Abstract
Although it is known that Candida albicans causes endothelial cell injury, in vitro and in vivo, the mechanism by which this process occurs remains unknown. Iron is critical for the induction of injury in many types of host cells. Therefore, we investigated the role of iron in Candida-induced endothelial cell injury. We found that pretreatment of endothelial cells with the iron chelators phenanthroline and deferoxamine protected them from candidal injury, even though the organisms germinated and grew normally. Loading endothelial cells with iron reversed the cytoprotective effects of iron chelation. Moreover, chelation of endothelial cell iron significantly reduced phagocytosis of C. albicans by these cells, while candidal adherence to chelator-treated endothelial cells was slightly enhanced. Since endothelial cell phagocytosis of C. albicans is required for endothelial cell injury to occur, inhibition of phagocytosis is likely the principal mechanism of the cytoprotective effects of iron chelation. The production of toxic reactive oxygen intermediates by host cells is known to be inhibited by iron chelation. Therefore, we investigated whether treating endothelial cells with antioxidants could mimic the cytoprotective effects of iron chelation. Neither extracellular nor membrane-permeative antioxidants reduced candidal injury of endothelial cells. Furthermore, depleting endothelial cells of the endogenous antioxidant glutathione did not render them more susceptible to damage by C. albicans. These results suggest that candidal injury of endothelial cells is independent of the production of reactive oxygen intermediates and that the cytoprotective effects of iron chelation are not due to inhibition of the synthesis of these toxic intermediates.
During the process of hematogenous dissemination, Candida albicans must first cross the endothelial cell lining of the vasculature to invade the tissue parenchyma. One mechanism by which the organism may escape from the vascular compartment is by causing endothelial cell injury. This injury likely results in exposure of the subendothelial cell basement membrane, which may enhance candidal adherence and facilitate tissue invasion (23). We have been investigating the mechanisms by which C. albicans injures endothelial cells in vitro. Previously, we found that phagocytosis of the organism by endothelial cells is required for endothelial cell damage to occur (8, 9). This phagocytosis requires both intact endothelial cell microfilaments and microtubules. In addition, although endothelial cells are able to phagocytize killed organisms (35), only the phagocytosis of live, germinating organisms causes endothelial cell injury (8).
After endothelial cells phagocytize C. albicans, the organism may injure the endothelial cells by several potential mechanisms. It is possible that phospholipases and/or proteinases secreted by C. albicans injure host cells (6, 18, 38). Another possibility is that C. albicans causes endothelial cell injury by an iron-dependent process. For example, endothelial cells are known to synthesize and release superoxide anions during phagocytic activity (11, 14). Iron is required for the assembly of enzymes, such as xanthine oxidase (33) and cytochromes (20), that catalyze the synthesis of these reactive oxygen intermediates. In addition, iron serves as a cofactor, which converts these anions to highly reactive hydroxyl radicals (21) which cause cellular damage (10, 24). Finally, iron is required for the function of nitric oxide synthase, an enzyme that can catalyze the synthesis of cytotoxic concentrations of nitric oxide in some cell types (31).
In this study, we used the iron chelators phenanthroline and deferoxamine to examine the role of iron in endothelial cell injury caused by C. albicans. We found that chelation of endothelial cell iron protected these cells from injury by C. albicans. The cytoprotective effects of iron chelation were likely due to reduced phagocytosis of this fungus by endothelial cells. Furthermore, we found that C. albicans damages endothelial cells by a process that is likely independent of the production of reactive oxygen intermediates.
(This work was presented in part at the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 1995, San Francisco, Calif.)
MATERIALS AND METHODS
Reagents.
All the reagents used, unless otherwise noted, were purchased from the Sigma Chemical Company, St. Louis, Mo.
Organism.
C. albicans ATCC 36082, originally a clinical isolate, was obtained from the American Type Culture Collection (Rockville, Md.). The organisms were grown overnight, at room temperature on a rotating drum, in yeast nitrogen base broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% dextrose as described previously (35). They were harvested by centrifugation and washed three times in 0.85% saline. The blastospores were counted in a hemacytometer and adjusted to the desired concentration in Hanks balanced salt solution (HBSS) (Irvine Scientific, Santa Ana, Calif.).
Endothelial cells.
Endothelial cells were harvested from human umbilical veins by the method of Jaffe et al. (19). The cells were grown in M-199 (Gibco, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (Intergen, Purchase, N.Y.), 10% calf serum (Hyclone, Logan, Utah), and 2 mM l-glutamine with penicillin and streptomycin (Irvine Scientific). For use in damage and adherence assays, third-passage endothelial cells were grown on a collagen matrix (Vitrogen; Celtrix, Palo Alto, Calif.) in either 24-well or 6-well tissue culture plates (Falcon, Lincoln Park, N.J.). All incubations were at 37°C in 5% CO2.
Quantification of endothelial cell damage.
