.Drożdż K, et al. Metabolic-associated fatty liver disease (MAFLD), diabetes, and cardiovascular disease: associations with fructose metabolism and gut microbiota. Nutrients. 2021;14(1):103. doi: 10.3390/nu14010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mandal C, et al. In utero alcohol exposure and the alteration of histone marks in the developing fetus: an epigenetic phenomenon of maternal drinking. Int J Biol Sci. 2017;13(9):1100–1108. doi: 10.7150/ijbs.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- 186.Rungratanawanich W, et al. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp Mol Med. 2021;53(2):168–188. doi: 10.1038/s12276-021-00561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16(4):667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paquot N. The metabolism of alcohol. Rev Med Liege. 2019;74(5–6):265–267. [PubMed] [Google Scholar]
- 189.Harjumäki R, Pridgeon CS, Ingelman-Sundberg M. CYP2E1 in alcoholic and non-alcoholic liver injury. Roles of ROS, reactive intermediates and lipid overload. Int J Mol Sci. 2021;22(15):8221. doi: 10.3390/ijms22158221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Namachivayam A, Valsala Gopalakrishnan A. A review on molecular mechanism of alcoholic liver disease. Life Sci. 2021;274:119328. doi: 10.1016/j.lfs.2021.119328. [DOI] [PubMed] [Google Scholar]
- 191.Chen J, et al. A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab Rev. 2019;51(2):178–195. doi: 10.1080/03602532.2019.1632889. [DOI] [PubMed] [Google Scholar]
- 192.Seitz HK. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem Biol Interact. 2020;316:108918. doi: 10.1016/j.cbi.2019.108918. [DOI] [PubMed] [Google Scholar]
- 193.Joshi-Barve S, et al. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cell Mol Gastroenterol Hepatol. 2015;1(4):356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bradford BU, et al. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41(2):336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 195.Osna NA, Donohue TM, Jr, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 196.Lieber CS. Alcohol and the liver: metabolism of alcohol and its role in hepatic and extrahepatic diseases. Mt Sinai J Med. 2000;67(1):84–94. [PubMed] [Google Scholar]
- 197.Moon KH, Lee YM, Song BJ. Inhibition of hepatic mitochondrial aldehyde dehydrogenase by carbon tetrachloride through JNK-mediated phosphorylation. Free Radic Biol Med. 2010;48(3):391–398. doi: 10.1016/j.freeradbiomed.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Moon KH, et al. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8(18):3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doorn JA, Hurley TD, Petersen DR. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem Res Toxicol. 2006;19(1):102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 200.Chaudhry KK, et al. ALDH2 deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol Clin Exp Res. 2015;39(8):1465–1475. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim YD, et al. Expression levels of hepatic cytochrome P450 enzymes in Aldh2-deficient mice following ethanol exposure: a pilot study. Arch Toxicol. 2005;79(4):192–195. doi: 10.1007/s00204-004-0630-8. [DOI] [PubMed] [Google Scholar]
- 202.Roberts BJ, et al. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun. 1994;205(2):1064–1071. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- 203.Cederbaum AI, et al. CYP2E1 sensitizes the liver to LPS- and TNF α-induced toxicity via elevated oxidative and nitrosative stress and activation of ASK-1 and JNK mitogen-activated kinases. Int J Hepatol. 2012;2012:582790. doi: 10.1155/2012/582790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cederbaum AI. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis. 2010;28(6):802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lu Y, et al. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 206.Butura A, et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J Hepatol. 2009;50(3):572–583. doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 207.Aleynik MK, et al. Polyenylphosphatidylcholine opposes the increase of cytochrome P-4502E1 by ethanol and corrects its iron-induced decrease. Alcohol Clin Exp Res. 1999;23(1):96–100. doi: 10.1111/j.1530-0277.1999.tb04028.x. [DOI] [PubMed] [Google Scholar]
- 208.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36(1):122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 209.Kathirvel E, et al. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21(9):973–983. doi: 10.1097/MEG.0b013e328328f461. [DOI] [PubMed] [Google Scholar]
- 210.Kathirvel E, et al. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J Gastroenterol Hepatol. 2010;25(6):1136–1143. doi: 10.1111/j.1440-1746.2009.06196.x. [DOI] [PubMed] [Google Scholar]
