Summary
Natural killer (NK) cells are cytotoxic innate lymphoid cells that participate in anti-tumour and anti-viral immune responses. Their ability to rapidly destroy abnormal cells and to enhance the anti-cancer function of dendritic cells, CD8+ T cells, and macrophages makes them an attractive target for immunotherapeutic strategies. The development of approaches that augment NK-cell activation against cancer is currently under intense preclinical and clinical research and strategies include chimeric antigen receptor NK cells, NK-cell engagers, cytokines, and immune checkpoint inhibitors. In this review, we highlight recent advances in NK-cell therapeutic development and discuss their potential to add to our armamentarium against cancer.
Keywords: natural killer cells (NK cells), chimeric antigen receptor (CAR), immunotherapy, CAR-NK cells, cancer, NK-cell engagers
Natural killers of cancer
Natural killer (NK) cells are innate lymphocytes that develop in the bone marrow from common lymphoid progenitor cells. NK-cell maturity and function are critically dependent upon the transcription factors T-BET and EOMES [1] and following maturation they express a diverse repertoire of germline-encoded, non-rearranged surface receptors [2, 3]. This diversity in the expression of activating and inhibitory receptors allows for the recognition of infected or tumour cells through complementary mechanisms in a combinatorial manner. As such, NK cells are fine-tuned to recognize changes in the balance of signals derived from those receptors. There are several different inhibitory receptors including NKG2A, inhibitory killer cell immunoglobulin-like receptors (KIR), TIGIT, and LILRB1. NKG2A binds human leukocyte antigen (HLA)-E, and upregulation of HLA-E on cancer cells can inhibit both CD8+ T cell and NK-cell function via NKG2A [4]. In addition, the diverse family of inhibitory killer cell immunoglobulin-like receptors (KIR) predominantly engage with HLA-A, -B, and -C alleles. Thus, the loss of HLA class I ligands for inhibitory receptors such as NKG2A or the inhibitory KIR proteins on cancerous cells leads to loss-of-self inhibitory signals and NK-cell activation [2]. The interaction of KIR and NKG2A with their ligands on healthy cells is also necessary for the production of functionally competent NK cells (termed NK-cell education). These ‘educated’ NK cells possess a superior ability to kill target cells than those that do not express a receptor for self-HLA class I. Interactions of NK cells with HLA class I determine global NK-cell function via fine tuning the quantity of releasable granzyme B which is crucial for cytotoxic activity [5]. Furthermore, the non-classical HLA class I molecules, HLA-F, and -G can also modulate NK-cell function. Upregulation of HLA-G in cancer can inhibit NK cells through engagement of LILRB1 and is associated with worse outcomes, suggesting potential as a target for therapeutic blockade [6–8]. In addition, HLA-F, which binds the activating receptor KIR3DS1 as an open conformer, may also be upregulated in cancer [9] and this may paradoxically be associated with worse survival [10–12].
There are many activating receptors expressed by NK cells, and some examples of relevance to cancer immunotherapy include NKp46, NKp30, DNAM-1 (CD226), 2B4 (CD244), activating KIR, NKG2D, NKG2C, and NKp44 [2, 3, 13, 14]. In cancer, the ligands for NKG2D and NKp46 are stress-induced ligands such as MICA/B, ULBPs, and ecto-calreticulin [3, 15]. Both the activating receptor NKG2C and the inhibitory receptor NKG2A bind to HLA-E. HLA-E can therefore have a dual role in both the promotion and inhibition of NK cell function. The effector functions of NKG2C have largely been demonstrated in the context of viral infections, however, they may also operate in cancer as NKG2C+ NK cells have recently demonstrated potent cytotoxicity against HLA-C:KIR mismatched leukemic cells [16]. Through their expression of CD16, NK cells mediate strong activation in response to target cells bound with IgG1 antibodies and this provides NK cells with the ability to respond to tumour targeting antibodies via antibody-dependent cellular cytotoxicity (ADCC) [17, 18].
Upon target cell recognition, NK cells can rapidly induce target cell lysis through the secretion of granzymes, granulysin, and perforin into the immunological synapse. Importantly, NK cells are protected from autolysis by densely packed lipid membranes [19]. Due to their expression of FASL and TRAIL, NK cells can also induce apoptosis of target cells through the engagement of death receptors. In addition to these direct cytotoxic actions, NK cells have an important role in shaping the multi-pronged anti-cancer immune response via the secretion of chemokines and cytokines. For example, NK cells recruit conventional type 1 dendritic cell (cDC1) into tumours due to their secretion of the chemokines XCL1, XCL2, and CCL5. These chemokines can also promote DC differentiation and DC survival via FLT3 ligand, and can promote CD8+ T-cell recruitment and activation at tumor sites [20–23]. Furthermore, NK-cell secretion of IFNγ can upregulate MHC class I expression which is critical for neoantigen recognition by CD8+ T cells. Finally, NK cells can promote antibody-dependent cellular phagocytosis of tumour cells by macrophages [21, 22, 24, 25].
The important role of NK cells in the anti-tumour response is well established in a multitude of murine and in vitro models, and in humans was illustrated by a landmark study showing that superior NK-cell function are associated with reduced cancer incidence [26]. Accordingly, enhanced NK function and infiltration is associated with improved prognosis in multiple solid and haematological malignancies (reviewed in [21, 27]). NK cells are less frequent in tumours than T cells [24], and NK-cell function can also be rapidly (within 48-72 h) suppressed following infiltration into tumours [28]. Thus in some solid organ cancers, CD56dim CD16high HSP40+ tumour-asssociated NK cells display a dysfunctional state with reduced perforin and granzyme B [29]. Therapeutic strategies must therefore overcome NK-cell exhaustion and augment NK-cell persistence, infiltration, and tumour cell recognition to have durable anti-cancer activity (reviewed in [30, 31]).
Although members of the innate immune system, recent work has highlighted that NK cells possess features of trained immunological memory [32]. This is relevant for NK cells generated through viral infection, contact hypersensitivity, and cytokine stimulation with IL-12, IL-15, and IL-18 to generate cytokine-induced memory-like (CIML) NK cells. These CIML NK cells have been shown to possess improved persistence, expansion, metabolic function, and activation against tumour cells both in vitro and in vivo in multiple solid and haematological cancer models [33–38]. Based on these promising pre-clinical data, CIML NK cells are now under assessment in early-stage clinical trials for patients with solid or hematological cancers (NCT05470140, NCT02782546, NCT04634435, NCT04290546, NCT04024761). Furthermore, genetic engineering strategies have shown promise in the recapitulation of the features of CMV-adapted NK cells. For example, induced pluripotent stem cell (iPSC)-derived NK cells expressing high affinity non-cleavable CD16, and membrane-bound IL-15/IL-15R in combination with silenced CD38 expression have demonstrated enhanced persistence and anti-tumour functions [39].
NK-cell therapy—an historical perspective
The observation that NK cells have cytolytic and anti-tumour activity in the mouse provided a rationale for using NK cells as immunotherapeutic agents. Studies in humans demonstrating that NK cells have intrinsic anti-tumour activities have been more challenging as genetic defects solely affecting the NK-cell lineage are relatively rare. Nevertheless, in a prospective longitudinal study of over 3000 individuals it was observed that those with low NK-cell cytotoxic activity have a higher incidence of cancer development [26]. Initial human immunotherapy studies were conducted by Rosenberg et al. using IL-2-activated peripheral blood mononuclear cells (PBMCs) to generate lymphokine-activated killer cells (LAKs). This cell preparation contains a heterogenous effector cell population of NK cells and T cells. Results from this study showed encouraging responses in 11 out of 25 individuals with a variety of metastatic cancers [40]. Subsequently a study by Ruggeri et al. more clearly demonstrated the potential for NK-cell therapy [41]. In this study, AML patients with enhanced donor-versus-recipient NK-cell alloreactivity had significantly lower levels of relapse. This concept of NK-cell alloreactivity was based on the ‘missing-self model’ of NK-cell activation, in which the donor NK cells are not inhibited by KIR engagement with the HLA-C molecules present in the recipient. This was taken forward by Miller et al., who used haploidentical family members to generate NK-cell products that were infused into patients with AML and maintained with low-dose subcutaneous IL-2 in the recipient [42]. Complete remissions were observed in 5 out of 19 individuals with AML, with significantly improved responses evident in patients who received KIR ligand mismatched grafts [42]. The concept of NK-cell alloreactivity has been further taken forward by the use of blocking antibodies against the MHC-I inhibitory receptors KIR and NKG2A, which is discussed further below. The alternative to blocking inhibitory receptors is to stimulate the activating receptors through antibody crosslinking and this forms the basis for the development of the NK-cell engagers (NKCE). These target antigens expressed on the cancer cell surface and NK-cell activating receptors including CD16, NKG2D, NKp30, and NKp46. This technology is evolving from engagers which have two moieties, targeting one on the NK cell and one on the tumour cell, to NKCE targeting two or more NK receptors simultaneously for enhanced potency [43]. Merging the opportunities of NK-cell adoptive transfer with tumour antigen-specific responses can be achieved using chimeric antigen receptor (CAR)-NK cell technology which is another rapidly evolving area discussed further in this article below.