The degree of endothelial cell injury caused by C. albicans was determined by measuring the release of 51Cr as described previously (8). Briefly, confluent endothelial cells in 24-well plates were incubated for 24 to 48 h in M-199 containing Na251CrO4 (ICN Biomedicals, Irvine, Calif.) (6 μCi per ml). The next day, the unincorporated chromium was aspirated, and the wells were washed three times with warm HBSS. Next, 106 C. albicans blastospores in 1 ml of HBSS were added to each well, and the plate was incubated for 3 h. At the end of the incubation period, 0.5 ml of the medium was removed from each well, after which 0.5 ml of 6 N NaOH was added to lyse the endothelial cells. The lysed cells were aspirated, and the wells were then washed twice with Radiac Wash (Biodex Medical Systems, Shirley, N.Y.). The rinses from each well were combined with the lysed cells. The amounts of 51Cr in the media and the lysates were determined by gamma counting. Control wells containing endothelial cells without C. albicans were processed in parallel to determine the spontaneous release of 51Cr. After correcting for the differences in the amount of 51Cr that was incorporated in each well, the specific release of 51Cr was calculated by the following formula: (2 × experimental release − 2 × spontaneous release)/(total incorporation − 2 × spontaneous release). We have determined previously that 51Cr released by the endothelial cells is not incorporated by C. albicans (7). All experiments were performed on at least three different days with endothelial cells from different umbilical cords.
Chelation of endothelial cell iron.
To evaluate the effects of chelating endothelial cell iron on injury caused by C. albicans, endothelial cells were incubated with selected concentrations of phenanthroline for 1 to 24 h or with deferoxamine for 48 h. The phenanthroline was prepared fresh daily by first dissolving it in methanol and then diluting it 1:100 in HBSS. Next, an aliquot of the diluted phenanthroline was added to the medium in which the endothelial cells were growing. Control wells of endothelial cells received an equal amount of diluted methanol without phenanthroline. The maximum final concentration of methanol was 0.06%. The deferoxamine was dissolved in dimethyl sulfoxide (DMSO) and then added to the endothelial cell growth medium so that the final concentration of deferoxamine was 0.4 mM. An equal amount of diluted DMSO (final concentration, 0.4%) without deferoxamine was added to control wells. To prevent chelation of the radioisotope, the 51Cr was added to the endothelial cells 24 h prior to the addition of phenanthroline or 24 h after the addition of deferoxamine. Treating endothelial cells with iron chelators does not interfere with quantifying cellular damage by the release of 51Cr (16, 33).
On the day of the experiment, unincorporated chelator and 51Cr were aspirated. Next, the endothelial cells were rinsed and then exposed to C. albicans suspended in HBSS in the absence of phenanthroline or deferoxamine as described above. Control wells of endothelial cells were incubated with either of the chelators and then exposed to HBSS alone to detect any toxicity caused by these agents.
To confirm that the effects of phenanthroline and deferoxamine on C. albicans-mediated endothelial cell injury were due to chelation of endothelial cell iron, exogenous iron in the form of hemin or ferric ammonium citrate was added to the endothelial cells (2, 30). Hemin was added to the endothelial cells at a final concentration of 20 μM, 2 h before the phenanthroline. The endothelial cells were incubated with 120 μM ferric ammonium citrate for 30 min before addition of phenanthroline to the growth medium. These concentrations were the highest that did not cause detectable endothelial cell toxicity in the absence of C. albicans and iron chelators (data not shown).
Antioxidants.
The antioxidants used to inhibit reactive oxygen intermediates produced by the Candida-infected endothelial cells are listed in Table 1. The effects of these antioxidants on endothelial cell injury caused by C. albicans were determined by 51Cr release assay as described above. Catalase, superoxide dismutase (SOD), and mannitol were added to the endothelial cells simultaneously with C. albicans. Endothelial cells were pretreated with allopurinol for 18 h and with dimethylpyrroline-N-oxide (DMPO) or dimethylthiourea (DMTU) for 2 h, prior to addition of the organisms. All of these inhibitors were present while the endothelial cells were infected with C. albicans. The toxicities of these antioxidants were determined by adding them to endothelial cells in the absence of C. albicans. None of these antioxidants were toxic to the endothelial cells, as determined by the release of 51Cr (data not shown).
TABLE 1.
Antioxidants used to inhibit oxidant-mediated endothelial cell injury
| Antioxidant | Final concn | Diluent | Mechanism of action | Site of action | Reference(s) |
|---|---|---|---|---|---|
| DMPO | 10 mM | HBSS | Spin trapping of hydroxyl radicals and superoxide anions | Intracellular | 1, 4, 40 |
| DMTU | 10 mM | Methanol | Scavenging of hydroxyl radicals | Intracellular | 1, 32 |
| Allopurinol | 0.1 mM | DMSO | Inhibition of XO | Intracellular | 16 |
| Catalase | 10 U/ml | HBSS | Reduction of hydroxyl radicals to water | Extracellular | 16, 24 |
| SOD | 100 U/ml | Water | Conversion of superoxide anions to hydrogen peroxide | Extracellular | 16, 24, 40 |
| Mannitol | 100 mM | HBSS | Scavenging of hydroxyl radicals | Extracellular | 24, 32 |
To ensure that these antioxidants could protect endothelial cells from oxidant damage, these cells were exposed to superoxide anions generated by xanthine oxidase (XO) and hypoxanthine (HX) (1, 16). Endothelial cells were incubated with phenanthroline, deferoxamine, or the antioxidants as mentioned above and exposed to 5 mU of XO per ml and 1 mM HX (1). Control wells were incubated with XO alone, HX alone, and XO plus HX in the absence of antioxidants and the iron chelators. In these experiments the incubation time was increased to 5 h because the release of 51Cr during oxidant-mediated endothelial cell damage does not occur with shorter incubations (1).