- 211.Song BJ, et al. Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J Hepatol. 2008;49(2):262–273. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hohmann N, et al. Effect of clomethiazole vs. clorazepate on hepatic fat and serum transaminase activities in alcohol-associated liver disease: results from a randomized, controlled phase II clinical trial. Alcohol Alcohol. 2023;58(2):134–141. doi: 10.1093/alcalc/agac068. [DOI] [PubMed] [Google Scholar]
- 213.Lu Y, Cederbaum AI. Cytochrome P450s and alcoholic liver disease. Curr Pharm Des. 2018;24(14):1502–1517. doi: 10.2174/1381612824666180410091511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33(2):191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gopal T, et al. A review of the role of ethanol-induced adipose tissue dysfunction in alcohol-associated liver disease. Alcohol Clin Exp Res. 2021;45(10):1927–1939. doi: 10.1111/acer.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Naim A, Pan Q, Baig MS. Matrix metalloproteinases (MMPs) in liver diseases. J Clin Exp Hepatol. 2017;7(4):367–372. doi: 10.1016/j.jceh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Born LJ, et al. Effects of ethanol administration on components of the ubiquitin proteolytic pathway in rat liver. Hepatology. 1996;23(6):1556–1563. doi: 10.1002/hep.510230636. [DOI] [PubMed] [Google Scholar]
- 218.Ge X, et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology. 2018;68(6):2380–2404. doi: 10.1002/hep.30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Puri P, et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology. 2018;67(4):1284–1302. doi: 10.1002/hep.29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tang W, et al. Role of Nrf2 in chronic liver disease. World J Gastroenterol. 2014;20(36):13079–13087. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 222.Ludwig J, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 223.Lu Z, et al. Amitriptyline inhibits nonalcoholic steatohepatitis and atherosclerosis induced by high-fat diet and LPS through modulation of sphingolipid metabolism. Am J Physiol Endocrinol Metab. 2020;318(2):E131–e144. doi: 10.1152/ajpendo.00181.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mazzolini G, et al. Significance of simple steatosis: an update on the clinical and molecular evidence. Cells. 2020;9(11):2458. doi: 10.3390/cells9112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Syn WK, et al. Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis. 2009;29(2):200–210. doi: 10.1055/s-0029-1214375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Joshi-Barve S, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46(3):823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 227.Malhi H, et al. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 228.Dallio M, et al. Immunity as cornerstone of non-alcoholic fatty liver disease: the contribution of oxidative stress in the disease progression. Int J Mol Sci. 2021;22(1):436. doi: 10.3390/ijms22010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Syn WK. Repair-associated inflammation in nonalcoholic fatty liver disease. Clin Med (Lond) 2013;13(Suppl 6):s15–s19. doi: 10.7861/clinmedicine.13-6-s15. [DOI] [PubMed] [Google Scholar]
- 230.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 231.Karkucinska-Wieckowska A, et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: a complex relationship. Eur J Clin Invest. 2022;52(3):e13622. doi: 10.1111/eci.13622. [DOI] [PubMed] [Google Scholar]
- 232.Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD)—pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49(2):197–211. doi: 10.1080/03602532.2017.1293683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 234.Semmler G, et al. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int. 2021;41(10):2249–2268. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76(1):99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ma T, et al. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J Pharm Pharmacol. 2011;63(4):587–593. doi: 10.1111/j.2042-7158.2011.01256.x. [DOI] [PubMed] [Google Scholar]
- 237.Di Ciaula A, et al. Nonalcoholic fatty liver disease (NAFLD). Mitochondria as players and targets of therapies? Int J Mol Sci. 2021;22(10):5375. doi: 10.3390/ijms22105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jaeschke H, et al. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 239.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281(30):21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 240.Rauchbach E, et al. Cholesterol induces oxidative stress, mitochondrial damage and death in hepatic stellate cells to mitigate liver fibrosis in mice model of NASH. Antioxidants (Basel) 2022;11(3):536. doi: 10.3390/antiox11030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xie J et al (2022) The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology [DOI] [PubMed]
