Abstract
Female mosquitoes use chemical and physical cues, including vision, smell, heat, and humidity, to orient toward hosts. Body odors are produced by skin resident bacteria that convert metabolites secreted in sweat into odorants that confer the characteristic body scent. Mosquitoes detect these compounds using olfactory receptors in their antennal olfactory receptor neurons. Such information is further integrated with the senses of temperature and humidity, as well as vision, processed in the brain into a behavioral output, leading to host finding. Knowledge of human scent components unveils a variety of odorants that are attractive to mosquitoes, but also odor-triggering repellency. Finding ways to divert human-seeking behavior by female mosquitoes using odorants can possibly mitigate mosquito-borne pathogen transmission.
Volatiles produced by human-skin resident microbiota attract mosquitoes
Mosquito host-seeking behavior is a multistep process triggered by different host cues [1]. Host-seeking behavioral steps include activation, odor-mediated long-range attraction, hovering, and landing [1]. The chemical cues that trigger these behavioral steps encompass carbon dioxide and skin odors (see Glossary) [1]. Visual stimuli act as a guide to orient the olfactory-mediated behavior towards the host, playing an important role in landing behavior [2], whereas temperature and humidity are thought to be close-range attractants [3]. Carbon dioxide (CO2) has been shown to activate resting mosquitoes [4,5] and drive long-range attraction (orientation; >1 m). Once close to a human host (<1 m), female mosquitoes hover around until they find the most attractive body part on which to land [6]. This short-range attraction can be induced by sweat-derived host odors [7,8] produced by the resident skin microbiota (Box 1; [9]).
Box 1. Sweat, skin microbiota, mosquito attraction.
Sweat is produced on the skin in mammals by three distinct glands: eccrine, apocrine, and sebaceous [102]. Eccrine is the most abundant gland in humans; it is present all over the body but with the highest density on hands and feet [102]. Apocrine glands are very limited and are found mostly on the human axilla and genitals [102]. Sebaceous glands are evenly distributed all over the body (Figure I; [102]). The sweat of eccrine glands consists of salts, proteins, amino acids, urea, ammonia, and L-(+)-lactic acid. Staphylococcus bacteria convert the aliphatic amino acids from eccrine gland secretions on the feet into short-chain carboxylic acids [9]. Apocrine secretions consist mostly of lipids, steroids, and proteins [9]. Sebaceous gland sweat consists primarily of lipids [9]. These lipids are converted into long-chain carboxylic acids by skin bacteria of the genera Propionibacterium, Corynebacterium, and Staphylococcus, which are, in turn, converted into short-chain carboxylic acids by Corynebacterium species [9]. As the different sweat glands are more concentrated on specific body parts [9], the skin volatiles isolated from each part also exhibit different compositions [102]. Whereas specific aldehydes and ketones are more abundant in hand and forearm odor bouquets [33], alkenes and carboxylic acids are more frequently found in foot and axilla odor bouquets [102].
The volatile bouquet collected from cultures of human foot microbiota revealed 15 predominant odorants [103], out of which five odorants were also present in the bouquet volatile of a reference strain of the predominant skin bacterium, Staphylococcus epidermidis [103]. Both human foot microbiota and S. epidermidis cultures were attractive to Anopheles gambiae [103]. The bouquet volatile of the other three bacterial species present in the human skin microbiome are also attractive to An. gambiae females [27]. These species encompass Corynebacterium minutissimum, Brevibacterium epidermidis, and Bacillus subtilis [27].
Differences in sweat gland distribution and skin microbiota composition have been observed amongst humans and apes [104–106]. Whereas the ratio of eccrine to apocrine glands in primates ranges between 50:50 and 60:40, eccrine glands are very limited in the human body [9]. Even though humans have the lowest quantity and richness of skin microbiota [104,106], Corynebacterium, Staphylococcus, and Pseudomonas bacteria have the highest relative abundance on the human skin compared with apes [104,105]. Compared with other primates, human skin surfaces display much higher amounts of L-(+)-lactic acid [9]; free carboxylic acids are nearly absent on the skin of other primates whereas these compounds are very abundant on the human skin [9].
Figure I. Sweat gland distribution in the human skin.