To further investigate the possibility that candidal damage of endothelial cells is oxidant mediated, endothelial cell production of the antioxidant glutathione was inhibited with buthionine sulfoximine (BSO), an irreversible inhibitor of γ-glutamylcysteine synthase (5, 37). In these experiments, endothelial cells were incubated with 1 mM BSO for 18 h. Next, unincorporated BSO was rinsed off the endothelial cells prior to the addition of C. albicans in the absence of additional BSO.
Candidal adherence to endothelial cells.
The adherence of C. albicans to endothelial cells was measured as described previously (35). Endothelial cells in six-well tissue culture plates were incubated with 60 μM phenanthroline for 24 h as described above. Next, the chelating agent was removed by rinsing, and 102 C. albicans blastospores in HBSS were added to each well. The inoculum was confirmed by culturing an aliquot of the suspension in Sabouraud agar (Difco). After incubation for 45 min, the unbound organisms were aspirated, and each well of endothelial cells was rinsed twice with 10 ml of warm HBSS. Each well was then overlaid with molten Sabouraud dextrose agar, and the plate was incubated overnight at 37°C. The following day, the number of adherent CFU was determined. Adherence was expressed as the percentage of the original inoculum.
Endothelial cell phagocytosis of C. albicans.
Endothelial cell phagocytosis of C. albicans blastospores was measured by using a minor modification of our previously described method (9). First, the organisms were killed by exposure to 20 mM periodate for 30 min (8). Killed blastospores were used in these experiments to obviate the problem of incomplete phagocytosis that occurs when live, germinating organisms are used. We have shown previously that periodate-killed organisms are phagocytized by endothelial cells similarly to live organisms (8, 35). Next, 105 blastospores in HBSS were added to endothelial cells that had been pretreated with 60 μM phenanthroline for 24 h. Cytochalasin D (0.6 μM) was added along with the C. albicans to parallel coverslips to inhibit phagocytosis and serve as a negative control. After 3 h, the media were aspirated, and the endothelial cells were fixed with 3% paraformaldehyde. Organisms that had not been internalized by the endothelial cells were visualized by staining with a Texas red-labeled goat anti-Candida antibody (Biodesign International, Kennebunkport, Maine). Next, the endothelial cells were permeabilized with 0.1% (vol/vol) Triton X-100 in Dulbecco’s phosphate-buffered saline (PBS), and all organisms were stained with 1% (vol/vol) Uvitex in PBS (a generous gift from Jay Isharani, Ciba-Geigy, Greensboro, N.C.) (26). The coverslips were examined under epifluorescence with a Zeiss Axiovert 10 microscope (Carl Zeiss Inc., Thornwood, N.Y.). The number of phagocytized organisms was determined by subtracting the number of C. albicans that were labeled with Texas red (nonphagocytized organisms) from the number of organisms that were labeled with Uvitex (total organisms). Each experiment was performed in triplicate, and at least 100 organisms per coverslip were evaluated.
Germ tube elongation.
To ascertain if chelating endothelial cell iron inhibited the growth of C. albicans, 3 × 103 blastospores were allowed to germinate for 3 h on endothelial cells that had been pretreated with either phenanthroline (60 μM for 24 h) or deferoxamine (0.4 mM for 48 h) as in the damage assays. Control wells with untreated endothelial cells were processed in parallel. After 3 h, the medium was gently aspirated and the wells were fixed with 2% (vol/vol) glutaraldehyde in PBS. The wells were examined with an inverted phase-contrast microscope, and the lengths of 100 germ tubes per condition were measured with a micrometer (17).
Data analysis.
The effects of the different conditions were analyzed by using the Kruskal-Wallis test with the Bonferroni correction for multiple comparisons. P values of ≤0.05 were considered significant.
RESULTS
Iron chelators protected endothelial cells from injury by C. albicans.
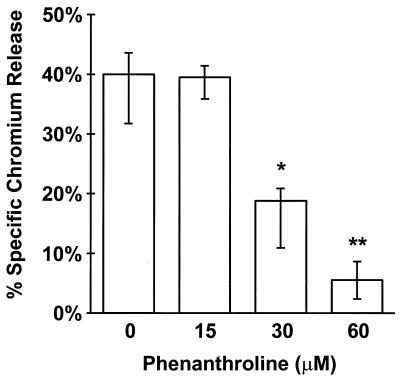
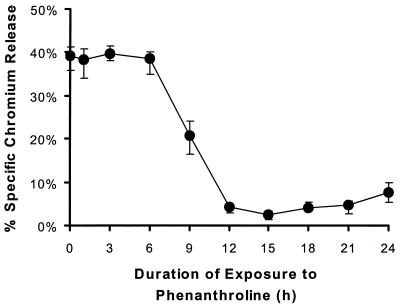
Pretreatment of endothelial cells with phenanthroline markedly reduced injury caused by C. albicans. The protective effect of phenanthroline was detectable at a concentration of 30 μM, and optimal protection occurred when endothelial cells were incubated with 60 μM phenanthroline (Fig. 1). At this concentration, candidal injury of endothelial cells was reduced by 78%. Higher concentrations of phenanthroline were not used because they were toxic to the endothelial cells (data not shown). At least 9 h of exposure to phenanthroline was required to prevent endothelial cell injury by C. albicans (Fig. 2). However, the maximal reduction in candidal damage did not occur until the endothelial cells had been incubated with phenanthroline for at least 12 h. The cytoprotective effect of iron chelation was confirmed by using deferoxamine. Preexposing endothelial cells to this iron chelator reduced Candida-mediated damage by a median of 48% compared to untreated control endothelial cells (Table 2).