- 242.Marušić M, et al. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827. doi: 10.1155/2021/6613827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nelson JE, Klintworth H, Kowdley KV. Iron metabolism in nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2012;14(1):8–16. doi: 10.1007/s11894-011-0234-4. [DOI] [PubMed] [Google Scholar]
- 244.Abdelmegeed MA, et al. Cytochrome P450-2E1 promotes fast food-mediated hepatic fibrosis. Sci Rep. 2017;7:39764. doi: 10.1038/srep39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119(5):1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 246.Harte AL, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Damms-Machado A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105(1):127–135. doi: 10.3945/ajcn.116.131110. [DOI] [PubMed] [Google Scholar]
- 249.Zhu L, et al. Gut microbiota produce alcohol and contribute to NAFLD. Gut. 2016;65(7):1232. doi: 10.1136/gutjnl-2016-311571. [DOI] [PubMed] [Google Scholar]
- 250.Dela Peña A, et al. NADPH oxidase is not an essential mediator of oxidative stress or liver injury in murine MCD diet-induced steatohepatitis. J Hepatol. 2007;46(2):304–313. doi: 10.1016/j.jhep.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 251.Davis NC. The influence of diet upon the liver injury produced by carbon tetrachloride. J Med Res. 1924;44(5):601–614.3. [PMC free article] [PubMed] [Google Scholar]
- 252.Bosma A, et al. Synergism between ethanol and carbon tetrachloride in the generation of liver fibrosis. J Pathol. 1988;156(1):15–21. doi: 10.1002/path.1711560106. [DOI] [PubMed] [Google Scholar]
- 253.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153(1):109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 254.Alkreathy HM, Esmat A. lycorine ameliorates thioacetamide-induced hepatic fibrosis in rats: emphasis on antioxidant, anti-inflammatory, and STAT3 inhibition effects. Pharmaceuticals (Basel) 2022;15(3):369. doi: 10.3390/ph15030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Enciso N, et al. Model of liver fibrosis induction by thioacetamide in rats for regenerative therapy studies. Anal Cell Pathol (Amst) 2022;2022:2841894. doi: 10.1155/2022/2841894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ghosh S, et al. Silymarin protects mouse liver and kidney from thioacetamide induced toxicity by scavenging reactive oxygen species and activating PI3K-Akt pathway. Front Pharmacol. 2016;7:481. doi: 10.3389/fphar.2016.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schyman P, et al. Concordance between thioacetamide-induced liver injury in rat and human in vitro gene expression data. Int J Mol Sci. 2020;21(11):4017. doi: 10.3390/ijms21114017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bashandy SAE, et al. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can J Physiol Pharmacol. 2018;96(4):337–344. doi: 10.1139/cjpp-2017-0247. [DOI] [PubMed] [Google Scholar]
- 259.Kang JS, et al. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol Appl Pharmacol. 2008;228(3):295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 260.Zhao Q, et al. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat Commun. 2023;14(1):1829. doi: 10.1038/s41467-023-37459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhao Q, et al. Celastrol protects from cholestatic liver injury through modulation of SIRT1-FXR signaling. Mol Cell Proteomics. 2019;18(3):520–533. doi: 10.1074/mcp.RA118.000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mohi-Ud-Din R, et al. Possible pathways of hepatotoxicity caused by chemical agents. Curr Drug Metab. 2019;20(11):867–879. doi: 10.2174/1389200220666191105121653. [DOI] [PubMed] [Google Scholar]
- 263.McGill MR, Jaeschke H. Biomarkers of drug-induced liver injury. Adv Pharmacol. 2019;85:221–239. doi: 10.1016/bs.apha.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Suk KT, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380–1387. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 265.Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63(2):590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 266.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761–794. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ramachandran A, Jaeschke H. Oxidant stress and acetaminophen hepatotoxicity: mechanism-based drug development. Antioxid Redox Signal. 2021;35(9):718–733. doi: 10.1089/ars.2021.