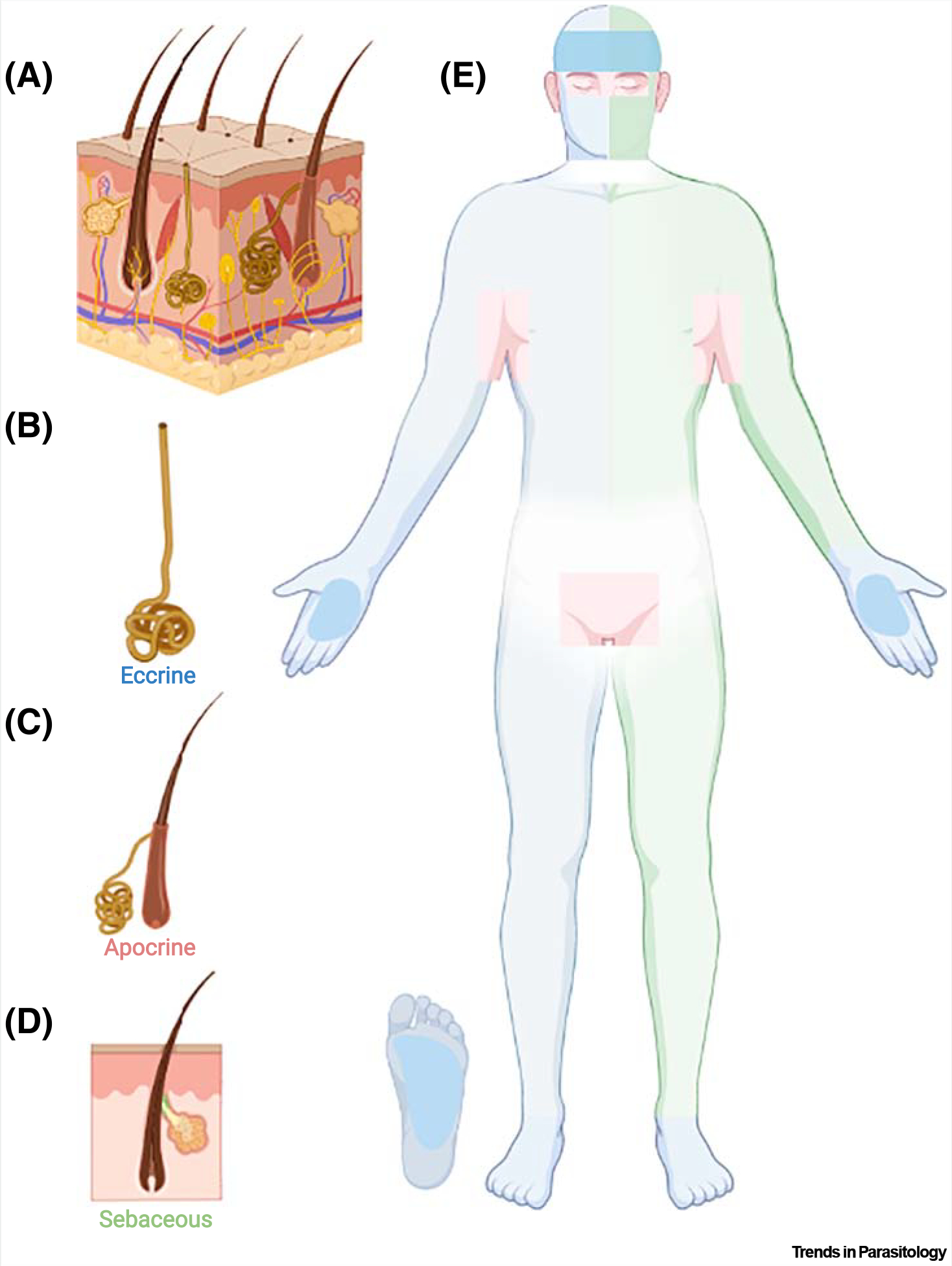
(A) Section of the human skin showing the sweat glands: (B) eccrine, (C) apocrine, and (D) sebaceous. (E) Eccrine glands are distributed all over the body (depicted on the right-half of the body in light blue), and more densely distributed on the palms, soles, and forehead (dark blue). Apocrine glands are mostly located on the axilla and genital regions, as highlighted in beige. Sebaceous glands are found all over the body (highlighted on the left side of the body in light green), with higher densities on the face (dark green). Figure created with BioRender.
The olfactory system of anthropophilic mosquito species is tuned to the formulation of human sweat [9]. Among the components of human sweat, L-(+)-lactic acid is a key synergist of CO2 and an attractive human cue for some mosquito species [4,10]. For other species, ammonia is the main attractive odorant that, along with CO2, ignites attraction to humans [11]. These mosquitoes prefer aged sweat, which has higher levels of ammonia produced upon the consumption of L-(+)-lactic acid by the growing skin microbiota [11]. Short-chain carboxylic acids are highly abundant on the human skin [12] and these compounds make mosquito attraction to L-(+)-lactic acid, ammonia, and CO2 even stronger [13].
In light of the development of insecticide resistance in mosquitoes [14] and drug resistance in malaria parasites [15], and filarial worms [16], new strategies to control mosquito-borne disease transmission are urgently needed. Despite significant advances in the identification of odor blends attractive to the main mosquito vectors [17,18], controlling mosquito populations using odor-baited traps seems to be a task nearly impossible to achieve. DEET has been used as a mosquito repellent for many decades [19], yet high cost and human health issues preclude its widespread use [20]. This review describes multiple aspects of the mosquito neuroethology that drive human-seeking behavior and highlights the odor-based approaches that effectively reduce mosquito bites. Based on this information, novel approaches could be developed to potentially reduce mosquito bites and prevent mosquito-borne diseases.