Advantages for targeting NK cells in cancer
NK cells possess multiple properties that complement CD8+ cytotoxic T-cell function against cancer. For example, while downregulation of HLA-A, -B, and -C alleles on tumour cells is a major resistance mechanism for CD8+ T cells [4], for NK cells this can be advantageous as loss of HLA-A, -B, and -C expression relieves their inhibition through the KIR. NK cells have multiple activating receptors to detect stress-induced ligands that are upregulated on tumour cells, such as MICA/B and ecto-calreticulin, and can therefore mediate killing in the absence of a specific tumour neoantigen. Furthermore, as already highlighted, NK cells promote DC recruitment and T-cell activation in tumours [21, 23, 44]. Adoptive NK-cell therapeutics also have off-the shelf capability and in clinical trials to date have not been associated with cytokine release syndrome (CRS), GVHD, or neurotoxicities which can plague T-cell therapies [45]. Inflammatory cytokines derived from activated macrophages are thought to drive CRS during T-cell therapy [46] and these cytokines remain low during adoptive NK therapy [47]. Furthermore, donor NK cells can lyse autologous donor T cells and thereby suppress T-cell-mediated GVHD [48].
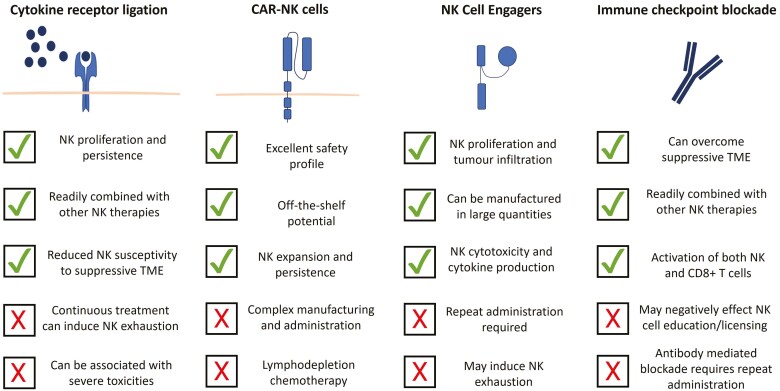
In this review, we highlight recent advances in the development of NK-targeted therapeutics including NK-cell engagers (NKCEs), cytokines, CAR-NK cells, and immune checkpoint inhibitors, and discuss their potential for the treatment of patients with cancer (Figure 1).
Figure 1.
Strategies under clinical development to enhance NK-cell activation against cancer.
Cytokine receptor engagement
NK-cell maturation, proliferation, and function are critically dependent upon cytokine receptor signalling. In accordance with this, the use of cytokine receptor ligation to potentiate anti-tumour NK-cell function is demonstrating promising efficacy in multiple different settings, including in combination with CAR-NK cells and NKCE strategies as described in the following sections.
The most widely targeted cytokine in development for NK-cell therapy is IL-15, with the IL-15R expressed on both NK cells and CD8+ T cells, but importantly, not regulatory T cells [49]. This is a key advantage over IL-2-based therapeutics [50] and importantly, IL-15 has been reported to prevent the loss of NK cell effector functions within the tumour microenvironment [28]. Continuous treatment of human NK cells with IL-15 can, however, induce NK-cell exhaustion [51], and therefore both the treatment schedule and route of administration must be carefully considered to achieve maximal benefit for patients. NIZ985 is a recombinant heterodimer of IL-15 and the IL-15 receptor alpha, mimicking the transpresentation of IL-15, which induces significant proliferation and IFNγ production in NK cells from patients with metastatic or unresectable solid tumours and is currently under evaluation in phase I/Ib trial in patients with advanced solid tumours or lymphoma (NCT04261439). The IL-15 superagonist complex N-803 (formerly termed ALT-803) induces potent activation of NK cells and has been shown to be safe when administered alone and in combination with the anti-CD20 antibody rituximab in patients with indolent non-Hodgkin lymphoma [52, 53]. In a phase I study, N-803 significantly increased the proliferation of NK cells, and to a lesser extent the proliferation of CD8+ T cells [52]. In accordance with this, N-803 administration in cynomolgus monkeys increased NK proliferation at lower concentrations than that required for CD8+ T-cell proliferation [54].
N-803 is currently under assessment in multiple active clinical trials, including a phase II/III trial for advanced non-small cell lung cancer in combination with the anti-PD1 antibody pembrolizumab (NCT05096663). This combination may be of particular value because the combination of N-803 with PD-1/PDL-1 blockade has recently been shown to overcome resistance to IL-15 therapy in preclinical models of ovarian cancer [55]. N-803 has also been used to support the expansion and persistence of memory-like NK cells in patients with leukaemia given reduced-intensity conditioning for HLA-haploidentical haematopoietic cell transplantation [56]. Importantly, in this study, there was over 1000-fold NK-cell expansion and NK cells persisted for over 2 months in patients [56]. This demonstrates the powerful capability of IL-15 to promote NK-cell expansion and persistence in patients. Conversely, a recent study has demonstrated that N-803 given during allogeneic cell therapy actually enhances clearance of donor NK cells by recipient CD8+ T cells [57]. This effect occurred in two independent clinical trial cohorts of relapsed/refractory AML patients treated with histocompatibility complex-haploidentical NK-cell therapy. This highlights that IL-15 receptor-mediated CD8+ cell activation must be carefully assessed to optimize IL-15 receptor-targeted therapeutics, particularly in the adoptive transfer setting. In accordance with CD8+ T-cell clearance being dependent upon MHC recognition, long-term >2-month expansion of NK cells was evident in HLA-mismatched CAR-NK-cell therapy [47]. This also highlights a possible advantage for NK persistence with autologous NK-cell therapies to circumvent CD8+ T-cell mediated clearance. Indeed, autologous NK cells have shown feasibility in treating multiple myeloma [58] and the ex vivo expanded autologous NK therapy (SNK01-US01) is currently under clinical evaluation for patients with solid tumours refractory to conventional therapy (NCT03941262).
It has recently been demonstrated that the ex vivo culture of NK cells with IL-15 in combination with nicotinamide, a vitamin B3 derivative involved in ATP generation, enhances NK-cell cytotoxicity and cytokine production, protects against oxidative stress, and, increases NK-cell persistence in vivo compared to IL-15 alone [59]. Furthermore, the combination of IL-15 with nicotinamide led to stable surface expression of CD62L, an L selectin critical for NK-cell adhesion and homing to the lymph nodes. In a phase 1 trial of patients with relapsed or refractory non-Hodgkin lymphoma (NCT03019666), the adoptive transfer of NK cells cultured with IL-15 and nicotinamide in combination with rituximab showed a complete response in 13 of 19 patients, with NK cells detected 14 days post-infusion [59].
The ex vivo stimulation of NK cells with IL-12, IL-15, and IL-18 to generate CIML NK cells has demonstrated superior anti-tumour efficacy in preclinical studies [34] and CIML NK cells are now under assessment in multiple clinical trials for both solid and haematological malignancies (Table 1). Utilization of CIML NK cells has previously involved ex vivo stimulation and infusion into patients. An alternative approach has recently been described however which allows NK memory cell formation in vivo by utilization of a fusion protein complex that combines IL-12, IL-15, and IL-18 to induce strong NK activation [60]. If transferred to the clinic, this approach would have the major benefit of utilizing CIML NK cells without the requirement for complex ex vivo culture conditions and adoptive transfer protocols.
Table 1.
Selected clinical trials utilising cytokine receptor ligation to promote NK-cell activation against cancer from www.clinicaltrials.gov
| Disease setting | Therapy | Clinical stage | Clinical trial number | Status | Sponsor |
|---|---|---|---|---|---|
| Advanced solid tumours and lymphoma | NIZ985 +/- spartalizumab or tislelizumab | Phase I/Ib | NCT04261439 | Active, not recruiting | Novartis Pharmaceuticals |
| Recurrent/metastatic gastric or head and neck cancer | N-803 + PD-L1 CAR-NK Cells + pembrolizumab | Phase II | NCT04847466 | Recruiting | National Cancer Institute |
| Advanced non-small cell lung cancer | N-803 + pembrolizumab | Phase II/III | NCT05096663 | Active, not recruiting | SWOG Cancer Research Network |
| Advanced or metastatic non-small cell lung cancer | N-803 + current standard of care or standard of care alone | Phase III | NCT03520686 | Active, not recruiting | ImmunityBio, Inc. |
| Relapsed acute myeloid leukaemia after HLA-matched related or unrelated allogeneic hematopoietic cell transplant | CIML NK Cell Infusion | Phase I/II | NCT03068819 | Recruiting | Washington University School of Medicine |
| Acute myeloid leukaemia | CIML NK cell infusion + N-803 | Phase II | NCT02782546 | Recruiting | Washington University School of Medicine |
| Refractory or relapsed acute myeloid leukaemia | CIML NK cell infusion | Phase I/II | NCT05580601 | Recruiting | Children’s Hospital Medical Center, Cincinnati |
| Advanced head and neck cancer | CIML NK cell infusion + N-803 with or without cetuximab or ipilimumab | Phase I | NCT04290546 | Recruiting | Dana-Farber Cancer Institute |
| Relapsed or refractory multiple myeloma or relapsed/refractory CD20-positive non-Hodgkin lymphoma | Nicotinamide expanded-natural killer cell-based therapy + elotuzumab or rituximab (IL-15 during ex vivo expansion followed by IL-2 in vivo) | Phase I | NCT03019666 | Completed | Masonic Cancer Center, University of Minnesota |
CAR-NK cells
CAR-T-cell therapies are now an established treatment option for patients with B-cell lymphoma, B-cell acute lymphoblastic leukaemia, or multiple myeloma [61]. However, these come at a high treatment cost and can be associated with severe toxicities. NK cells are under clinical assessment for solid and haematological malignancies in multiple phases 1 and 2 trials as an alternative cellular source for CAR-based therapies (Table 2) [45, 62]. Importantly, CAR-NK cells have the potential for off-the-shelf utility (allowing for reduced product cost and time) and reduced toxicities compared to CAR-T therapy (reviewed in [45, 63, 64]). A variety of sources of NK cells are being investigated for CAR-NK-cell production and these include induced pluripotent stem cells (iPSC), cord blood, peripheral blood from healthy donors, immortalized NK-like cell lines, and autologous cancer patient-derived NK cells [65]. Specific CAR-mediated recognition of surface tumour antigens is usually achieved by NK-cell expression of single-chain variable fragment (scFv) and the potential of CAR-NK cells to treat cancer was demonstrated in a landmark study published in 2020, in which anti-CD19 CAR-NK cells derived from cord blood were administered to 11 HLA-mismatched patients with CLL or non-Hodgkin lymphoma [47]. In this study, there were no incidences of CRS, neurotoxicity, or GVHD and furthermore, an overall 73% response rate was achieved, with complete remissions in 7/11 patients at a median follow-up of 13.8 months. Importantly, expansion of CAR-NK cells was evident following infusion and CAR-NK cells could be detected for at least 12 months by PCR [47] demonstrating the potential for long-term persistence of CAR-NK cells. Interestingly, in the two patients available for assessment of lymph nodes, CAR-NK cells preferentially homed to the lymph nodes compared to the blood and bone marrow [47] indicating that CAR-NK cells may be home to sites with high tumour burden.