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ramachandran A, Jaeschke H. Acetaminophen toxicity: novel insights into mechanisms and future perspectives. Gene Expr. 2018;18(1):19–30. doi: 10.3727/105221617X15084371374138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis. 2019;39(2):221–234. doi: 10.1055/s-0039-1679919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–156. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ye H, et al. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol. 2018;24(13):1373–1385. doi: 10.3748/wjg.v24.i13.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee WM. Acetaminophen toxicity: a history of serendipity and unintended consequences. Clin Liver Dis (Hoboken) 2020;16(Suppl 1):34–44. doi: 10.1002/cld.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Matthews AM, et al. Comparison of covalent binding of acetaminophen and the regioisomer 3′-hydroxyacetanilide to mouse liver protein. Toxicol Lett. 1997;90(1):77–82. doi: 10.1016/S0378-4274(96)03831-3. [DOI] [PubMed] [Google Scholar]
- 274.Michael SL, et al. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30(1):186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 275.Win S, et al. Hepatic mitochondrial SAB deletion or knockdown alleviates diet-induced metabolic syndrome, steatohepatitis, and hepatic fibrosis. Hepatology. 2021;74(6):3127–3145. doi: 10.1002/hep.32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity: a mitochondrial perspective. Adv Pharmacol. 2019;85:195–219. doi: 10.1016/bs.apha.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gunawan BK, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 278.Kaplowitz N, et al. How to protect against acetaminophen: don't ask for JUNK. Gastroenterology. 2008;135(4):1047–1051. doi: 10.1053/j.gastro.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 279.Hanawa N, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.He X, et al. Mitoquinone protects against acetaminophen-induced liver injury in an FSP1-dependent and GPX4-independent manner. Toxicol Appl Pharmacol. 2023;465:116452. doi: 10.1016/j.taap.2023.116452. [DOI] [PubMed] [Google Scholar]
- 281.Begriche K, et al. Acetaminophen-induced hepatotoxicity in obesity and nonalcoholic fatty liver disease: a critical review. Livers. 2023;3(1):33–53. doi: 10.3390/livers3010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McClain CJ, et al. Potentiation of acetaminophen hepatotoxicity by alcohol. JAMA. 1980;244(3):251–253. doi: 10.1001/jama.1980.03310030027020. [DOI] [PubMed] [Google Scholar]
- 283.Lee WM, Kaplowitz N. Alcohol, fasting, and therapeutic dosing of acetaminophen: a perfect storm. Hepatology. 2021;73(5):1634–1636. doi: 10.1002/hep.31747. [DOI] [PubMed] [Google Scholar]
- 284.Kumar S, et al. Hepatic, extrahepatic and extracellular vesicle cytochrome P450 2E1 in alcohol and acetaminophen-mediated adverse interactions and potential treatment options. Cells. 2022;11(17):2620. doi: 10.3390/cells11172620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49(4):291–301. doi: 10.1046/j.1365-2125.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Seeff LB, et al. Acetaminophen hepatotoxicity in alcoholics. A therapeutic misadventure. Ann Intern Med. 1986;104(3):399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- 287.Schiødt FV, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337(16):1112–1117. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 288.Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA. 1994;272(23):1845–1850. doi: 10.1001/jama.1994.03520230055038. [DOI] [PubMed] [Google Scholar]
- 289.Louvet A, et al. Acute liver injury with therapeutic doses of acetaminophen: a prospective study. Hepatology. 2021;73(5):1945–1955. doi: 10.1002/hep.31678. [DOI] [PubMed] [Google Scholar]
- 290.Pateria P, de Boer B, MacQuillan G. Liver abnormalities in drug and substance abusers. Best Pract Res Clin Gastroenterol. 2013;27(4):577–596. doi: 10.1016/j.bpg.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 291.Valente MJ, et al. Contribution of oxidative metabolism to cocaine-induced liver and kidney damage. Curr Med Chem. 2012;19(33):5601–5606. doi: 10.2174/092986712803988938. [DOI] [PubMed] [Google Scholar]
- 292.Labib R, Turkall R, Abdel-Rahman MS. Endotoxin potentiates cocaine-mediated hepatotoxicity by nitric oxide and reactive oxygen species. Int J Toxicol. 2003;22(4):305–316. doi: 10.1080/10915810305117. [DOI] [PubMed] [Google Scholar]
- 293.Dean RA, et al. Effects of ethanol on cocaine metabolism: formation of cocaethylene and norcocaethylene. Toxicol Appl Pharmacol. 1992;117(1):1–8. doi: 10.1016/0041-008X(92)90210-J. [DOI] [PubMed] [Google Scholar]