Chemical and physical cues attractive to the main mosquito vectors
Anopheles gambiae females are attracted to compounds found in skin odor in the presence of CO2 [4,21,22]. The need for these mosquitoes to have contact with CO2 to trigger host-seeking behavior seems to be an important adaptation to life indoors where these mosquitoes are in constant exposure to skin odors even in the absence of a host [23]. In this sense, such mosquitoes could rest while the human subjects are absent and attack when the subjects are found indoors releasing CO2 [23]. Along these lines, in the absence of CO2, female An. gambiae is weakly attracted to ammonia alone, and the addition of L-(+)-lactic acid to ammonia has no additive attractive effect (Figure 1A and Table 1) [24]. In the presence of CO2, by contrast, female attraction is enhanced by addition of ammonia and L-(+)-lactic acid [24,25]. Addition of seven specific carboxylic acids only enhances attraction to a blend of CO2, L-(+)-lactic acid, and ammonia, but not to a similar blend lacking L-(+)-lactic acid (Figure 2A and Table 1) [13,17,24–27].
Figure 1. Human odorants attractive to female mosquitoes.
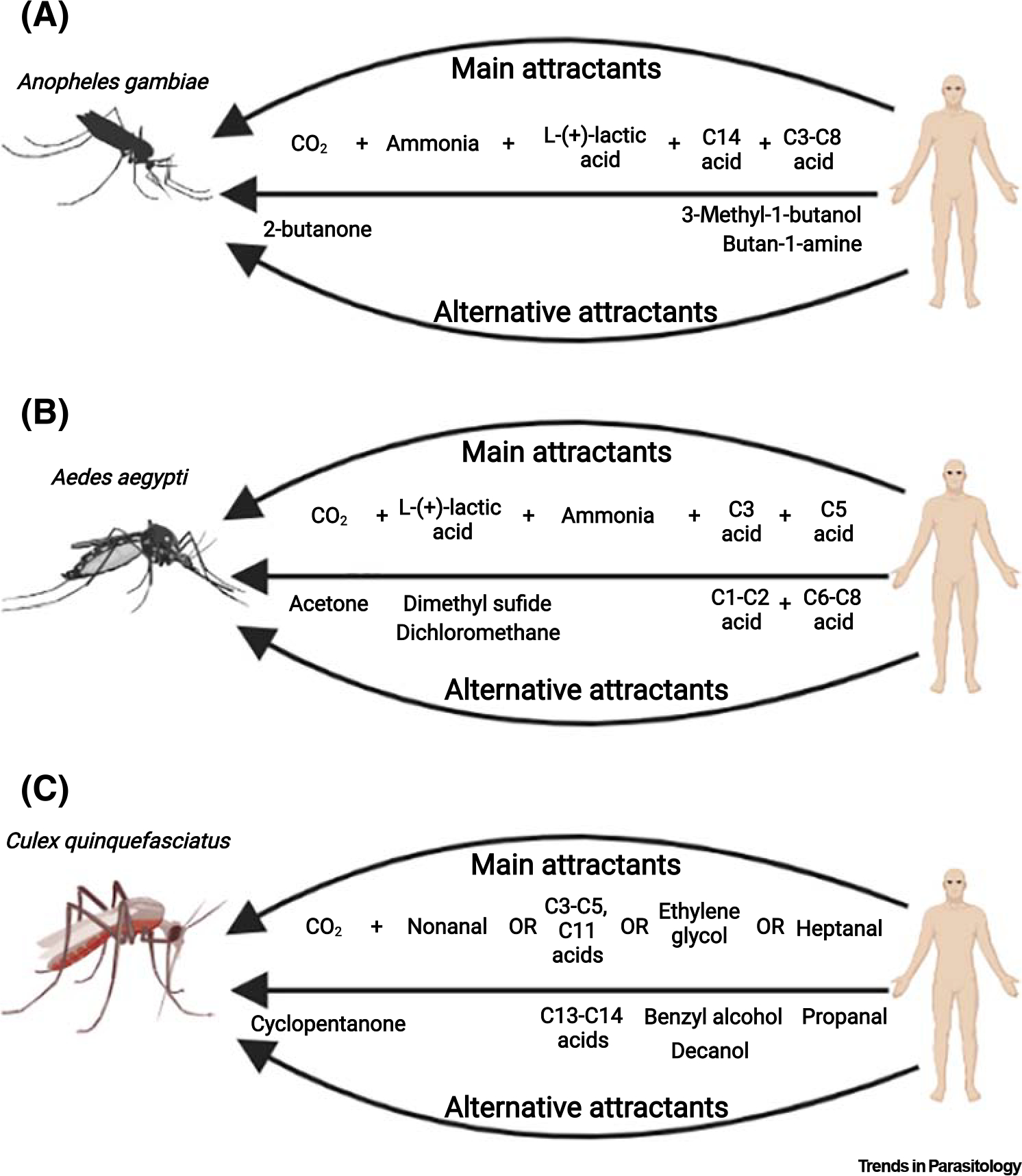
(A) Attraction of female Anopheles gambiae is evoked by sensing carbon dioxide (CO2) and ammonia, followed by the detection of L-(+)-lactic acid that triggers the perception of different carboxylic acids. Attractive behavior is similarly evoked when CO2 is replaced by 2-butanone, and short-chain carboxylic acids are replaced by 3-methyl-1-butanol along with butan-1-amine. (B) Combinations of CO2, L-(+)-lactic acid, ammonia, one C1–C3 as well as one C5–C8 carboxylic acid are sufficient to trigger Aedes aegypti female attraction. The substitutive odorants acetone and dimethyl sulfide or dichloromethane can play the roles of CO2 and L-(+)-lactic, respectively. (C) Even though less comprehensively investigated, Culex quinquefasciatus female attractive behavior can be evoked by a combination of CO2 and nonanal, and individually by short-chain (C1), middle-chain (C6–C11) and long-chain (C13–C14) carboxylic acids, specific alcohols, and aldehydes. Figure created with BioRender.