Table 2.
Selected clinical trials for CAR-NK cells in solid and haematological malignancies from www.clinicaltrials.gov
| Disease setting | Target antigen | Cellular source | Clinical phase | Clinical trial number | Status | Sponsor |
|---|---|---|---|---|---|---|
| Advanced solid tumours | Claudin6 | Autologous PBMC | Phase I/II | NCT05410717 | Recruiting | Second Affiliated Hospital of Guangzhou Medical University |
| Relapsed or refractory haematological malignancies | CD70 | Cord blood | Phase I/II | NCT05092451 | Recruiting | M.D. Anderson Cancer Center |
| Relapsed or refractory B-cell non-Hodgkin lymphoma | CD19 | Cord blood | Phase II | NCT05020015 | Recruiting | Takeda |
| Recurrent/metastatic gastric or head and neck cancer | PD-L1 | Engineered NK-92 cells | Phase II | NCT04847466 | Recruiting | National Cancer Institute |
| Relapsed or refractory B-cell lymphoma or chronic lymphocytic leukemia | CD19 | iPSC | Phase I | NCT04245722 | Active, not recruiting | Fate Therapeutics |
| Relapsed or refractory multiple myeloma | BCMA | iPSC | Phase I | NCT05182073 | Recruiting | Fate Therapeutics |
| Acute myeloid leukaemia | CD33/ CLL1 | Information not available | Phase I | NCT05215015 | Recruiting | Wuxi People’s Hospital |
| Relapsed and refractory multiple myeloma | BCMA | NK92 | Phase I/II | NCT03940833 | Unknown | Asclepius Technology Company Group (Suzhou) Co., Ltd. |
| Recurrent HER2-positive glioblastoma | HER2 | NK-92/5.28.z | Phase I | NCT03383978 | Recruiting | Johann Wolfgang Goethe University Hospital |
| Relapsed or refractory CD19-positive B-cell malignancies | CD19 | iPSC | Phase I | NCT05336409 | Recruiting | Century Therapeutics, Inc. |
| Recurrent or refractory CD19 positive B-cell malignant tumours | CD19 | Information not available | Phase I | NCT05410041 | Recruiting | Beijing Boren Hospital |
| MUC1 positive advanced refractory or relapsed solid tumours | MUC1 | Information not available | Phase I/II | NCT02839954 | Unknown | PersonGen BioTherapeutics (Suzhou) Co., Ltd. |
| Locally advanced or metastatic pancreatic cancer | PD-L1 | Engineered NK-92 cells | Phase II | NCT04390399 | Recruiting | ImmunityBio, Inc. |
| Patients who have previously received treatment with PD-1/PD-L1 immune checkpoint inhibitors | PD-L1 | Engineered NK-92 cells | Phase II | NCT03228667 | Active, not recruiting | ImmunityBio, Inc. |
An important mechanism for relapse during CAR-T-cell therapy is the loss of CAR-target antigen expression. CAR-NK cells can potentially kill tumour cells independently of the CAR-target antigen via their retention of native activating receptors and via ADCC in combination with antibodies that target different tumour associated antigens. For example, anti-CD19 CAR-NK cells obtained from iPSC engineered to express high affinity non-cleavable CD16 have demonstrated potent anti-lymphoma efficacy in murine models and were able to target both CD19- and CD19+ target cells in combination with anti-CD20 antibodies [66]. In addition, a multi-antigen targeting strategy has been assessed for multiple myeloma, with anti-BCMA CAR-NK cells engineered with a high-affinity non-cleavable CD16 demonstrating strong efficacy in murine models of myeloma in combination with the anti-CD38 antibody daratumumab [67]. This study also demonstrated the importance of minimizing NK-cell fratricide to enhance CAR-NK-cell efficacy, with the silencing of CD38 expression able to inhibit CAR-NK-cell fratricide during combination with daratumumab [67]. An alternative strategy developed to reduce CAR-NK-cell fratricide is via the co-expression of an inhibitory CAR construct which recognizes an antigen expressed on NK cells [68]. This dual CAR approach allowed for CAR-NK mediated lysis of tumour cells that expressed the tumour antigen, while CAR-NK cells which co-expressed the target antigen (via trogocytosis) and the target antigen for the inhibitory CAR were spared from lysis. Importantly, this approach enhanced CAR-NK-cell persistence and efficacy in pre-clinical models [68] demonstrating its strong potential for translation to the clinic.
In patients with solid tumours, intracranial injection of anti-HER2 CAR-NK cells in patients with glioblastoma demonstrated that CAR-NK cells are safe and well tolerated when given by this route and can have clinical activity, with stable disease being the best response in this phase 1 trial [69]. Anti-GD2 CAR-NK cells have also been shown to inhibit tumour growth in a xenograft murine model of paediatric brainstem gliomas [70], highlighting the potential utility of CAR-NK cells for brain tumours which are notoriously hard to treat effectively. In addition to these tumour types, CAR-NK cells are now under clinical evaluation in early-phase trials for patients with a number of different solid tumours, including head and neck cancer and pancreatic cancer (Table 2).
NK cells have also been engineered to express a functional T-cell receptor (TCR)/CD3 construct, as opposed to a scFv, termed TCR-NK cells [71]. This allows for NK-cell-mediated targeting of specific peptide: MHC cancer-associated antigens and targeting of antigens that are unsuitable for scFv CAR NK cells. Examples of TCR-NK-cell therapeutics under investigation include targeting the MAGE-A4 antigen for solid tumours [72] and the NY-ESO-1 antigen in multiple myeloma [73]. Building on this approach, dual expression of a TCR specific to human papillomavirus E7 antigen in combination with a CAR targeting the trophoblast cell surface antigen 2 (TROP2) has been shown to synergistically enhance NK-cell function compared to TCR-NK cells alone in preclinical models [74].
Using single-cell RNA sequencing and mass cytometry in combination with murine models and patient samples, Li et al. recently demonstrated that loss of metabolic fitness of CAR-NK cells is a major cause of tumour resistance to therapy [75]. Incorporation of IL-15 into the CAR construct improved NK-cell function. However, over time these CAR+IL-15-NK cells still lost their anti-tumour functions against highly metabolic tumours, suggested to be due to local depletion of nutrients by the tumours [75]. Importantly, a second infusion of CAR+IL-15-NK cells showed increased efficacy and allowed for more effective control of tumours [75] demonstrating that the treatment schedule of CAR-NK cells will need to be carefully assessed to optimize patient outcomes. This work is in agreement with previous studies of patients treated with CAR-NK cells, which demonstrated that the metabolic fitness of CAR-NK cells is an important determinant of clinical outcome [75].
An alternative approach to enhance CAR-NK-cell function includes the targeting of the cytokine-inducible SH2-containing (CISH) protein, a crucial inhibitory regulator of IL-15-mediated signalling. This strategy led to augmented metabolic fitness of CAR-NK cells, increased expansion, more effective lysis of tumour cells in vitro, and enhanced anti-tumour efficacy in vivo [76, 77]. The utilization of protocols that generate CIML has been applied to CAR technology, making CAR-memory-like NK cells. This enhances CAR-NK persistence and multiple studies have demonstrated the promising efficacy of this approach, including for AML [78] and lymphoma [79] and solid tumours including head and neck cancer [38]. Furthermore, CMV-induced memory-like NK cells typically lack SYK expression and it has recently been shown that CRISPR-Cas9 silencing of SYK enhances NK-cell cytotoxicity and cytokine production, indicating that genetic engineering approaches to recapitulate key features of memory-like NK cells could potentially be used to improve CAR-NK-cell function [80]. Furthermore, CAR-NK-cell function may be improved by rational drug combination strategies such as epigenetic modulators, oncolytic viruses, small molecule inhibitors, [63] or with stimulator of interferon genes (STING) agonists which have recently been demonstrated to potentiate CAR-NK-cell killing of tumour cells [81, 82]. Additional promising approaches to improve CAR-NK-cell function include: tailoring the intracellular signalling proteins within the CAR construct for NK cells rather than using a CAR construct designed initially for T cells [83]; and optimizing the affinity of the CAR for the target antigen to minimize on-target off-tumour cytotoxicity [84, 85].