- 294.Upreti VV, et al. Drug interaction between ethanol and 3,4-methylenedioxymethamphetamine ("ecstasy") Toxicol Lett. 2009;188(2):167–172. doi: 10.1016/j.toxlet.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mai HN, et al. Genetic depletion of p53 attenuates cocaine-induced hepatotoxicity in mice. Biochimie. 2019;158:53–61. doi: 10.1016/j.biochi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 296.Carvalho M, et al. Mechanisms underlying the hepatotoxic effects of ecstasy. Curr Pharm Biotechnol. 2010;11(5):476–495. doi: 10.2174/138920110791591535. [DOI] [PubMed] [Google Scholar]
- 297.Cajanding RJM. MDMA-associated liver toxicity: pathophysiology, management, and current state of knowledge. AACN Adv Crit Care. 2019;30(3):232–248. doi: 10.4037/aacnacc2019852. [DOI] [PubMed] [Google Scholar]
- 298.Mudd TW, Guddati AK. Management of hepatotoxicity of chemotherapy and targeted agents. Am J Cancer Res. 2021;11(7):3461–3474. [PMC free article] [PubMed] [Google Scholar]
- 299.Abd Rashid N, et al. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed Pharmacother. 2021;144:112328. doi: 10.1016/j.biopha.2021.112328. [DOI] [PubMed] [Google Scholar]
- 300.Quintanilha JCF, et al. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol. 2017;80(2):223–233. doi: 10.1007/s00280-017-3358-x. [DOI] [PubMed] [Google Scholar]
- 301.Gong S, et al. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J Transl Med. 2021;19(1):147. doi: 10.1186/s12967-021-02814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fang CY, et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 2021;42(12):1951–1969. doi: 10.1038/s41401-021-00620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. 2006;89(2):515–523. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- 304.Eltamany EE, et al. The antioxidant Carrichtera annua DC. Ethanolic extract counteracts cisplatin triggered hepatic and renal toxicities. Antioxidants (Basel) 2021;10(6):825. doi: 10.3390/antiox10060825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sun T, et al. Melatonin attenuates cisplatin-induced acute kidney injury in mice: involvement of PPARα and fatty acid oxidation. Food Chem Toxicol. 2022;163:112970. doi: 10.1016/j.fct.2022.112970. [DOI] [PubMed] [Google Scholar]
- 306.Hwang DB, et al. Ccrn4l as a pre-dose marker for prediction of cisplatin-induced hepatotoxicity susceptibility. Free Radic Biol Med. 2020;148:128–139. doi: 10.1016/j.freeradbiomed.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 307.Eid BG, El-Shitany NA. Captopril downregulates expression of Bax/cytochrome C/caspase-3 apoptotic pathway, reduces inflammation, and oxidative stress in cisplatin-induced acute hepatic injury. Biomed Pharmacother. 2021;139:111670. doi: 10.1016/j.biopha.2021.111670. [DOI] [PubMed] [Google Scholar]
- 308.Paech F, et al. Sunitinib induces hepatocyte mitochondrial damage and apoptosis in mice. Toxicology. 2018;409:13–23. doi: 10.1016/j.tox.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 309.Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3(1):37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Boelsterli UA, Lee KK. Mechanisms of isoniazid-induced idiosyncratic liver injury: emerging role of mitochondrial stress. J Gastroenterol Hepatol. 2014;29(4):678–687. doi: 10.1111/jgh.12516. [DOI] [PubMed] [Google Scholar]
- 311.Ezhilarasan D (2023) Antitubercular drugs induced liver injury: an updated insight into molecular mechanisms. Drug Metab Rev 1–15 [DOI] [PubMed]
- 312.Jia ZL, et al. Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere. 2019;227:541–550. doi: 10.1016/j.chemosphere.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 313.Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8(3):207–223. doi: 10.2174/1574886311308030011. [DOI] [PubMed] [Google Scholar]
- 314.Todorović Vukotić N, et al. Antidepressants- and antipsychotics-induced hepatotoxicity. Arch Toxicol. 2021;95(3):767–789. doi: 10.1007/s00204-020-02963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Carrier P, et al. Liver illness and psychiatric patients. Hepat Mon. 2016;16(12):e41564. doi: 10.5812/hepatmon.41564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Labenz C, et al. Nonalcoholic fatty liver disease increases the risk of anxiety and depression. Hepatol Commun. 2020;4(9):1293–1301. doi: 10.1002/hep4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sonali S, et al. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells. 2022;11(8):1362. doi: 10.3390/cells11081362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chidambaram SB, et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacol Ther. 2022;231:107988. doi: 10.1016/j.pharmthera.2021.107988. [DOI] [PubMed] [Google Scholar]