Table 1.
Odor blends and single odorants attractive or aversive to Anopheles gambiae females
| Anopheles gambiae | ||||
|---|---|---|---|---|
| Odor blend | Dose/concentration | Effect | Behavior (assay) | Refs |
| Carbon dioxidea Ammonia L-(+) lactic acid | 0–1030 parts per million (ppm) 2.5% 90% | Attraction | Activation Long-range attraction (wind tunnel - dual port) | [22] |
| Ammonia L-(+) lactic acid Carboxylic acids (C3-C8 andC14) | 2.5% Pure Pure | Attraction 3mC4 reduces attraction to blend | Short-range attraction/ Trap-catch (wind tunnel - dual port; MM-X trap) | [13,24] |
| Carbon dioxide Ammonia L-(+) lactic acid Carboxylic acids: C3 C4 C5 C7 C8 C14 | 500 ml/min 2.5% 85% 0.1% 1% 0.01% 0.01% 0.01% 0.01% | Attractiona 3mC4 inert to blend | Trap-catch (MM-X trap - semi-field) | [17] |
| 1-Butanol 2,3-Butanedione 2-Methyl-1-butanol 2-Methylbutanal 2-Methylbutanoic acid 3-Hydroxy-2-butanone 3-Methyl-1-butanol 3-Methylbutanal 3-Methylbutanoic acid benzene ethanol | Pure Pure Pure Pure Pure Pure Pure Pure Pure Pure | Attraction | Trap-catch (MM-X trap) | [103] |
| Ammonia L-(+) lactic acid Carboxylic acid (C14) + 2-Methylbutanoate OR butyl butyrate OR butyl acetate OR butyl isobutyrate OR dimethylsulfide | 2.5% Pure Pure 1% 1% 1% 0.1% 0.01–0.001% | Attractiona 2-Pentadecanone (1%) reduces attraction to blend | Short-range attraction (wind tunnel - dual port) | [27] |
| Ammonia L-(+) lactic acid Carboxylic acid (C14) + 3-Methyl-1-butanol OR 3-methylbutanal | 25% Pure Pure 0.01% 1% | Attraction | Short-range attraction (wind tunnel - dual port) | [26] |
| Carbon dioxide Ammonia L-(+) lactic acid Carboxylic acid (C14) + 3-Methyl-1-butanol OR 3-methylbutanoic acid | 4.5% 25% Pure Pure 0.1% (pure for traps) 0.1% | Attraction | Trap-catch/ Short-range attraction (MM-X trap - semi-field; wind tunnel - dual port; MM-X trap - open-field) | [25,26,107] |
| Carbon dioxide Ammonia L-(+) lactic acid Carboxylic acid (C14) + butan-1-amine | 4.5% 25% Pure Pure 0.00001–1% | Attraction | Short-range attraction (wind tunnel - dual port) | [26] |
| Carbon dioxide Ammonia L-(+) lactic acid Carboxylic acid (C14) + 3-methyl-1-butanol + butan-1-amine | 4.5% 25% Pure Pure 0.01% or 0.001 % 0.01 to 0.004% | Attraction | Short-range attraction (wind tunnel - dual port) | [26] |
| Carbon dioxide Ammonia L-(+) lactic acid Carboxylic acid (C14) 3-Methyl-1-butanol + butan-1-amine OR 1-dodecanol | 63 ml/min 2.5% 85% 0.00025% 0.000001% 0.0000001% 0.0001% | Attraction | Trap-catch (MM-X trap - semi-field) | [108] |
| Ammonia L-(+) lactic acid Carboxylic acid (C14) 3-Methyl-1-butanol butan-1-amine 2-Butanone | 2.5% 85% 0.00025% 0.000001% 0.001% Pure | Attraction | Trap-catch (MM-X trap - semi-field) | [40] |
| Individual odor | Dose/concentration | Effect | Behavior | Refs |
| Ammonia | 13.7–13 700 ppm | Attraction | Short-range attraction (wind tunnel - dual port) | [24] |
Turbulent plume.
Figure 2. Mosquito olfactory receptor families expressed in odorant receptor neurons (ORNs) and housed in distinct sensilla.