NK-cell engagers (NKCEs)
NK cells express multiple activating receptors which can synergize to enhance the level of NK cell activation. The FcγRIII receptor (CD16) allows NK cells to mediate ADCC and thus contribute to the therapeutic efficacy of tumour targeting antibodies [18, 86–89]. To exploit this, a new generation of antibody constructs, termed NK-cell engagers (NKCEs) are now under development which combine antibody variable domains that bind tumour antigens with antibody domains that bind NK cell receptors. Receptors targeted alone or in combination with this strategy include CD16, NKp30, NKp46, NKG2D, or cytokine receptors [90]. Therefore NKCEs which simultaneously engage one or more activating receptors and/or cytokine receptors on NK cells allow for more potent activation against tumour cells. This can enhance NK-cell proliferation compared to ligation of CD16 alone [90, 91]. These bi-specific (BiKE), tri-specific (TriKe), and tetra-specific NKCEs are under development against both haematological malignancies and solid tumours, with examples of clinical trials for these shown in Table 3.
Table 3.
Selected clinical trials for NKCEs in cancer from www.clinicaltrials.gov
| Disease | Tumour antigen target | NKCE | Clinical phase | Clinical trial number | Status | Sponsor |
|---|---|---|---|---|---|---|
| Relapsed or refractory acute myeloid leukaemia, B-cell acute lymphoblastic leukemia, or high risk-myelodysplasia | CD123 | SAR443579 | Phase I/II | NCT05086315 | Recruiting | Sanofi |
| Relapsed or refractory multiple myeloma, relapsed or refractory light-chain amyloidosis | BCMA | SAR445514 | Phase I/II | NCT05839626 | Recruiting | Sanofi |
| Relapsed or refractory acute myeloid leukaemia | CD123 | AFM28 | Phase I | NCT05817058 | Recruiting | Affimed GmbH |
| Advanced solid malignancies | EGFR | AFM24 | Phase I/II | NCT04259450 | Active, not recruiting | Affimed GmbH |
| Relapsed or refractory CD30-positive T-cell lymphoma | CD30 | AFM13 | Phase II | NCT04101331 | Active, not recruiting | Affimed GmbH |
| Advanced or metastatic EGFR-expressing cancers | EGFR | AFM24 | Phase I/II | NCT05099549 | Active, not recruiting | NKGen Biotech, Inc. |
| Recurrent or refractory CD30-positive Hodgkin or non-Hodgkin lymphomas | CD30 | AFM13 | Phase I/II | NCT04074746 | Active, not recruiting | M.D. Anderson Cancer Center |
| CD33-expressing high-risk myelodysplastic syndromes, refractory/relapsed acute myeloid leukaemia, or advanced systemic mastocytosis | CD33 | GTB-3550 | Phase I/II | NCT03214666 | Terminated due to development of the second-generation TriKE GTB-3650 | GT Biopharma, Inc. |
| Relapsed and/or refractory multiple myeloma | BCMA | CC-92328 | Phase I | NCT04975399 | Recruiting | Celgene |
| Advanced solid tumours | HER2 | DF1001 | Phase I/II | NCT04143711 | Recruiting | Dragonfly Therapeutics |
| Relapsed or refractory acute myeloid leukaemia | CD33 | CC-96191 | Phase I | NCT04789655 | Recruiting | Celgene |
NKCEs have been tested predominantly against haematological malignancies, targeting antigens such as CLEC12A, CD19, CD20, CD33, CD123, and BCMA. The benefit of targeting multiple NK receptors simultaneously is exemplified by an NKCE targeting CD16, NKp46, the β-chain of the interleukin-2 receptor (IL-2R), and CD20 [91]. This tetra-specific NKCE showed significantly improved control of lymphoma growth in a murine model compared to the anti-CD20 therapeutic antibody obinutuzumab. Importantly, the incorporation of the IL-2R stimulating domain induced NK-cell proliferation in vivo and led to increased NK infiltration into lymphoma tumours [91]. In this NKCE, NKp46/CD16 and tumour antigen engagement induced strong NK cytotoxicity, while IL-2R ligation was required to drive NK expansion and infiltration [91]. This highlights the benefit of incorporating cytokine receptor ligation into NKCE development. Incorporation of an IL-15 moiety into a TRiKE targeting CD16 and CD19 has also been shown to induce NK-cell expansion and to improve the killing of CLL cells compared to rituximab [92]. In AML, a TriKe targeting the activating receptor NKG2C, the IL-15R, and the tumour-associated antigen CD33 showed potent NK-cell degranulation and cytokine production in response to primary CD33+ AML blasts [93]. Targeting the myeloid lineage antigen CLEC12A in combination with CD16 and IL-15R ligation by a TriKe stimulated NK-cell proliferation and cytotoxicity against primary AML blasts whilst sparing normal haematopoietic stem cells [94]. Furthermore, in a patient-derived xenograft model, this TriKe reduced AML burden in the bone marrow of mice [94].
A TriKe targeting NKp46, CD16, and CD20 provided superior efficacy against CD20+ lymphoma cells in vivo and in vitro compared to obinutuzumab, an approved anti-CD20 therapeutic antibody [95]. Against pediatric B-ALL cells which express low levels of CD20, a CD19 targeting NKCE which ligates CD16 in combination with either NKp46 or NKp30 showed potent activation of NK cells against primary B-ALL cells and could overcome inhibitory HLA-mediated interactions [96]. Furthermore, targeting NKp46, CD16, and the tumour-associated antigen CD123 (IPH6101/SAR’579) induced NK-cytokine secretion and activation against primary AML cells [97] demonstrating improved efficacy in vivo compared to an ADCC-enhanced therapeutic antibody targeting CD123 [97]. IPH6101/SAR’579 is now in a phase 1/2 trial for patients with relapsed or refractory AML, B-ALL, or high risk-myelodysplastic syndrome (NCT05086315), with initial reports indicating good tolerance and clinical benefit in patients with relapsed/refractory AML [98]. Furthermore, the NKCE IPH6401/SAR’514 targeting BCMA, CD16, and NKp46 is in a phase 1/2 trial for patients with relapsed/refractory multiple myeloma or light-chain amyloidosis (NCT05839626). Other NKCEs under assessment in haematological malignancies included a TriKE (GTB-3550) targeting CD16, IL-15, and the tumour-associated antigen CD33 in AML and high-risk myelodysplastic syndromes in a phase 1 trial (NCT03214666). This therapeutic is now being superseded by the second-generation camelid nanobody NKCE GTB-3650.
Administration of NKCEs into cancer patients relies on the effector mechanisms of patient NK cells which can be dysregulated and or have low frequency. A promising strategy to overcome this is to combine NKCEs with adoptive transfer of healthy donor-derived ex vivo expanded NK cells. For example, combining the tetravalent bispecific engager AFM13 which targets CD30 on lymphoma cells and CD16 on NK cells, with pre-activated cord blood-derived NK cells demonstrated improved control of CD30+ lymphoma in vivo compared to AFM13 or pre-activated NK cells alone [99]. AFM13 in combination with cord blood-derived NK cells is now under assessment in a phase I/II trial for patients with recurrent or refractory CD30+ Hodgkin or non-Hodgkin lymphoma (NCT04074746). Donor selection may also be important and ‘superdonors’ with large subpopulations of NKG2C+ adaptive NK cells provide a ready source of allogeneic NK cells which express single inhibitory KIR, such that they would be predicted to be potently alloreactive. These have been combined with an anti-CD33 TriKE to good effect in a preclinical AML model [16].
NKCEs have also been studied in solid tumours, but less extensively. For instance, a CD16/IL-15R/HER2 targeting TRiKE induced NK-cell proliferation and demonstrated promising efficacy against ovarian cancer cells in vitro and in vivo [100]. A CD16/IL15R/mesothelin TriKE enhanced the proliferation, cytotoxicity, and cytokine production of patient-derived NK cells against lung cancer cell lines [101]. In addition, this NKCE significantly improved control of tumour growth in a metastatic xenograft model [101]. Furthermore, to overcome NK-cell dysfunction in patients, the combination of NKCE with adoptively transferred NK cells in solid tumours is currently under clinical assessment (Table 3). For example, the autologous NK-cell product SNK01 in combination with AFM24, a CD16 and EGFR engager, is currently in a phase I/II trial for patients with advanced or metastatic ERGF-expressing solid tumours (NCT05099549).