FIG. 1.
Chelating endothelial cell iron with phenanthroline reduced C. albicans-mediated damage. Endothelial cells in 24-well tissue culture plates were loaded with 51Cr and then incubated with the indicated concentrations of phenanthroline for 24 h. The unabsorbed 51Cr and phenanthroline were rinsed off the endothelial cells, after which 106 C. albicans blastospores suspended in HBSS without phenanthroline were added to each well. The amount of C. albicans-mediated injury was measured after 3 h by the specific release of 51Cr. The results represent the medians from at least three experiments. The error bars indicate the first and third quartiles. ∗, P < 0.05 compared to the control; ∗∗, P < 0.001 compared to the control.
FIG. 2.
Effect of the duration of pretreatment with phenanthroline on protection of endothelial cells from C. albicans-mediated injury. Endothelial cells were incubated with 60 μM phenanthroline for the indicated times. Next, the unincorporated phenanthroline was removed, and the endothelial cells were exposed to C. albicans in the absence of chelator as described for Fig. 1. The extent of C. albicans-mediated injury was measured as the specific release of 51Cr. The results represent the medians from at least three experiments. The error bars indicate the first and third quartiles.
TABLE 2.
Effect of iron chelation on oxidant-mediated endothelial cell damage
| Treatment | Endothelial cell damage (median % specific release of 51Cr)a by:
|
|
|---|---|---|
| C. albicansb | XO-HXc | |
| Control | 39.7 (35.3–42.6) | 27.4 (20.7–35.3) |
| Catalase | 37.8 (34.3–41.4) | −0.1 (−0.9–0.5)d |
| SOD | 38.4 (35.3–41.4) | 22.7 (12.6–23.7)e |
| DMTU | 35.2 (33.8–39.4) | 4.2 (0.5–15.7)d |
| DMPO | 43.3 (39.2–45.2) | 11.3 (5.6–17.4)d |
| Allopurinol | 31.5 (24.7–32.8) | 11.5 (0.8–15.7)d |
| Mannitol | 47.5 (44.2–49.5) | 15.5 (13.2–16.4)e |
| BSO | 40.2 (36.8–41.7) | 32.2 (24.9–51.5)e |
| Deferoxamine | 20.7 (17.0–21.4)d | 7.9 (3.2–10.3)d |
| Phenanthroline (1 h) | 38.3 (33.9–40.7) | 11.8 (7.3–19.2)d |
| Phenanthroline (24 h) | 7.6 (5.4–9.9)d | 52.6 (37.6–61.6)e |
The numbers in parentheses indicate the first to third quartiles.
Endothelial cells were incubated for 3 h with 106 C. albicans blastospores/ml.
Endothelial cells were incubated for 5 h with 5 mU of XO per ml and 1 mM HX.
P < 0.001 versus the control.
P = 0.05 versus the control.
Incubation of the endothelial cells with either phenanthroline or deferoxamine had no visible effect on the growth and morphology of C. albicans when it was added to these cells. The median germ tube length of organisms grown on control endothelial cells was 42.0 μm (interquartile range, 35.0 to 50.0 μm), whereas the median germ tube lengths of organisms grown on endothelial cells exposed to phenanthroline and deferoxamine were 40.5 μm (interquartile range, 35.8 to 50.0 μm), and 40.0 μm (interquartile range, 33.0 to 45.0 μm), respectively (P > 0.05 for each comparison).
Phenanthroline and deferoxamine protected endothelial cells by chelating iron.
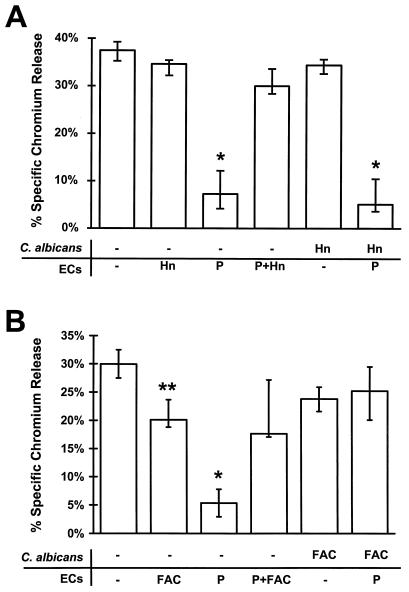
To determine if the protective effects of phenanthroline and deferoxamine were due to chelation of iron, the endothelial cells were supplied with exogenous iron in the form of hemin or ferric ammonium citrate, either before or after the addition of the chelators. When hemin or ferric ammonium citrate was added to endothelial cells prior to phenanthroline, the cytoprotective effects of this chelator were reversed (Fig. 3). Pretreating endothelial cells with hemin in the absence of chelator had no effect on Candida-mediated damage. However, endothelial cells that had been exposed to ferric ammonium citrate were slightly less susceptible to injury by C. albicans. Adding hemin along with deferoxamine to the endothelial cells also reversed the cytoprotective effects of this chelator (data not shown). These results suggest that both phenanthroline and deferoxamine reduce endothelial cell injury by an iron-dependent mechanism.