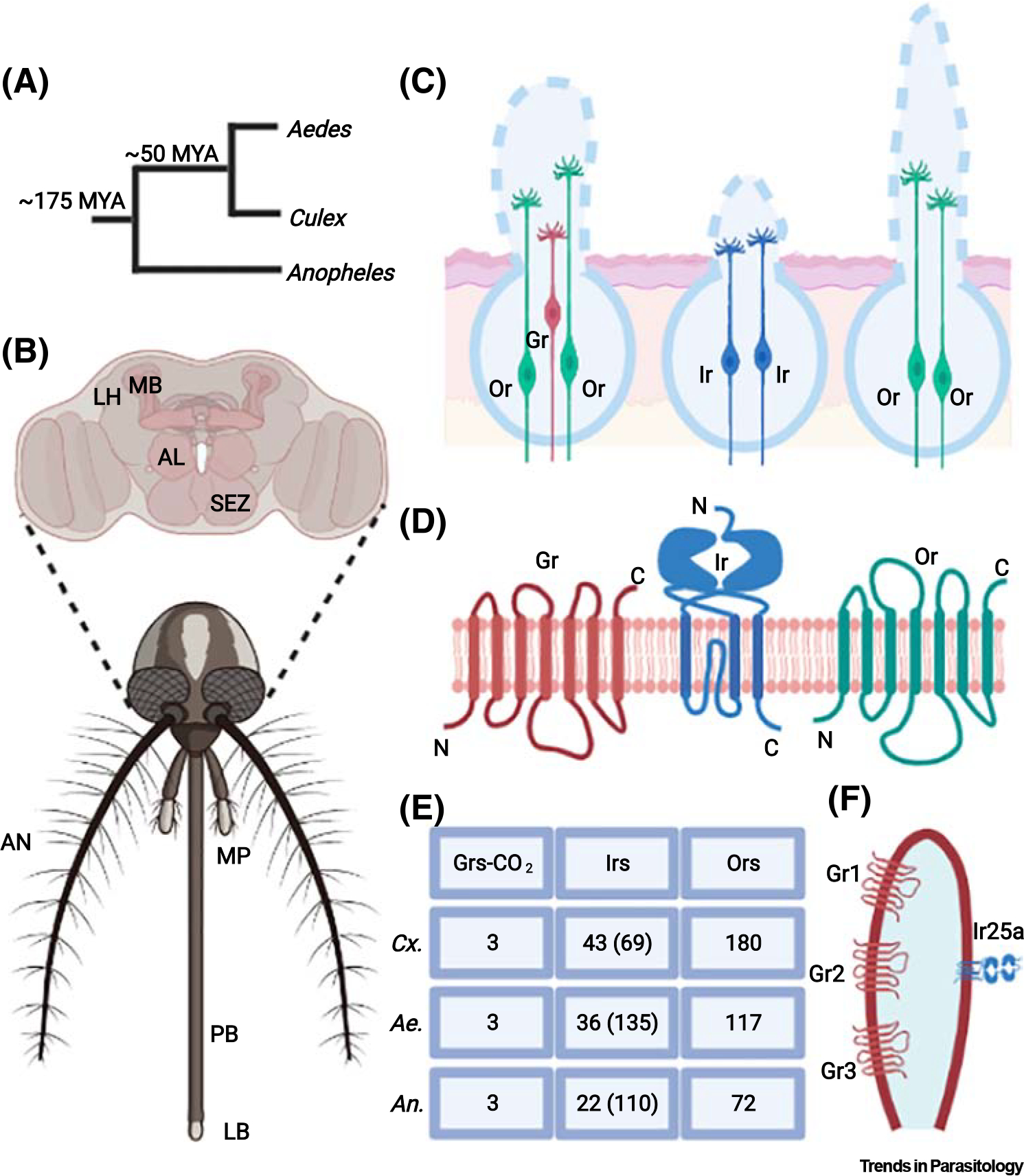
(A) Cladogram showing the divergence time between Culicini and Aedini subtribes and Anophelinae and Culicinae subfamilies (B) Head of a Culicinae mosquito exhibiting the olfactory appendages [antennae (AN), maxillary palps (MP), and labella (LB)], along with the brain’s primary olfactory and taste centers [antennal lobes (AL), subesophageal zone (SEZ)], and high-order centers [lateral horns (LH) and mushroom bodies (MB)]. (C) Classical view of the olfactory system showing one receptor per neuron. Left. In mosquitoes, capitate-peg sensilla on the maxillary palps house the cpA neuron that expresses the CO2 sensors Gr1–3 (or Gr22–24). These sensilla also house two other neurons expressing predominantly Ors. On the antennae, grooved peg (middle) and trichoid (right) sensilla house ORNs expressing either Ors or Irs or both. The exact distribution of such neurons in the different sorts of antennal sensilla is not known. (D) Molecular structures of Gr (left), Ir (middle) and Or (right). Whereas Ors and Grs are seven-pass transmembrane proteins that resemble G-protein-coupled receptors but function as ion channels, Irs emerged from the ionotropic glutamate receptor (iGluRs) gene family [63]. (E) The number of genes belonging to the gustatory receptor (Gr - CO2 sensors), ionotropic receptors (Ir), and odorant receptors (Or) in Culex quinquefasciatus (Cx.), Aedes aegypti (Ae.), and Anopheles gambiae (An.) is depicted. For the Irs, genes expressed in olfactory appendages along with the number of genes in the genome (parenthesis) are shown. (F) Modern view of the olfactory system depicting more than one receptor per neuron for the mosquito capitate peg sensillum neuron cpA [75]. Figure created with BioRender. Abbreviation: MYA, million years ago.