In addition to targeting antigens specifically associated with tumour cells, NKCEs have also shown utility against antigens that are shared by both tumour cells and tumour-associated stromal cells [102]. This is important because tumour-associated stromal cells can aid tumour growth and mediate immunosuppression [102], and therefore targeting these cells may be advantageous by modulating the tumour microenvironment, especially for solid tumours which can rapidly cause NK dysfunction [28]. An example of this is TEM8, which is expressed by tumour associated stromal cells, fibroblasts, endothelial cells, and various cancer types including breast cancer cells. A CD16/IL-15R/TEM8 targeting TRiKE stimulated NK-cell cytotoxicity against both tumour cells and tumour endothelial cells in vivo, with a decrease in endothelial density in tumours evident [102]. Interestingly, this NKCE was also able to increase NK-cell infiltration into subcutaneous tumours formed from breast cancer cell lines compared to functionally equivalent IL-15 and could enhance the proliferation of NK cells [102]. This study reveals the possibility of using NKCE to target not only the tumour cells themselves but also the surrounding tumour microenvironment and angiogenesis to enhance their efficacy.
Immune checkpoint blockade
NK-cell activation is tightly regulated by an array of inhibitory receptors and disruption of these receptor: ligand interactions can release NK cells from immunosuppression in cancer. The KIR family of receptors is a critical component of NK recognition of self and plays an important role in NK-cell education [2]. The inhibitory KIR recognize HLA-A/B/C and disruption of KIR: HLA interactions allows for potent NK-cell activation against target cells. However, the KIR have proved difficult to target effectively to date. Innate Pharma developed the anti-KIR blocking antibody lirilumab to potentiate NK-cell effector functions and this approach showed promising preclinical activity in vitro and in vivo with enhanced NK-cell cytotoxicity and ADCC [103]. However, in phase 2 clinical trial for patients with multiple myeloma lirilumab was ineffective, reducing NK-cell function and KIR2D expression by trogocytosis [104, 105]. Further evaluation of lirilumab is currently ongoing in a phase 2 clinical trial for squamous cell carcinoma of the head and neck in combination with the anti-PD-1 antibody nivolumab (NCT03341936) and these results are eagerly anticipated.
NK cells are under constitutive inhibition by inhibitory receptors for MHC class I, and genome-scale CRISPR-based gene editing screens have revealed that HLA-E interactions with NKG2A are a key determinant for tumour cell sensitivity to NK-cell mediated lysis [106]. Upon ligation, NKG2A suppresses NK-cell activation via SHP-1/2 making it an attractive target to release NK cells from inhibition [107]. HLA-E is widely expressed in solid tumours and haematological malignancies [108, 109] and can be further increased upon IFNγ stimulation [110, 111], or by lymph node associated signals on CLL cells [112]. Furthermore, NKG2A-positive cells are detected within solid tumour tissues [108], and in the lymph nodes of patients with lymphoma [113]. Importantly, it has recently been shown that circulating tumour cells are protected from NK-cell mediated clearance via NKG2A:HLA-E interactions, with HLA-E expression promoted by platelet-derived RGS18 and NKG2A blockade able to restore NK function and prevent tumour metastasis in vivo [114]. In accordance with this, NKG2A+ NK cells are detected in tumour draining lymph nodes, the first site for metastases in patients with breast cancer [115]. Additionally, disruption of NKG2A function may have particular importance in combination with adoptive NK-cell therapies because clinically relevant NK-cell expansion techniques lead to significantly increased surface NKG2A expression (reviewed in [116]).
The NKG2A blocking antibody monalizumab promotes NK-cell effector function in vitro and in vivo against tumour cells and enhances ADCC, as well as the efficacy of cancer vaccines [108, 117]. Monalizumab is currently in several clinical trials for solid tumours, including a phase 3 clinical trial in combination with durvalumab (anti-PD-L1) in patients with unresectable, stage III non-small cell lung cancer (NCT05221840) (Table 4). However, a trial of monalizumab and cetuximab in head and neck cancer was recently discontinued. NKG2A is also expressed on CD8+ T cells [108, 117] and NKG2A expression identifies a subset of the innate-like Vδ2 T cells with enhanced anti-tumour functions [118]. This indicates that NKG2A blockade in patients may simultaneously release the effector functions of NK cells as well as CD8+ T cells and Vδ2 T cells. In addition to monalizumab, the NKG2A blocking antibody BMS-986315 is currently in a phase 1/2 trial for patients with advanced solid tumours either alone or in combination with nivolumab or cetuximab (NCT04349267). In addition to direct antibody-mediated blockade of NKG2A, other approaches under investigation include antibody-mediated blockade of HLA-E, as well as CRISPR-Cas9 mediated silencing of NKG2A expression [116, 119, 120]. Interestingly, tumour cell surface expression of HLA-E is sensitive to downregulation by various clinically relevant pharmacological agents such as the XPO1 inhibitor selinexor, the proteosome inhibitor bortezomib, and the cyclin-dependent kinase inhibitor dinaciclib [112, 121–123]. This indicates that the combination of NK cells with approved small molecules which modulate HLA-E levels on tumour cells may have synergistic anti-tumour effects.
Table 4.
Selected clinical trials for immune checkpoint inhibitors in cancer from www.clinicaltrials.gov
| Disease | Inhibitory receptor target | Antibody | Clinical phase | Clinical trial number | Status | Sponsor |
|---|---|---|---|---|---|---|
| Locally advanced, unresectable non-small cell lung cancer, who have not progressed following platinum-based cCRT | NKG2A | Monalizumab | Phase III | NCT05221840 | Recruiting | AstraZeneca |
| Resectable, early-stage non-small cell lung cancer | NKG2A | Monalizumab | Phase II | NCT05061550 | Recruiting | AstraZeneca |
| Advanced solid tumours | NKG2A | BMS-986315 | Phase I/II | NCT04349267 | Recruiting | Bristol-Myers Squibb |
| Recurrent squamous cell carcinoma of the head and neck | KIR2DL1/2/3 | Lirilumab | Phase II | NCT03341936 | Active, not recruiting | Dana-Farber Cancer Institute |
CISH is a critical negative regulator of IL-15 signalling in NK cells which acts via inhibition of JAK1 enzymatic activity [124, 125]. CRISPR-Cas9 mediated silencing of CISH in human NK cells enhances cytokine production capacity and NK cytotoxicity against K562 cells and improves the control of tumours in murine models [126]. Importantly, however, CISH-deleted NK cells are still sensitive to TGFβ mediated suppression and the combined suppression of both CISH and TGFβ substantially improved anti-tumour immunity compared to suppression of either protein alone [127]. In glioblastoma multiforme (GBM), the release of TGFβ from glioblastoma stem cells was mediated by αv integrins and the targeting of αv integrin or blockade of the TGF-β receptor improved NK-cell function and improved tumour control in a murine model [128]. TGFβR ligation induces SMAD2/3-mediated NK-cell suppression and interestingly, TGFβ-independent suppression of NK cells via SMAD2/3 has also been identified. SMAD2/3-mediated suppression of NK cells occurred via activin-A mediated ligation of the type I activin receptor ALK4 that is expressed on NK cells [129]. This highlights the requirement to consider the contribution of other receptors in addition to TGFβR to fully overcome SMAD2/3-mediated suppression of NK function. Furthermore, hypoxia is a characteristic feature of the tumour microenvironment and NK cells are more sensitive to hydrogen peroxide compared to T or B cells due to decreased expression of peroxiredoxin-1 (PRDX1) [130]. In accordance with this, overexpression of PRDX1 can augment NK cell and CAR-NK-cell anti-tumour activity under oxidative stress conditions [130].
The immune checkpoint receptors PD-1 and TIGIT are classically targeted to augment T-cell function and both TIGIT and PD-1 are expressed in NK cells. TIGIT is associated with NK-cell exhaustion [131] and TIGIT blockade augmented NK-cell activation and CD8+ T-cell effector function in murine models of cancer [131]. Furthermore, expression of functional PD-1 is detected on both resting and activated human NK cells [132, 133], and a single chain fragment of pembrolizumab increased NK cell cytokine production up to 4-fold [132]. In accordance with this, murine models have shown that NK cells are required to mediate the full therapeutic efficacy of PDL-1 blockade [133] and iPSC-derived NK cells can co-operate with T cells in combination with anti-PD1 antibodies to control tumour growth in vivo [20]. In cancer patients, NK-cell frequency associated with stimulatory dendritic cells, the response to anti-PD-1 therapies, and overall survival [23]. In addition, it has recently been identified that NK-cell production of the DC chemoattractant CCL5 is important for the response to anti-PD-1 therapy [44]. This highlights the important role of NK cells not only in the direct lysis of tumour cells but also in their ability to shape the adaptive anti-tumour immune response via cross-talk with DC and CD8+ T cells [21].
Conclusions and future perspectives
NK cells hold significant potential for cancer immunotherapy due to their excellent safety record, potent cytotoxic activities, and ability to kill tumour cells with downregulated MHC expression. The unique features of NK cells also make them attractive for either combination with T-cell-directed therapies, or for use in T-cell therapy refractory settings. The potential for adoptive NK-cell therapies to provide an off-the-shelf product also provides the opportunity for reduced cost and production time compared to CAR-T cells. Specific challenges exist for different cancer types, and these will need to be assessed in pre-clinical and clinical studies to optimize anti-cancer NK-cell immunity in patients.