- 319.Bizerra PFV, et al. The harmful acute effects of clomipramine in the rat liver: impairments in mitochondrial bioenergetics. Toxicol Lett. 2023;383:1–16. doi: 10.1016/j.toxlet.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 320.Yadav D, et al. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97(10):2634–2639. doi: 10.1111/j.1572-0241.2002.06041.x. [DOI] [PubMed] [Google Scholar]
- 321.Ando M, et al. Mitochondrial electron transport inhibition in full genomic hepatitis C virus replicon cells is restored by reducing viral replication. Liver Int. 2008;28(8):1158–1166. doi: 10.1111/j.1478-3231.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 322.Otani K, et al. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128(1):96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 323.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24(1):19. doi: 10.1186/s12929-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Brooks PJ, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6(3):e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yao S, et al. ALDH2 is a prognostic biomarker and related with immune infiltrates in HCC. Am J Cancer Res. 2021;11(11):5319–5337. [PMC free article] [PubMed] [Google Scholar]
- 328.Park KS, et al. Proteomic alterations of the variants of human aldehyde dehydrogenase isozymes correlate with hepatocellular carcinoma. Int J Cancer. 2002;97(2):261–265. doi: 10.1002/ijc.1585. [DOI] [PubMed] [Google Scholar]
- 329.Cho YM, et al. Differential expression of the liver proteome in senescence accelerated mice. Proteomics. 2003;3(10):1883–1894. doi: 10.1002/pmic.200300562. [DOI] [PubMed] [Google Scholar]
- 330.Kim J, et al. Proteome analysis of human liver tumor tissue by two-dimensional gel electrophoresis and matrix assisted laser desorption/ionization-mass spectrometry for identification of disease-related proteins. Electrophoresis. 2002;23(24):4142–4156. doi: 10.1002/elps.200290032. [DOI] [PubMed] [Google Scholar]
- 331.Taniai M. Alcohol and hepatocarcinogenesis. Clin Mol Hepatol. 2020;26(4):736–741. doi: 10.3350/cmh.2020.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Song BJ, et al. Contributing roles of CYP2E1 and other cytochrome p450 isoforms in alcohol-related tissue injury and carcinogenesis. Adv Exp Med Biol. 2019;1164:73–87. doi: 10.1007/978-3-030-22254-3_6. [DOI] [PubMed] [Google Scholar]
- 333.Yokoyama A, et al. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998;19(8):1383–1387. doi: 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- 334.Stice CP, Xia H, Wang XD. Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development. Chronic Dis Transl Med. 2018;4(4):211–224. doi: 10.1016/j.cdtm.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hohmann N, et al. Clomethiazole inhibits cytochrome P450 2E1 and improves alcoholic liver disease. Gut. 2022;71(4):842–844. doi: 10.1136/gutjnl-2021-324727. [DOI] [PubMed] [Google Scholar]
- 336.Liu SY, Tsai IT, Hsu YC. Alcohol-related liver disease: basic mechanisms and clinical perspectives. Int J Mol Sci. 2021;22(10):5170. doi: 10.3390/ijms22105170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ponziani FR, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69(1):107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 338.Ayares G, et al. Current medical treatment for alcohol-associated liver disease. J Clin Exp Hepatol. 2022;12(5):1333–1348. doi: 10.1016/j.jceh.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Devarbhavi H et al (2023) Global burden of liver disease: 2023 update. J Hepatol [DOI] [PubMed]
- 340.Crabb DW, et al. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 341.Matteoni CA, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 342.Raynard B, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35(3):635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 343.Åberg F, et al. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67(6):2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 344.Hart CL, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chang Y, et al. Low levels of alcohol consumption, obesity, and development of fatty liver with and without evidence of advanced fibrosis. Hepatology. 2020;71(3):861–873. doi: 10.1002/hep.30867. [DOI] [PubMed] [Google Scholar]
- 346.Lewis JH. Digitizing DILI: who can? RUCAM? RECAM? Hepatology. 2022;76(1):3–5. doi: 10.1002/hep.32312. [DOI] [PubMed] [Google Scholar]