Combinations of skin odor with CO2 synergistically affect Aedes aegypti attractive behavior (Figure 1B and Table 2) [28]. Female Ae. aegypti are not attracted to L-(+)-lactic acid alone [10,28], and they are weakly attracted to CO2 or human skin odor alone [28]. Furthermore, combinations of L-(+)-lactic acid or skin odor with CO2 synergistically affect attractive behavior [28]. A blend with CO2 and odorants presented on the human skin [L-(+)-lactic acid, ammonia, and short (C1–C3) and middle (C5–C8) length carboxylic acids] was shown to be nearly as attractive as CO2 along with human skin odor [7]. Female Ae. aegypti mosquitoes are also attracted to different combinations of L-(+)-lactic acid with specific ketones, sulfides, and/or chloroalkanes, indicating that such odorants can replace CO2 as an attractant [29–31]. Some of these combinations are more attractive to these mosquitoes than human skin odor or mixtures of CO2 and L-(+)-lactic acid [18]. Specific carboxylic acids and sulfur compounds can alone induce landing for this mosquito species (Figure 1B and Table 2) [32].
Table 2.
Odor blends and single odorants attractive or aversive to Aedes aegypti females
| Aedes aegypti | ||||
|---|---|---|---|---|
| Odor blend | Dose/concentration | Effect | Behavior (assay) | Refs |
| Carbon dioxide | 0.05–1% | Attraction | Activation Long-range attraction (wind tunnel) | [5] |
| Carbon dioxide Ammonia L-(+) lactic acid (C1-C3) carboxylic acid (C5-C8) carboxylic acid | 4% 0.13 mol/l Pure 0.01–1% 0.01–1% | Attraction | Short-range attraction (Y-olfactometer) | [7] |
| L-(+) lactic acid Acetone Dimethyl sulfide | Pure Pure Pure | Attraction | Short-range attraction (Y-olfactometer) | [30] |
| L-(+) lactic acid Acetone OR dimethyl sulfide OR dichloromethane | 2 mg/ml Pure Pure Pure | Attraction | Short-range attraction (Y-olfactometer) | [29] |
| Acetone OR dimethyl sulfide OR dichloromethane | Pure Pure Pure | Attraction | Short-range attraction (Y-olfactometer) | [31] |
| L-(+) lactic acid Acetone OR butanone OR dimethyl sulfide OR dimethyl disulfide OR allyl methyl sulfide OR carbon disulfide OR chloroform OR 1,1,2-trichloroethane OR dichloromethane OR carbon tetrachloride OR trichloroethylene OR tetrachloroethylene OR 1,1,1-trichloroethane | 2 mg/ml Pure Pure Pure Pure Pure Pure Pure Pure Pure Pure Pure Pure Pure | Attraction | Short-range attraction (Y-olfactometer) | [18] |
| 2,3-Butanedione 1-Hexanol 1-Butanal 1-Pentanal | 3% 3% 3% 3% | Repellent (reduced attraction to carbon dioxide source) | Short-range repellency (wind tunnel) | [36] |
| Carbon dioxide L-(+) lactic acid | 2000–3000 ppm Pure | Attraction | Short-range attraction (two-port olfactometer) | [28] |
| Individual odor | Dose/concentration | Effect | Behavior | Refs |
| L-(+) lactic acid | 200 μg | Attraction | Short-range attraction (Y-olfactometer) | [32] |
| Dimethyl sulfide | 200 μg | Attraction | Short-range attraction (Y-olfactometer) | [32] |
| Dichloromethane | 200 μg | Attraction | Short-range attraction (Y-olfactometer) | [32] |
| Acetic acid | 200 μg/1 μg | Attraction | Short-range attraction/ Landing (Y-olfactometer; Cage assay) | [32] |
| Methyl sulfide (dimethyl sulfide) | 200 μg/1 μg | Attraction | Short-range attraction/ Landing on perfumed object (Y-olfactometer; Cage assay) | [32] |
| Carbon disulfide | 200 μg/1 μg | Attraction | Short-range attraction/ landing on perfumed object (Y-olfactometer; Cage assay) | [32] |
| Methyl propyl disulfide | 200 μg/1 μg | Attraction | Short-range attraction/ landing on perfumed object (Y-olfactometer; Cage assay) | [32] |
| Butanoic acid | 1 μg | Attraction | Landing on perfumed object (Cage assay) | [32] |
| 3-Methyl butanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Heptanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Tetradecanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Hexadecanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Octadecanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Benzoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| 2-hydrobenzoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Spermidine (human odorant analog) | 10% | Repellent (reduced landing on skin odor) | Landing on perfumed object (cage assay) | [41] |
| 6-Methyl-5-hapten-2-one (sulcatone) | 10 ng 1 μg | Reduced probing activity | Short-range attraction/ probing on perfumed hand (Y-olfactometer) | [53] |
| Octanal | 10 ng 100 ng 1 μg 10 μg | Reduced attraction | Short-range attraction/ probing on perfumed hand (Y-olfactometer) | [53] |
| Nonanal | 1μg 10 μg | Reduced attraction | Short-range attraction/ probing on perfumed hand (Y-olfactometer) | [53] |
| Decanal | 1 μg 10 μg 100 μg | Reduced attraction | Short-range attraction/ probing on perfumed hand (Y-olfactometer) | [53] |
| Geranylacetone | 10 ng (probing only) 100 ng 1 μg 10 μg 100 μg (attraction only) | Reduced attraction and probing activity | Short-range attraction/ probing on perfumed hand (Y-olfactometer) | [53] |
Human skin odor, particular specific carboxylic acids and sulfur compounds were found to be very important landing cues for Culex quinquefasciatus (Figure 1C and Table 3) [6,22,32]. Nonanal, found at high abundance in human skin odor, can synergize with CO2 to attract these mosquitoes to baited traps [33]. Cx. quinquefasciatus females are also attracted to a variety of carboxylic acids (C3, C6–C11, and C13–C14), alcohols (ethylene glycol, benzyl alcohol, cholesterol, and decanol), as well as aldehydes (heptenal, propanal, and nonanal) present in the human skin volatile bouquet (Figure 1C and Table 3) [34].