Therapeutic strategies which enhance the expansion, persistence, and infiltration of NK cells into areas of high tumour burden will be crucial to realize the full potential of NK cells. This is of particular importance in patients with solid tumours, with pre-clinical and clinical evidence indicating that NK cells have impaired tumour infiltration, as well as reduced activation within the immunosuppressive tumour microenvironment. In addition, sensitization of tumour cells to NK-cell killing in a drug combination strategy, as has recently been shown with the BH3-mimetic venetoclax [134], may prove useful to augment the efficacy of NK therapies. Furthermore, mRNA-encoded cytokines can enhance NK-cell activation and promote the control of tumours in vivo [135], highlighting the promising potential for mRNA-based therapeutics to improve NK-cell activity in cancer patients. Finally, because the chronic stimulation of NK cells without concomitant negative receptor signalling can induce exhaustion of NK cells [136], therapeutic strategies must be based on a deep understanding of NK-cell biology to realize their full potential against cancer.
Acknowledgments
The Editor-in-Chief, Tim Elliott, and handling editor, Yiwei Chu, would like to thank Megat Hamid and two anonymous reviewers, for their contribution to the publication of this article.
Contributor Information
Matthew D Blunt, School of Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, UK.
Salim I Khakoo, School of Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, UK.
Author Contributions
M.D.B and S.I.K wrote the first draft of the manuscript and then edited and reviewed this.
Funding
This work was supported by a John Goldman Fellowship from Leukaemia UK and funding from the Medical Research Council (MR/M019829/1) and Cancer Research UK (ECRIN-M3 accelerator award C42023/A29370).
Conflict of interest
M.D.B and S.I.K have applied for a patent for peptide mediated NK cell activation. M.D.B has received research funding from Karyopharm Therapeutics.
Ethics approval
Not applicable.
Data Availability
No new data were generated or analysed in support of this research.
References
- 1. Wong P, Foltz JA, Chang Let al.. T-BET and EOMES sustain mature human NK cell identity and antitumor function. J Clin Invest 2023; 133(13):e162530. 10.1172/JCI162530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Djaoud Z, Parham P.. HLAs, TCRs, and KIRs, a triumvirate of human cell-mediated immunity. Annu Rev Biochem 2020; 89:717–39. 10.1146/annurev-biochem-011520-102754 [DOI] [PubMed] [Google Scholar]
- 3. Medjouel Khlifi H, Guia S, Vivier Eet al. Role of the ITAM-bearing receptors expressed by natural killer cells in cancer. Front Immunol 2022; 13:898745. 10.3389/fimmu.2022.898745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hazini A, Fisher K, Seymour L.. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J ImmunoTher Cancer 2021; 9(8):e002899. 10.1136/jitc-2021-002899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Philippon C, Tao S, Clement Det al.. Allelic variation of KIR and HLA tunes the cytolytic payload and determines functional hierarchy of NK cell repertoires. Blood Adv 2023; 7:4492–4504. 10.1182/bloodadvances.2023009827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, Chen Y, Deng Met al. Antagonistic anti-LILRB1 monoclonal antibody regulates antitumor functions of natural killer cells. J ImmunoTher Cancer 2020; 8(2):e000515. 10.1136/jitc-2019-000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartolome J, Molto C, Benitez-Fuentes JDet al. Prognostic value of human leukocyte antigen G expression in solid tumors: a systematic review and meta-analysis. Front Immunol 2023; 14:1165813. 10.3389/fimmu.2023.1165813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loustau M, Anna F, Dréan Ret al. HLA-G neo-expression on tumors. Front Immunol 2020; 11:1685. 10.3389/fimmu.2020.01685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Beltran WF, Hölzemer A, Martrus Get al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat Immunol 2016; 17(9):1067–74. 10.1038/ni.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Han H, Zhang Fet al. Lesion human leukocyte antigen-F expression is associated with a poor prognosis in patients with hepatocellular carcinoma. Oncol Lett 2015; 9(1):300–4. 10.3892/ol.2014.2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng E, Liang T, Wang Xet al. Correlation of alteration of HLA-F expression and clinical characterization in 593 brain glioma samples. J Neuroinflammation 2019; 16(1):33. 10.1186/s12974-019-1418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu B, Yang H, Ying Set al. High HLA-F expression is a poor prognosis factor in patients with nasopharyngeal carcinoma. Anal Cell Pathol (Amst) 2018; 2018:7691704. 10.1155/2018/7691704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blunt MD, Khakoo SI.. Activating killer cell immunoglobulin-like receptors: detection, function and therapeutic use. Int J Immunogenet 2020; 47(1):1–12. 10.1111/iji.12461 [DOI] [PubMed] [Google Scholar]
- 14. Cifaldi L, Melaiu O, Giovannoni Ret al. DNAM-1 chimeric receptor-engineered NK cells: a new frontier for CAR-NK cell-based immunotherapy. Front Immunol 2023; 14:1197053. 10.3389/fimmu.2023.1197053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sen Santara S, Lee D-J, Crespo Aet al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 2023; 616(7956):348–56. 10.1038/s41586-023-05912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haroun-Izquierdo A, Vincenti M, Netskar Het al. Adaptive single-KIR(+)NKG2C(+) NK cells expanded from select superdonors show potent missing-self reactivity and efficiently control HLA-mismatched acute myeloid leukemia. J ImmunoTher Cancer 2022; 10(11):e005577. 10.1136/jitc-2022-005577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruhns P, Iannascoli B, England Pet al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113(16):3716–25. 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 18. Sopp J, Cragg MS.. Deleting malignant B cells with second-generation anti-CD20 antibodies. J Clin Oncol 2018; 36(22):2323–5. 10.1200/JCO.2018.78.7390 [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Orange JS.. Degranulation enhances presynaptic membrane packing, which protects NK cells from perforin-mediated autolysis. PLoS Biol 2021; 19(8):e3001328. 10.1371/journal.pbio.3001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cichocki F, Bjordahl R, Gaidarova Set al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med 2020; 12(568):eaaz5618. 10.1126/scitranslmed.aaz5618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huntington ND, Cursons J, Rautela J.. The cancer-natural killer cell immunity cycle. Nat Rev Cancer 2020; 20(8):437–54. 10.1038/s41568-020-0272-z [DOI] [PubMed] [Google Scholar]
- 22. Bottcher JP, Bonavita E, Chakravarty Pet al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018; 172(5):1022–1037.e14. 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry KC, Hsu J, Broz MLet al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 2018; 24(8):1178–91. 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kyrysyuk O, Wucherpfennig KW.. Designing cancer immunotherapies that engage T cells and NK cells. Annu Rev Immunol 2023; 41:17–38. 10.1146/annurev-immunol-101921-044122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turaj AH, Hussain K, Cox KLet al. Antibody tumor targeting is enhanced by CD27 agonists through myeloid recruitment. Cancer Cell 2017; 32(6):777–791.e6. 10.1016/j.ccell.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai K, Matsuyama S, Miyake Set al. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000; 356(9244):1795–9. 10.1016/S0140-6736(00)03231-1 [DOI] [PubMed] [Google Scholar]
- 27. Cozar B, Greppi M, Carpentier Set al. Tumor-infiltrating natural killer cells. Cancer Discov 2021; 11(1):34–44. 10.1158/2159-8290.CD-20-0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dean IW, Lee CYC, Tuong ZKet al. Rapid establishment of a tumor-retained state curtails the contribution of conventional NK cells to anti-tumor immunity in solid cancers. bioRxiv 2023:2023.08.10.552797. 10.1101/2023.08.10.552797 [DOI] [Google Scholar]
- 29. Tang F, Li J, Qi Let al. A pan-cancer single-cell panorama of human natural killer cells. Cell 2023; 186(19):4235–4251.e20. [DOI] [PubMed] [Google Scholar]
- 30. Tong L, Jiménez-Cortegana C, Tay AHMet al. NK cells and solid tumors: therapeutic potential and persisting obstacles. Mol Cancer 2022; 21(1):206. 10.1186/s12943-022-01672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tarannum M, Romee R, Shapiro RM.. Innovative strategies to improve the clinical application of NK cell-based immunotherapy. Front Immunol 2022; 13:859177. 10.3389/fimmu.2022.859177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau CM, Wiedemann GM, Sun JC.. Epigenetic regulation of natural killer cell memory. Immunol Rev 2022; 305(1):90–110. 10.1111/imr.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gang M, Wong P, Berrien-Elliott MMet al. Memory-like natural killer cells for cancer immunotherapy. Semin Hematol 2020; 57(4):185–93. 10.1053/j.seminhematol.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Romee R, Rosario M, Berrien-Elliott MMet al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016; 8(357):357ra–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bednarski JJ, Zimmerman C, Berrien-Elliott MMet al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022; 139(11):1670–83. 10.1182/blood.2021013972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marin ND, Krasnick BA, Becker-Hapak Met al. Memory-like differentiation enhances NK cell responses to melanoma. Clin Cancer Res 2021; 27(17):4859–69. 10.1158/1078-0432.CCR-21-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shapiro RM, Birch GC, Hu Get al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J Clin Invest 2022; 132(11):e154334. 10.1172/JCI154334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacobs MT, Wong P, Zhou AYet al. Memory-like differentiation, tumor targeting monoclonal antibodies, and chimeric antigen receptors enhance natural killer cell responses to head and neck cancer. Clin Cancer Res 2023;29(20):4196-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woan KV, Kim H, Bjordahl Ret al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 2021; 28(12):2062–2075.e5. 10.1016/j.stem.2021.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenberg SA, Lotze MT, Muul LMet al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985; 313(23):1485–92. 10.1056/NEJM198512053132327 [DOI] [PubMed] [Google Scholar]