- 347.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362–376. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Vall A, et al. The promise of AI for DILI prediction. Front Artif Intell. 2021;4:638410. doi: 10.3389/frai.2021.638410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nassir F. NAFLD: mechanisms, treatments, and biomarkers. Biomolecules. 2022;12(6):824. doi: 10.3390/biom12060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kim D, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Singh S, Osna NA, Kharbanda KK. Treatment options for alcoholic and non-alcoholic fatty liver disease: a review. World J Gastroenterol. 2017;23(36):6549–6570. doi: 10.3748/wjg.v23.i36.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Szabo G, et al. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. 2022;76(4):1058–1068. doi: 10.1002/hep.32478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Higuera-de la Tijera F, et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol. 2015;21(16):4975–4985. doi: 10.3748/wjg.v21.i16.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Arab JP, et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: a worldwide study. J Hepatol. 2021;75(5):1026–1033. doi: 10.1016/j.jhep.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Vatsalya V, et al. Association of serum zinc with markers of liver injury in very heavy drinking alcohol-dependent patients. J Nutr Biochem. 2018;59:49–55. doi: 10.1016/j.jnutbio.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Liangpunsakul S, et al. Interaction between the patatin-like phospholipase domain-containing protein 3 genotype and coffee drinking and the risk for acute alcoholic hepatitis. Hepatol Commun. 2018;2(1):29–34. doi: 10.1002/hep4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Samala N, et al. Posttraumatic stress disorder in patients with heavy alcohol consumption and alcoholic hepatitis. Alcohol Clin Exp Res. 2018;42(10):1933–1938. doi: 10.1111/acer.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hernandez-Tejero M, Clemente-Sanchez A, Bataller R. Spectrum, screening, and diagnosis of alcohol-related liver disease. J Clin Exp Hepatol. 2023;13(1):75–87. doi: 10.1016/j.jceh.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ha Y, Jeong I, Kim TH. Alcohol-related liver disease: an overview on pathophysiology, diagnosis and therapeutic perspectives. Biomedicines. 2022;10(10):2530. doi: 10.3390/biomedicines10102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ramkissoon R, Shah VH. Alcohol use disorder and alcohol-associated liver disease. Alcohol Res. 2022;42(1):13. doi: 10.35946/arcr.v42.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Moreno C, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology. 2016;150(4):903–10.e8. doi: 10.1053/j.gastro.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 362.Loomba R, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25(5):1054–1062.e5. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yan T, et al. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm Sin B. 2020;10(1):3–18. doi: 10.1016/j.apsb.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients. 2018;10(9):1153. doi: 10.3390/nu10091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yang W, et al. Silymarin protects against acute liver injury induced by acetaminophen by downregulating the expression and activity of the CYP2E1 enzyme. Molecules. 2022;27(24):8855. doi: 10.3390/molecules27248855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Luo DD, et al. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem Toxicol. 2019;123:349–362. doi: 10.1016/j.fct.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 367.Barcelo S, et al. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis. 1996;17(2):277–282. doi: 10.1093/carcin/17.2.277. [DOI] [PubMed] [Google Scholar]
- 368.Cui Z, et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. doi: 10.3389/fimmu.2022.943321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dai H, et al. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol Ther. 2018;188:140–154. doi: 10.1016/j.pharmthera.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ramirez T, et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66(3):601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Li Y, et al. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine. 2013;43(2):376–386. doi: 10.1007/s12020-012-9761-5. [DOI] [PubMed] [Google Scholar]
- 372.Norman BH. Drug induced liver injury (DILI). Mechanisms and medicinal chemistry avoidance/mitigation strategies. J Med Chem. 2020;63(20):11397–11419. doi: 10.1021/acs.jmedchem.0c00524. [DOI] [PubMed] [Google Scholar]