Table 3.
Odor blends and single odorants attractive or aversive to Culex quinquefasciatus females
| Culex quinquefasciatus | ||||
|---|---|---|---|---|
| Odor blend | Dose/concentration | Effect | Behavior (assay) | Refs |
| Carbon dioxide | 4% | Attraction | Activation Long-range attraction (wind tunnel) | [6] |
| 2,3Bbutanedione 1-Hexanol 1-Butanal 1-Pentanal | 3% 3% 3% 3% | Repellent (reduced attraction to carbon dioxide source) | Short-range repellency (MM-X trap - semi-field) | [36] |
| Carbon dioxide Nonanal | Released from dry ice 0.1 mg | Attraction | Trap-catch (MM-X trap - open-field) | [33] |
| Individual odor | Dose/concentration | Effect | Behavior | Refs |
| L-(+)-lactic acid | 200 μg | Attraction | Short-range attraction (Y-olfactometer) | [32] |
| Acetic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Hexadecanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Octadecanoic acid | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Methyl sulfide (dimethyl sulfide) | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Methyl propyl disulfide | 1 μg | Attraction | Landing on perfumed object (cage assay) | [32] |
| Propanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Hexanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Heptanoic acid | 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Octanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Nonanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Decanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Undecanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Tridecanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Tetradecanoic acid | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Pentadecanoic acid | 10 μg | Repellent | Short-range attraction (Y-olfactometer) | [34] |
| Hexapentanoic acid | 0.1 μg, 1 μg, 10 μg | Repellent | Short-range attraction (Y-olfactometer) | [34] |
| Heptapentanoic acid | 0.1 μg, 1 μg (10 μg) | Attractant (repellent) | Short-range attraction (repellency) (Y-olfactometer) | [34] |
| Octapentanoic acid | 0.1 μg, 1 μg, 10 μg | Repellent | Short-range attraction (Y-olfactometer) | [34] |
| Glycerol | 0.1 μg, 1 μg | Repellent | Short-range attraction (Y-olfactometer) | [34] |
| Ethylene glycol | 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Benzyl alcohol | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Cholesterol | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Decanol | 0.1 μg (10 μg) | Repellent (attractant) | Short-range repellency (attraction) (Y-olfactometer) | [34] |
| Heptenal | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Propanal | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Nonanal | 0.1 μg, 1 μg, 10 μg | Attraction | Short-range attraction (Y-olfactometer) | [34] |
| Cyclopentanone (human odorant analog) | 5–20% | Attraction | Trap-catch (MM-X trap - semi-field) | [37] |
| Ethyl butyrate (human odorant analog) | 10% | Repellent (reduced attraction to carbon dioxide source) | Trap-catch (MM-X trap - semi-field) | [37] |
An interesting aspect of the olfactory system of mosquitoes is the fact that some skin odorants can also bind to the CO2 receptor, evoking neuronal responses and inducing behavior [35–39]. Skin odors and their chemical analogs have been used to activate, superactivate, and inhibit the CO2 receptor and, in turn, manipulate the host-seeking behavior of mosquitoes (Tables 1–3) [36,37,40,41]. At nonphysiological concentrations the human skin odor 2,3-butanedione triggers prolonged firing of the CO2 neuron, disorienting the host-seeking behavior of Ae. aegypti and Cx. quinquefasciatus mosquitoes [36]. Cyclopentanone and ethyl pyruvate are two odorants with a chemical structure similar to that of skin odors and capable of activating and inhibiting the CO2 receptor neuron, respectively [37]. Whereas the former odorant acts as an attractant, the latter can reduce CO2 source attraction of female Ae. aegypti and Cx. quinquefasciatus mosquitoes [37]. Along the same lines, acetone is another CO2 receptor neuron activator [39], and Ae. aegypti females show similar attraction to acetone and L-(+)-lactic acid as to CO2 and L-(+)-lactic acid [18,29]. Additionally, a human body odor called spermidine inhibits CO2 activation and reduces Ae. aegypti landing evoked by human skin odor [41]. Lastly, replacing CO2 in an attractive blend of odorants with the CO2 receptor activator 2-butanone [36] sustains similar rates of trap catches of An. gambiae and Anopheles funestus in field settings in Kenya [40].