- 41. Ruggeri L, Capanni M, Urbani Eet al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295(5562):2097–100. 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 42. Miller JS, Soignier Y, Panoskaltsis-Mortari Aet al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105(8):3051–7. 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 43. Demaria O, Gauthier L, Debroas Get al. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol 2021; 51(8):1934–42. 10.1002/eji.202048953 [DOI] [PubMed] [Google Scholar]
- 44. Kirchhammer N, Trefny MP, Natoli Met al. NK cells with tissue-resident traits shape response to immunotherapy by inducing adaptive antitumor immunity. Sci Transl Med 2022; 14(653):eabm9043. 10.1126/scitranslmed.abm9043 [DOI] [PubMed] [Google Scholar]
- 45. Berrien-Elliott MM, Jacobs MT, Fehniger TA.. Allogeneic natural killer cell therapy. Blood 2023; 141(8):856–68. 10.1182/blood.2022016200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morris EC, Neelapu SS, Giavridis Tet al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2022; 22(2):85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu E, Marin D, Banerjee Pet al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020; 382(6):545–53. 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olson JA, Leveson-Gower DB, Gill Set al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010; 115(21):4293–301. 10.1182/blood-2009-05-222190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rautela J, Huntington ND.. IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol 2017; 44:1–6. 10.1016/j.coi.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 50. Ahmadzadeh M, Rosenberg SA.. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107(6):2409–14. 10.1182/blood-2005-06-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Felices M, Lenvik AJ, McElmurry Ret al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018; 3(3):e96219. 10.1172/jci.insight.96219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romee R, Cooley S, Berrien-Elliott MMet al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018; 131(23):2515–27. 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Foltz JA, Hess BT, Bachanova Vet al. Phase I trial of N-803, an IL15 receptor agonist, with rituximab in patients with indolent non-Hodgkin lymphoma. Clin Cancer Res 2021; 27(12):3339–50. 10.1158/1078-0432.CCR-20-4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rhode PR, Egan JO, Xu Wet al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res 2016; 4(1):49–60. 10.1158/2326-6066.CIR-15-0093-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Felices M, Wesley E, Bendzick LEet al. Reverse translation identifies the synergistic role of immune checkpoint blockade and IL15 to enhance immunotherapy of ovarian cancer. Cancer Immunol Res 2023; 11(5):674–86. 10.1158/2326-6066.CIR-22-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berrien-Elliott MM, Foltz JA, Russler-Germain DAet al. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci Transl Med 2022; 14(633):eabm1375. 10.1126/scitranslmed.abm1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berrien-Elliott MM, Becker-Hapak M, Cashen AFet al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood 2022; 139(8):1177–83. 10.1182/blood.2021011532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nahi H, Chrobok M, Meinke Set al. Autologous NK cells as consolidation therapy following stem cell transplantation in multiple myeloma. Cell Rep Med 2022; 3(2):100508. 10.1016/j.xcrm.2022.100508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cichocki F, Zhang B, Wu C-Yet al. Nicotinamide enhances natural killer cell function and yields remissions in patients with non-Hodgkin lymphoma. Sci Transl Med 2023; 15(705):eade3341. 10.1126/scitranslmed.ade3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Becker-Hapak MK, Shrestha N, McClain Eet al. A fusion protein complex that combines IL-12, IL-15, and IL-18 signaling to induce memory-like NK cells for cancer immunotherapy. Cancer Immunol Res 2021; 9(9):1071–87. 10.1158/2326-6066.CIR-20-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cappell KM, Kochenderfer JN.. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol 2023; 20(6):359–71. 10.1038/s41571-023-00754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Y, Rezvani K, Rafei H.. Next-generation chimeric antigen receptors for T- and natural killer-cell therapies against cancer. Immunol Rev 2023; 320(1):217–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Laskowski TJ, Biederstadt A, Rezvani K.. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer 2022; 22(10):557–75. 10.1038/s41568-022-00491-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kilgour MK, Bastin DJ, Lee S-Het al. Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy. Front Immunol 2023; 14:1166038. 10.3389/fimmu.2023.1166038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Merino A, Maakaron J, Bachanova V.. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood Rev 2023; 60:101073. 10.1016/j.blre.2023.101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cichocki F, Goodridge JP, Bjordahl Ret al. Dual antigen-targeted off-the-shelf NK cells show durable response and prevent antigen escape in lymphoma and leukemia. Blood 2022; 140(23):2451–62. 10.1182/blood.2021015184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cichocki F, Bjordahl R, Goodridge JPet al. Quadruple gene-engineered natural killer cells enable multi-antigen targeting for durable antitumor activity against multiple myeloma. Nat Commun 2022; 13(1):7341. 10.1038/s41467-022-35127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li Y, Basar R, Wang Get al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med 2022; 28(10):2133–44. 10.1038/s41591-022-02003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burger MC, Forster M, Romanski Aet al. Intracranial injection of NK cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro Oncol 2023;25(11):2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zuo P, Li Y, He Cet al. Anti-tumor efficacy of anti-GD2 CAR NK-92 cells in diffuse intrinsic pontine gliomas. Front Immunol 2023; 14:1145706. 10.3389/fimmu.2023.1145706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mensali N, Dillard P, Hebeisen Met al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine 2019; 40:106–17. 10.1016/j.ebiom.2019.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. https://www.zelluna.com/pipeline; 2023. [06 September 2023, date last accessed].
- 73. https://replay.bio/news/replay-and-md-anderson-announce-fda-clearance-of-ind; 2023. [06 September 2023, date last accessed].
- 74. Poorebrahim M, Quiros-Fernandez I, Marmé Fet al. A costimulatory chimeric antigen receptor targeting TROP2 enhances the cytotoxicity of NK cells expressing a T cell receptor reactive to human papillomavirus type 16 E7. Cancer Lett 2023; 566:216242. 10.1016/j.canlet.2023.216242 [DOI] [PubMed] [Google Scholar]
- 75. Li L, Mohanty V, Dou Jet al. Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering. Sci Adv 2023; 9(30):eadd6997. 10.1126/sciadv.add6997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Daher M, Basar R, Gokdemir Eet al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 2021; 137(5):624–36. 10.1182/blood.2020007748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu H, Blum RH, Bernareggi Det al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 2020; 27(2):224–237.e6. 10.1016/j.stem.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dong H, Ham JD, Hu Get al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc Natl Acad Sci U S A 2022; 119(25):e2122379119. 10.1073/pnas.2122379119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gang M, Marin ND, Wong Pet al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020; 136(20):2308–18. 10.1182/blood.2020006619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dahlvang JD, Dick JK, Sangala JAet al. Ablation of SYK kinase from expanded primary human NK cells via CRISPR/Cas9 enhances cytotoxicity and cytokine production. J Immunol 2023; 210(8):1108–22. 10.4049/jimmunol.2200488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Knelson EH, Ivanova EV, Tarannum Met al. Activation of tumor-cell STING primes NK-cell therapy. Cancer Immunol Res 2022; 10(8):947–61. 10.1158/2326-6066.CIR-22-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Da Y, Liu Y, Hu Yet al. STING agonist cGAMP enhances anti-tumor activity of CAR-NK cells against pancreatic cancer. Oncoimmunology 2022; 11(1):2054105. 10.1080/2162402X.2022.2054105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhuang X, Long EO.. NK cells equipped with a chimeric antigen receptor that overcomes inhibition by HLA class I for adoptive transfer of CAR-NK cells. Front Immunol 2022; 13:840844. 10.3389/fimmu.2022.840844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gurney M, Stikvoort A, Nolan Eet al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 2022; 107(2):437–45. 10.3324/haematol.2020.271908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Drent E, Themeli M, Poels Ret al. A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Mol Ther 2017; 25(8):1946–58. 10.1016/j.ymthe.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hatjiharissi E, Xu L, Santos DDet al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the FcgammaRIIIa-158 V/V and V/F polymorphism. Blood 2007; 110(7):2561–4. 10.1182/blood-2007-01-070656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scott AM, Wolchok JD, Old LJ.. Antibody therapy of cancer. Nat Rev Cancer 2012; 12(4):278–87. 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 88. Klanova M, Oestergaard MZ, Trněný Met al. Prognostic impact of natural killer cell count in follicular lymphoma and diffuse large B-cell lymphoma patients treated with immunochemotherapy. Clin Cancer Res 2019; 25(15):4634–43. 10.1158/1078-0432.CCR-18-3270 [DOI] [PubMed] [Google Scholar]