- 373.Stine JG, Lewis JH. Current and future directions in the treatment and prevention of drug-induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol. 2016;10(4):517–536. doi: 10.1586/17474124.2016.1127756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Grattagliano I, et al. Targeting mitochondria to oppose the progression of nonalcoholic fatty liver disease. Biochem Pharmacol. 2019;160:34–45. doi: 10.1016/j.bcp.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 375.Esler WP, Bence KK. Metabolic targets in nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol. 2019;8(2):247–267. doi: 10.1016/j.jcmgh.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Leo S, Szabadkai G, Rizzuto R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann NY Acad Sci. 2008;1147:264–274. doi: 10.1196/annals.1427.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sulaimon LA et al (2021) Molecular mechanism of mitoquinol mesylate in mitigating the progression of hepatocellular carcinoma-in silico and in vivo studies. J Cell Biochem [DOI] [PubMed]
- 378.Virgana R, et al. MitoTEMPOL modulates mitophagy and histopathology of Wistar rat liver after streptozotocin injection. Iran J Basic Med Sci. 2022;25(11):1382–1388. doi: 10.22038/IJBMS.2022.65285.14375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Zapata J et al (2022) Targeting mitochondria for the prevention and treatment of nonalcoholic fatty liver disease: polyphenols as a non-pharmacological approach. Curr Med Chem 30(26):2977-2995 [DOI] [PubMed]
- 380.Zhao X, et al. Quercetin as a protective agent for liver diseases: a comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021;35(9):4727–4747. doi: 10.1002/ptr.7104. [DOI] [PubMed] [Google Scholar]
- 381.Wang K, et al. New insight and potential therapy for NAFLD: CYP2E1 and flavonoids. Biomed Pharmacother. 2021;137:111326. doi: 10.1016/j.biopha.2021.111326. [DOI] [PubMed] [Google Scholar]
- 382.Matsura T, et al. Mechanisms of protection by melatonin against acetaminophen-induced liver injury in mice. J Pineal Res. 2006;41(3):211–219. doi: 10.1111/j.1600-079X.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 383.Liang YL, et al. Melatonin protects against apoptosis-inducing factor (AIF)-dependent cell death during acetaminophen-induced acute liver failure. PLoS ONE. 2012;7(12):e51911. doi: 10.1371/journal.pone.0051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Madhu P, Reddy KP, Reddy PS. Melatonin reduces oxidative stress and restores mitochondrial function in the liver of rats exposed to chemotherapeutics. J Exp Zool A Ecol Genet Physiol. 2015;323(5):301–308. doi: 10.1002/jez.1917. [DOI] [PubMed] [Google Scholar]
- 385.Luo C, et al. The multiple protective roles and molecular mechanisms of melatonin and its precursor N-acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radic Biol Med. 2019;130:215–233. doi: 10.1016/j.freeradbiomed.2018.10.402. [DOI] [PubMed] [Google Scholar]
- 386.Kanda T, et al. Molecular mechanisms: connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int J Mol Sci. 2020;21(4):1525. doi: 10.3390/ijms21041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Chen B, et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature. 2022;610(7932):562–568. doi: 10.1038/s41586-022-05299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ritze Y, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS ONE. 2014;9(1):e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Grander C, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 390.Ballway JW, Song BJ. Translational approaches with antioxidant phytochemicals against alcohol-mediated oxidative stress, gut dysbiosis, intestinal barrier dysfunction, and fatty liver disease. Antioxidants (Basel) 2021;10(3):384. doi: 10.3390/antiox10030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Hao S, et al. Fecal microbiota transplantation research over the past decade: current status and trends. Can J Infect Dis Med Microbiol. 2023;2023:6981721. doi: 10.1155/2023/6981721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Fang W, et al. Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high-fat diet-induced obesity in mice. J Nutr. 2019;149(5):747–754. doi: 10.1093/jn/nxy324. [DOI] [PubMed] [Google Scholar]
- 394.Degirolamo C, et al. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7(1):12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 395.Chen ML, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7(2):e02210–e2215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Liu S, et al. Ligustrum robustum alleviates atherosclerosis by decreasing serum TMAO, modulating gut microbiota, and decreasing bile acid and cholesterol absorption in mice. Mol Nutr Food Res. 2021;65(14):e2100014. doi: 10.1002/mnfr.202100014. [DOI] [PubMed] [Google Scholar]
- 397.Sener G, Sehirli AO, Ayanoğlu-Dülger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res. 2003;35(1):61–68. doi: 10.1034/j.1600-079X.2003.00050.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.