FIG. 3.
Iron reversed the cytoprotective effect of C. albicans-mediated injury to endothelial cells (ECs). (A) Endothelial cells were exposed to buffer (−), 60 μM phenanthroline (P), and/or 20 μM hemin (Hn) as described in Materials and Methods. After being rinsed, these cells were exposed for 3 h to C. albicans suspended in either HBSS alone or HBSS containing 20 μM hemin. The extent of endothelial cell injury was determined by the specific release of 51Cr. (B) Endothelial cells were exposed to the same conditions as for panel A except that 120 μM ferric ammonium citrate (FAC) was substituted for hemin. Results represent the medians from at least three experiments. The error bars indicate the first and third quartiles. ∗, P < 0.005 compared to control endothelial cells exposed to C. albicans alone; ∗∗, P = 0.04 compared to control endothelial cells.
Next, we examined the possibility that chelating endothelial cell iron prevented C. albicans from utilizing these cells as an iron source. Endothelial cells were pretreated with phenanthroline, and then C. albicans plus either hemin or ferric ammonium citrate was added simultaneously to these cells. Adding hemin in this manner had no effect on the ability of phenanthroline to protect endothelial cells from candidal injury (Fig. 3). However, adding ferric ammonium citrate and C. albicans simultaneously to phenanthroline-treated cells reversed the protective effect of iron chelation. Neither hemin nor ferric ammonium citrate altered the amount of candidal damage to untreated, control endothelial cells.
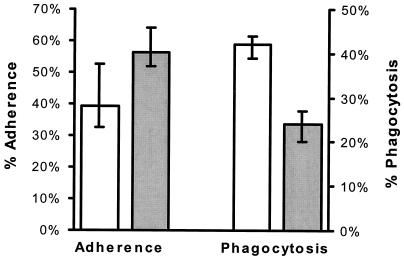
Phenanthroline enhanced adherence of C. albicans to endothelial cells while reducing endothelial cell phagocytosis of the organism.
We next investigated potential mechanisms by which iron chelation reduced candidal injury of endothelial cells. Since intimate contact between C. albicans and endothelial cells is required for endothelial cell damage to occur (8), we examined the effects of phenanthroline on candidal adherence to endothelial cells as well as on endothelial cell phagocytosis of C. albicans. Pretreating endothelial cells with phenanthroline enhanced their ability to bind C. albicans; 30% more organisms adhered to these cells than to untreated control cells (P = 0.005) (Fig. 4). In contrast to its effects on adherence, phenanthroline reduced endothelial cell phagocytosis of C. albicans by 43% (P = 0.002) (Fig. 4). Phenanthroline was at least as effective in inhibiting endothelial cell phagocytosis as was cytochalasin D. The latter agent reduced endothelial cell phagocytosis of the organism by a median of 33% (interquartile range, 26 to 40%) compared with untreated endothelial cells.
FIG. 4.
Phenanthroline had disparate effects on candidal adherence to endothelial cells and endothelial cell phagocytosis of C. albicans. Endothelial cells were exposed to either medium alone (open bars) or 60 μM phenanthroline (solid bars) for 24 h, after which adherence and phagocytosis were measured as described in Materials and Methods. The results represent the medians from three experiments, and the error bars indicate the first and third quartiles.
Iron chelation did not protect endothelial cells by inhibiting oxidant-mediated damage.
Iron plays a critical role in the synthesis of reactive oxygen intermediates. Therefore, we also examined the possibility that chelation of endothelial cell iron protected these cells from damage by C. albicans by inhibiting the production of these toxic anions. In these experiments, the abilities of different antioxidants to mimic the cytoprotective effects of iron chelation were determined. The extracellular oxidant scavengers catalase, SOD, and mannitol, as well as the intracellular scavengers allopurinol, DMPO, and DMTU, were used to prevent any oxidant-mediated damage induced by C. albicans. None of these antioxidants significantly inhibited candidal injury of endothelial cells (Table 2).
To ensure that these compounds could actually protect endothelial cells against oxidant-mediated damage, we incubated these cells with a mixture of XO and HX to generate superoxide anions. Damage caused by XO and HX was not detectable until the endothelial cells were incubated for 5 h with the reaction mixture (Table 2). Each of the antioxidants protected endothelial cells from injury caused by XO and HX.
In other experiments, the endothelial cells were depleted of glutathione by treatment with BSO to enhance their susceptibility to oxidant-mediated injury. We found that pretreating the endothelial cells with BSO did not alter the amount of injury caused by C. albicans (Table 2). However, BSO augmented the amount of damage caused by XO and HX by 38%. These data suggest that C. albicans likely injures endothelial cells by a mechanism that is independent of the production of reactive oxygen intermediates.
Phenanthroline had disparate effects on candidal and oxidant-mediated damage of endothelial cells, depending on the duration of exposure to the chelator. Pretreating the endothelial cells with phenanthroline for 24 h inhibited C. albicans-mediated injury, while damage caused by oxidants was actually augmented (Table 2). In contrast, when endothelial cells were pretreated with phenanthroline for 1 h, damage by C. albicans was not affected, while oxidant-mediated damage was inhibited. Unlike the case for phenanthroline, prolonged exposure (48 h) to deferoxamine reduced oxidant-mediated injury to endothelial cells by 75%.