- 89. Varchetta S, Gibelli N, Oliviero Bet al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res 2007; 67(24):11991–9. 10.1158/0008-5472.CAN-07-2068 [DOI] [PubMed] [Google Scholar]
- 90. Phung SK, Miller JS, Felices M.. Bi-specific and Tri-specific NK cell engagers: the new avenue of targeted NK cell immunotherapy. Mol Diagn Ther 2021; 25(5):577–92. 10.1007/s40291-021-00550-6 [DOI] [PubMed] [Google Scholar]
- 91. Demaria O, Gauthier L, Vetizou Met al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell Rep Med 2022; 3(10):100783. 10.1016/j.xcrm.2022.100783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Felices M, Kodal B, Hinderlie Pet al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv 2019; 3(6):897–907. 10.1182/bloodadvances.2018029371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chiu E, Felices M, Cichocki Fet al. Anti-NKG2C/IL-15/anti-CD33 killer engager directs primary and iPSC-derived NKG2C(+) NK cells to target myeloid leukemia. Mol Ther 2021; 29(12):3410–21. 10.1016/j.ymthe.2021.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arvindam US, van Hauten PMM, Schirm Det al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 2021; 35(6):1586–96. 10.1038/s41375-020-01065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gauthier L, Morel A, Anceriz Net al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019; 177(7):1701–1713.e16. 10.1016/j.cell.2019.04.041 [DOI] [PubMed] [Google Scholar]
- 96. Colomar-Carando N, Gauthier L, Merli Pet al. Exploiting natural killer cell engagers to control pediatric B-cell precursor acute lymphoblastic leukemia. Cancer Immunol Res 2022; 10(3):291–302. 10.1158/2326-6066.CIR-21-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gauthier L, Virone-Oddos A, Beninga Jet al. Control of acute myeloid leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat Biotechnol 2023; 41(9):1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.https://meetings.asco.org/abstracts-presentations/219910
- 99. Kerbauy LN, Marin ND, Kaplan Met al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30(+) malignancies. Clin Cancer Res 2021; 27(13):3744–56. 10.1158/1078-0432.CCR-21-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vallera DA, Oh F, Kodal Bet al. A HER2 tri-specific NK cell engager mediates efficient targeting of human ovarian cancer. Cancers (Basel) 2021; 13(16):3994. 10.3390/cancers13163994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kennedy PR, Vallera DA, Ettestad Bet al. A tri-specific killer engager against mesothelin targets NK cells towards lung cancer. Front Immunol 2023; 14:1060905. 10.3389/fimmu.2023.1060905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kaminski MF, Bendzick L, Hopps Ret al. TEM8 tri-specific killer engager binds both tumor and tumor stroma to specifically engage natural killer cell anti-tumor activity. J ImmunoTher Cancer 2022; 10(9):e004725. 10.1136/jitc-2022-004725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kohrt HE, Thielens A, Marabelle Aet al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123(5):678–86. 10.1182/blood-2013-08-519199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Felices M, Miller JS.. Targeting KIR blockade in multiple myeloma: trouble in checkpoint paradise? Clin Cancer Res 2016; 22(21):5161–3. 10.1158/1078-0432.CCR-16-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Carlsten M, Korde N, Kotecha Ret al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin Cancer Res 2016; 22(21):5211–22. 10.1158/1078-0432.CCR-16-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sheffer M, Lowry E, Beelen Net al. Genome-scale screens identify factors regulating tumor cell responses to natural killer cells. Nat Genet 2021; 53(8):1196–206. 10.1038/s41588-021-00889-w [DOI] [PubMed] [Google Scholar]
- 107. van Hall T, André P, Horowitz Aet al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J ImmunoTher Cancer 2019; 7(1):263. 10.1186/s40425-019-0761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Andre P, Denis C, Soulas Cet al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK Cells. Cell 2018; 175(7):p. 1731–1743 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marin R, Ruiz-Cabello F, Pedrinaci Set al. Analysis of HLA-E expression in human tumors. Immunogenetics 2003; 54(11):767–75. 10.1007/s00251-002-0526-9 [DOI] [PubMed] [Google Scholar]
- 110. Levy EM, Bianchini M, Von Euw EMet al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol 2008; 32(3):633–41. [PubMed] [Google Scholar]
- 111. Nguyen S, Beziat V, Dhedin Net al. HLA-E upregulation on IFN-gamma-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant 2009; 43(9):693–9. 10.1038/bmt.2008.380 [DOI] [PubMed] [Google Scholar]
- 112. Fisher JG, Doyle ADP, Graham LVet al. XPO1 inhibition sensitises CLL cells to NK cell mediated cytotoxicity and overcomes HLA-E expression. Leukemia 2023; 37:2036–49. 10.1038/s41375-023-01984-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Enqvist M, Jacobs B, Junlén HRet al. Systemic and intra-nodal activation of NK cells after rituximab monotherapy for follicular lymphoma. Front Immunol 2019; 10:2085. 10.3389/fimmu.2019.02085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liu X, Song J, Zhang Het al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell 2023; 41(2):272–287.e9. 10.1016/j.ccell.2023.01.001 [DOI] [PubMed] [Google Scholar]
- 115. Frazao A, Messaoudene M, Nunez Net al. CD16(+)NKG2A(high) Natural Killer Cells Infiltrate Breast Cancer-Draining Lymph Nodes. Cancer Immunol Res 2019; 7(2):208–18. 10.1158/2326-6066.CIR-18-0085 [DOI] [PubMed] [Google Scholar]
- 116. Fisher JG, Doyle ADP, Graham LVet al. Disruption of the NKG2A:HLA-E immune checkpoint axis to enhance NK cell activation against cancer. Vaccines (Basel) 2022; 10(12):1993. 10.3390/vaccines10121993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van Montfoort N, Borst L, Korrer MJet al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 2018; 175(7):1744–1755.e15. 10.1016/j.cell.2018.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cazzetta V, Bruni E, Terzoli Set al. NKG2A expression identifies a subset of human Vdelta2 T cells exerting the highest antitumor effector functions. Cell Rep 2021; 37(3):109871. 10.1016/j.celrep.2021.109871 [DOI] [PubMed] [Google Scholar]
- 119. Ravindranath MH, Filippone EJ, Devarajan Aet al. Enhancing natural killer and CD8(+) T cell-mediated anticancer cytotoxicity and proliferation of CD8(+) T cells with HLA-E monospecific monoclonal antibodies. Monoclon Antib Immunodiagn Immunother 2019; 38(2):38–59. 10.1089/mab.2018.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bexte T, Alzubi J, Reindl LMet al. CRISPR-Cas9 based gene editing of the immune checkpoint NKG2A enhances NK cell mediated cytotoxicity against multiple myeloma. Oncoimmunology 2022; 11(1):2081415. 10.1080/2162402X.2022.2081415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yun HD, Schirm DK, Felices Met al. Dinaciclib enhances natural killer cell cytotoxicity against acute myelogenous leukemia. Blood Adv 2019; 3(16):2448–52. 10.1182/bloodadvances.2019000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fisher JG, Walker CJ, Doyle ADet al. Selinexor enhances NK cell activation against malignant B cells via downregulation of HLA-E. Front Oncol 2021; 11:785635. 10.3389/fonc.2021.785635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Carlsten M, Namazi A, Reger Ret al. Bortezomib sensitizes multiple myeloma to NK cells via ER-stress-induced suppression of HLA-E and upregulation of DR5. Oncoimmunology 2019; 8(2):e1534664. 10.1080/2162402X.2018.1534664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Delconte RB, Kolesnik TB, Dagley LFet al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016; 17(7):816–24. 10.1038/ni.3470 [DOI] [PubMed] [Google Scholar]
- 125. Sudholz H, Delconte RB, Huntington ND.. Interleukin-15 cytokine checkpoints in natural killer cell anti-tumor immunity. Curr Opin Immunol 2023; 84:102364. 10.1016/j.coi.2023.102364 [DOI] [PubMed] [Google Scholar]
- 126. Bernard PL, Delconte R, Pastor Set al. Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity. J ImmunoTher Cancer 2022; 10(5):e004244. 10.1136/jitc-2021-004244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Souza-Fonseca-Guimaraes F, Rossi GR, Dagley LFet al. TGFbeta and CIS inhibition overcomes NK-cell suppression to restore antitumor immunity. Cancer Immunol Res 2022; 10(9):1047–54. 10.1158/2326-6066.CIR-21-1052 [DOI] [PubMed] [Google Scholar]
- 128. Shaim H, Shanley M, Basar Ret al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest 2021; 131(14):e142116. 10.1172/JCI142116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rautela J, Dagley LF, de Oliveira CCet al. Therapeutic blockade of activin-A improves NK cell function and antitumor immunity. Sci Signal 2019; 12(596):eaat7527. 10.1126/scisignal.aat7527 [DOI] [PubMed] [Google Scholar]
- 130. Klopotowska M, Bajor M, Graczyk-Jarzynka Aet al. PRDX-1 supports the survival and antitumor activity of primary and CAR-modified NK cells under oxidative stress. Cancer Immunol Res 2022; 10(2):228–44. 10.1158/2326-6066.CIR-20-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Q, Bi J, Zheng Xet al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018; 19(7):723–32. 10.1038/s41590-018-0132-0 [DOI] [PubMed] [Google Scholar]
- 132. Davis Z, Felices M, Lenvik Tet al. Low-density PD-1 expression on resting human natural killer cells is functional and upregulated after transplantation. Blood Adv 2021; 5(4):1069–80. 10.1182/bloodadvances.2019001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hsu J, Hodgins JJ, Marathe Met al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest 2018; 128(10):4654–68. 10.1172/JCI99317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pan R, Ryan J, Pan Det al. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell 2022; 185(9):1521–1538.e18. 10.1016/j.cell.2022.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hotz C, Wagenaar TR, Gieseke Fet al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci Transl Med 2021; 13(610):eabc7804. 10.1126/scitranslmed.abc7804 [DOI] [PubMed] [Google Scholar]
- 136. Myers JA, Schirm D, Bendzick Let al. Balanced engagement of activating and inhibitory receptors mitigates human NK cell exhaustion. JCI Insight 2022; 7(15):e150079. 10.1172/jci.insight.150079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.