DISCUSSION
In this study we found that pretreatment of endothelial cells with phenanthroline or deferoxamine prevented candidal injury to these cells. These effects were likely to be due to the chelation of endothelial cell iron, because the protective effects of the chelators could be reversed by adding excess iron to the endothelial cells in the form of either hemin or ferric ammonium citrate. Previously, other investigators have reported that chelating iron in host cells reduces the virulence of other fungal pathogens, such as Histoplasma capsulatum (25), Rhizopus species (3), and Pneumocystis carinii (39). The mechanism of protection in these experiments was the inhibition of fungal growth. Iron is also required for optimal growth and germination of C. albicans (22, 27). However, in the current study candidal growth was not inhibited when the organisms were exposed to endothelial cells that had been pretreated with iron chelators. This finding suggests that the cytoprotective effects of the iron chelators were due to their action on the endothelial cells and not their action on C. albicans.
Hemin and ferric ammonium citrate had disparate effects on endothelial cell injury when added along with C. albicans to the endothelial cells, after the iron chelators had been removed. Ferric ammonium citrate reversed the protective effect of phenanthroline, whereas hemin did not. One possible explanation for this finding is that endothelial cells absorbed ferric ammonium citrate faster than hemin, so the former compound was able to reverse the protective effects of phenanthroline during the 3 h when the organisms were in contact with the endothelial cells. Alternatively, it is possible that chelating endothelial cell iron down-regulates some candidal factor, other than germination, that is required for endothelial cell injury. Such a factor could potentially be up-regulated to a greater extent by ferric ammonium citrate than by hemin. Therefore, although it is likely that the cytoprotective effect of iron chelation is due mainly to the action of the chelators on the endothelial cells, we cannot completely exclude the possibility that exposure to chelator-treated endothelial cells may also have had subtle effects on the C. albicans.
Several potential mechanisms by which chelation of endothelial cell iron protects these cells from candidal injury were investigated. We examined whether iron chelation inhibited the adherence of C. albicans to endothelial cells and/or the phagocytosis of this organism by these cells, because we have found previously that the phagocytosis of C. albicans is required for endothelial cell injury to occur (8, 9). In addition, it is highly likely that the organisms must first adhere to endothelial cells before phagocytosis can occur. Surprisingly, we found that C. albicans exhibited enhanced adherence to endothelial cells that had been exposed to iron chelators. The mechanism by which this phenomenon occurred is unclear. It is possible that iron chelation up-regulates the receptors on endothelial cells to which C. albicans binds. However, these receptors have not yet been characterized.
Another finding was that phenanthroline inhibited endothelial cell phagocytosis of C. albicans. This reduction in phagocytosis is probably the principle mechanism of the cytoprotective effects of iron chelation. For example, we have found previously that inhibiting endothelial cell phagocytosis of C. albicans with cytochalasin D or gamma interferon also inhibits candidal injury of these cells (8, 9). To our knowledge, the effect of chelating iron on the phagocytic activity of endothelial cells has not been reported previously. However, other investigators have found that the phagocytic activities of both neutrophils and macrophages are reduced in the presence of iron deficiency (15, 28).
Although phagocytosis of C. albicans is a prerequisite for the induction of endothelial cell injury, additional factors are required for the injury process to occur. For instance, we have found that only the phagocytosis of live, germinated organisms causes endothelial cell injury. Neither nongerminating nor killed C. albicans is able to induce this process, even though they are phagocytized by the endothelial cells (8). Therefore, we examined whether chelation of endothelial cell iron influenced any postphagocytic events that may contribute to the development of endothelial cell injury. The role of reactive oxygen intermediates in Candida-induced endothelial cell injury was investigated because iron is critical for the production of these potentially toxic intermediates, and the phagocytosis of particulate stimuli, such as latex beads, is known to induce endothelial cells to generate them (14). In addition, Garcia et al. (11) have reported that the phagocytosis of asbestos fibers by endothelial cells causes significant injury to these cells. This fiber-induced endothelial cell injury is likely mediated by the production of reactive oxygen intermediates, because it can be blocked by SOD and catalase, as well as deferoxamine. However, unlike fiber-induced injury, candidal damage of endothelial cells occurs by a mechanism that is likely independent of the production of reactive oxygen intermediates. This conclusion is supported by our findings that (i) neither extracellular nor membrane-permeative antioxidants protected the endothelial cells from Candida-induced injury and (ii) depleting endothelial cells of the endogenous antioxidant glutathione did not enhance their susceptibility to candidal damage. Finally, the inability of the antioxidants to mimic the cytoprotective effects of iron chelation on endothelial cell injury caused by C. albicans suggests that the salutary effects of iron chelation occur independently of any inhibition of the synthesis of reactive oxygen intermediates.
While performing these experiments, we found that pretreatment with phenanthroline for only 1 h was required to inhibit cellular damage caused by exogenously generated superoxide, whereas this duration of pretreatment was completely ineffective in preventing injury by C. albicans. Moreover, when endothelial cells were exposed to this iron chelator for 24 h, oxidant-mediated cellular damage was actually increased even though Candida-mediated damage was inhibited. Why prolonged exposure to phenanthroline increased the susceptibility of the endothelial cells to damage by exogenously generated oxidants is unclear. It is possible that prolonged exposure of the endothelial cells to phenanthroline caused some subtle toxicity, such as the depletion of endogenous antioxidants, that rendered them more susceptible to oxidant-mediated injury. Nevertheless, enhanced susceptibility to exogenous oxidants was not observed with deferoxamine, even after 48 h of incubation. In addition, our finding that exogenously generated reactive oxygen intermediates caused chelator-treated endothelial cells to release 51Cr indicates that pretreating these cells with the iron chelators did not interfere with the measurement of endothelial cell injury.
A potential mechanism by which iron chelation may protect endothelial cells from injury by C. albicans is by inhibiting the activity of inducible nitric oxide synthase. Phagocytosis has been found to stimulate the activity of this iron-dependent enzyme in host cells (13). Therefore, it is possible that the phagocytosis of C. albicans induces nitric oxide synthase activity in endothelial cells. However, although murine endothelial cells are capable of producing toxic concentrations of nitric oxide (31), these cells synthesize at least 100-fold more nitric oxide than do human umbilical vein endothelial cells (29, 34). Therefore, it is unlikely that candidal damage of the endothelial cells used in the current study was mediated by nitric oxide.
In conclusion, the data from this study suggest that normal endothelial cell iron metabolism is required for endothelial cells to phagocytize and be damaged by C. albicans. Unlike mechanisms of protection seen in other studies, reduction in endothelial cell injury by iron chelation did not appear to be mediated by inhibition of the production of reactive oxygen intermediates or by restriction of candidal germination and growth. Future investigations on the mechanism by which iron chelation reduces endothelial cell phagocytosis of and injury by C. albicans will focus on other iron-dependent endothelial cell processes, such as cytochrome activity (20), metalloproteinase synthesis (36), and mitochondrial function (12).
ACKNOWLEDGMENTS
We thank the perinatal nurses at Harbor-UCLA and Torrance Memorial Medical Centers for collecting umbilical cords, Alison Orozco and Brad Spellberg for helping with tissue culture, and Toyota USA for donating the Olympus phase-contrast microscope.
This work was supported in part by Public Health Service grants AI-19990, AI-37194, and MO1 RR00425 from the National Institutes of Health and by a grant-in-aid to S.G.F. from the American Heart Association, Greater Los Angeles Affiliate.
REFERENCES
- 1.Andreoli S P, McAteer J A. Reactive oxygen molecule mediated injury in endothelial and renal tubular epithelial cells in vitro. Kidney Int. 1990;38:785–794. doi: 10.1038/ki.1990.272. [DOI] [PubMed] [Google Scholar]
- 2.Balla J, Jacob H S, Balla G, Nath K, Eaton J W. Endothelial-cell heme uptake from proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelaert J R, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt H W, Schneider J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buettner G R. The spin trapping of superoxide and hydroxyl free radicals with DMPO (5,5-dimethylpyrroline-N-oxide): more about iron. Free Radical Res Commun. 1993;19:S79–S87. doi: 10.3109/10715769309056s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotgreave I A, Constantin-Teodosiu D, Moldeus P. Nonxenobiotic manipulation and sulfur precursor specificity of human endothelial cell glutathione. J Appl Physiol. 1991;70:1220–1227. doi: 10.1152/jappl.1991.70.3.1220. [DOI] [PubMed] [Google Scholar]
- 6.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 7.Edwards J E, Jr, Rotrosen D, Fontaine J W, Haudenschild C C, Diamond R D. Neutrophil-mediated protection of cultured human vascular endothelial cells from damage by growing Candida albicans hyphae. Blood. 1987;69:1450–1457. [PubMed] [Google Scholar]
- 8.Filler S F, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fratti R A, Ghannoum M A, Edwards J E, Jr, Filler S G. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect Immun. 1996;64:4714–4718. doi: 10.1128/iai.64.11.4714-4718.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon D E, Varani J, Phan S H, Ward J H, Kaplan J, Till G O, Simon R H, Ryan U S, Ward P. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987;57:37–44. [PubMed] [Google Scholar]
- 11.Garcia J G N, Dodson R F, Callahan K S. Effect of environmental particulates on cultured human and bovine endothelium. Cellular injury via an oxidant-dependent pathway. Lab Invest. 1989;61:53–60. [PubMed] [Google Scholar]
- 12.Gerber E, Bredy A, Kahl R. Ortho-phenanthroline modulates enzymes of cellular energy metabolism. Toxicology. 1996;110:85–93. doi: 10.1016/0300-483x(96)03331-8. [DOI] [PubMed] [Google Scholar]
- 13.Goodrum K J, McCormick L L, Schneider B. Group B streptococcus-induced nitric oxide production in murine macrophages is CR3 (CD11b/CD18) dependent. Infect Immun. 1994;62:3102–3107. doi: 10.1128/iai.62.8.3102-3107.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorog P, Pearson J D, Kakkar V V. Generation of reactive oxygen metabolites by phagocytosing endothelial cells. Atherosclerosis. 1988;72:19–27. doi: 10.1016/0021-9150(88)90058-5. [DOI] [PubMed] [Google Scholar]
- 15.Hasan S M, Aziz M, Ahmad P, Aggarwal M. Phagocyte metabolic functions in iron deficiency anaemia of Indian children. J Trop Ped. 1989;35:6–9. doi: 10.1093/tropej/35.1.6-a. [DOI] [PubMed] [Google Scholar]
- 16.Hiraishi H, Terano A, Razandi M, Pedram A, Sugimoto T, Harada T, Ivey K J. Reactive oxygen metabolite-induced toxicity to cultured bovine endothelial cells: status of cellular iron mediating injury. J Cell Physiol. 1994;160:132–140. doi: 10.1002/jcp.1041600116. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim A S, Filler S G, Ghannoum M A, Edwards J E., Jr Interferon-gamma protects endothelial cells from damage by Candida albicans. J Infect Dis. 1993;167:2239–2244. doi: 10.1093/infdis/167.6.1467. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim A S, Mirbod F, Filler S G, Banno Y, Cole G T, Kitajima Y, Edwards J E, Jr, Nozawa Y, Ghannoum M A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–1998. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe E A, Nachman R L, Becker C G, Ninick C R. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones C W, Poole R K. The analysis of cytochromes. Methods Microbiol. 1985;18:285–328. [Google Scholar]
- 21.Karwatowska-Prokopczuk E, Czarnowska E, Beresewicz A. Iron availability and free radical induced injury in the isolated ischaemic/reperfused rat heart. Cardiovasc Res. 1992;26:58–66. doi: 10.1093/cvr/26.1.58. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick C H, Green I, Rich R R, Schade A L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971;124:539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- 23.Klotz S A, Maca R D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988;56:2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvietys P R, Inauen W, Bacon B R, Grisham M B. Xanthine oxidase-injury to endothelium: role of intracellular iron and hydroxyl radical. Am J Physiol. 1989;257:H1640–H1646. doi: 10.1152/ajpheart.1989.257.5.H1640. [DOI] [PubMed] [Google Scholar]
- 25.Lane T E, Wu-Hsieh B A, Howard D H. Iron limitation and the gamma interferon-mediated antihistoplasma state of murine macrophages. Infect Immun. 1991;59:2274–2278. doi: 10.1128/iai.59.7.2274-2278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitz S M, DiBenedetto D J, Diamond R D. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J Immunol Methods. 1987;101:37–42. doi: 10.1016/0022-1759(87)90213-4. [DOI] [PubMed] [Google Scholar]
- 27.Moors M A, Stull T L, Blank K J, Buckley H R, Mosser D M. A role for complement receptor-like molecules in iron acquisition by Candida albicans. J Exp Med. 1992;175:1643–1651. doi: 10.1084/jem.175.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omara F O, Blakley B R, Huang H S. Effect of iron status on endotoxin-induced mortality, phagocytosis and interleukin-1 alpha and tumor necrosis factor-alpha production. Vet Human Toxicol. 1994;36:423–428. [PubMed] [Google Scholar]
- 29.Oswald I P, Eltoum I, Wynn T A, Schwartz B, Caspar P, Paulin D, Sher A, James S L. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakala R, Pakala R, Benedict C R. Novel 21-aminosteroidlike compounds prevent iron-induced free radical-mediated injury to vascular endothelial cells. J Cardiovasc Pharmacol. 1995;25:871–879. doi: 10.1097/00005344-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Palmer R M, Bridge J L, Foxwell N A, Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharm. 1992;105:11–12. doi: 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker N B, Berger E M, Curtis W E, Muldrow M E, Linas S L, Repine J E. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes dimethyl sulfoxide consumption in vitro. J Free Radicals Biol Med. 1985;1:415–419. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldo J E, Gorry M. Protection by deferoxamine from endothelial injury: a possible link with inhibition of intracellular xanthine oxidase. Am J Respir Cell Mol Biol. 1990;3:525–533. doi: 10.1165/ajrcmb/3.6.525. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz-Weiss W, Sessa C, Milstien S, Kaufman S, Watson C A, Pober J S. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J Clin Invest. 1994;93:2236–2243. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotrosen D, Edwards J E, Jr, Gibson T R, Moore J D, Cohen A H, Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985;152:1264–1273. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- 36.Shaughnessy S G, Whaley M, Lafrenie R M, Orr F W. Walker 256 tumor cell degradation of extracellular matricies involves a latent gelatinase activated by reactive oxygen species. Arch Biochem Biophys. 1993;304:314–321. doi: 10.1006/abbi.1993.1356. [DOI] [PubMed] [Google Scholar]
- 37.Tsan M F, Danis E H, Del Vecchio P J, Rosano C L. Enhancement of intracellular glutathione protects endothelial cells against oxidant damage. Biochem Biophys Res Commun. 1985;127:270–276. doi: 10.1016/s0006-291x(85)80154-6. [DOI] [PubMed] [Google Scholar]
- 38.Vartivarian, S. E. 1992. Virulence properties and nonimmune pathogenetic mechanisms of fungi. Clin. Infect. Dis. 14(Suppl. 1):S30–36. [DOI] [PubMed]
- 39.Weinberg G A. Iron chelators as therapeutic agents against Pneumocystis carinii. Antimicrob Agents Chemother. 1994;38:997–1003. doi: 10.1128/aac.38.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweier J L, Broderick R, Kuppusamy P, Thompson-Gorman S, Lutty G A. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J Biol Chem. 1994;269:24156–24162. [PubMed] [Google Scholar]