Physical cues, such as heat and humidity, are also perceived by female mosquitoes, playing important roles in short-range attraction [1]. Upon activation by CO2, female Ae. aegypti mosquitoes are attracted to heat [2,28], without any evidence of habituation [42]. These mosquitoes respond to heat at 2.5°C above ambient temperatures and remain attracted to temperatures up to 40°C [42]. Attraction to warmed objects is further enhanced when humidity is increased [3], triggering the hovering of mosquitoes over a 6–8 cm distance [2].
Visual cues are also involved with female mosquito short-range attraction and landing behaviors [1]. Ae. aegypti is aided by visual cues, or heat, to land when activated by CO2 [2,28,42]. When heat and visual cues are present, females still prefer warmed dark objects, yet this attraction is only induced when the warmed object exhibits a high contrast against the background [2]. Visual cues can induce landing without CO2 activation, even though CO2 switches attention from visual cues and helps mosquitoes to focus on the thermal cue [43]. In the presence of CO2, mosquitoes also discriminate against color hues [44]. Ae. aegypti shows preference for cyan, orange, and red objects; Cx. quinquefasciatus prefers blue and red objects; Anopheles stephensi is more attracted to black and red objects [44].
As aforementioned, the integration of multiple host cues triggers a synergistic effect regarding mosquito host-finding [1]. Heat along with skin odors evoked a higher An. gambiae landing response than such cues alone [45], and the combination of heat, skin odor, and vision increases trap catches drastically [46]. CO2 gates Ae. aegypti attraction to heat and skin odor [28]. Also, CO2 gates An. gambiae responses to skin odor [4], evoking landing behavior [23]. By contrast, Cx. quinquefasciatus females land on warmed beads perfumed with skin odor regardless of contact with CO2 [6]. This species also lands into individual skin odors without previous exposure to CO2 [32,34].
The multistep behavioral output that leads to host finding in different species is triggered by a few similar components, such as CO2, visual cues, heat, humidity, and skin odor. Nonetheless, skin odor is differentially sensed by different mosquito species. These findings highlight the evolutionary differences amongst mosquito species, which share common ancestors approximately 145–200 million years ago (MYA) between the subfamilies Anophelinae and Culicinae [47] and 52–54 MYA between the subtribes Aedini and Culicini (Figure 2A) [48,49]. This evolutionary divergence led to the differences in behavioral responses to olfactory cues and likely defined the ecological niche of each mosquito species [50].
Mosquito host choice
Mosquitoes can exhibit species-specific or seasonal attraction to humans, and such behaviors are triggered by human-skin volatiles [33,51]. Different subspecies of Ae. aegypti show distinct mosquito host choice (host preferences) [51], with the domestic subspecies Ae. aegypti aegypti displaying a strong preference to feed on humans, and the forest-dwelling subspecies Ae. aegypti formosus exhibiting zoophilic behavior [51]. Such preferences may be associated with the ability of these subspecies to sense the human-skin-emanating volatile sulcatone [51]. This volatile is present in much greater amounts in human skin volatiles than in the scent of other animals [51], and the olfactory receptor neurons (ORNs) of the anthropophilic subspecies display a higher sensitivity to sulcatone than do the olfactory neurons of the zoophilic subspecies [51].
Differences in human-host perception are also observed between the anthropophilic mosquito Ae. aegypti and the anthropo(zoo)philic mosquito Cx. quinquefasciatus [33,52]. Ae. aegypti shows stronger attraction to human odors than to other animals [52]. Such attraction is mediated by the enrichment of specific ketones (sulcatone and geranylacetone) and long-chain aldehydes (decanal) in the human scent, contrasting with the greater abundance of short-chain aldehydes (hexanal and heptanal) in the scent of other animals [52]. As decanal activates the Ae. aegypti olfactory system at levels released by human skin, it possibly mediates mosquito attractive behavior towards humans [52]. By contrast, Cx. quinquefasciatus prefers to feed on both humans and birds, switching hosts upon season and availability [33]. To do so, these mosquitoes rely upon chemical volatiles that are commonly present in both the human and bird volatile bouquets [33]. Among the volatiles commonly found in the chicken and pigeon odor bouquets, nonanal was also found at higher proportions in human skin emanations [33]. This compound evokes strong responses by specific classes of olfactory sensilla and synergizes with CO2 in attracting this mosquito species to traps [33].
Mosquito human discrimination
Mosquitoes exhibit differential attraction to different human individuals [53,54], and quantitative and qualitative differences in skin microbiota and skin odors among individuals are likely the factors tuning attractiveness [53,54]. Differences in human attractiveness by An. gambiae are correlated with the quantity and diversity of the skin microbiota [54]. A greater abundance of Staphylococcus spp. in a less diverse skin microbiome is associated with highly attractive individuals whereas enrichment with Pseudomonas spp. in a more diverse skin microbiome correlates with reduced attractiveness to An. gambiae females [54]. In addition, reduced attractiveness to human hands has been associated with increased releases of specific human skin volatiles [53]. While perfuming a human hand with different quantities of sulcatone were inert to Ae. aegypti females, wearing specific doses of octanal, nonanal, decanal, and geranylacetone on the skin reduced attractiveness [53]. In fact, decanal can even reduce mosquito attractiveness to a human hand to levels similar to that of clean air, and geranylacetone is capable of reducing even probing activity [53].
Odorant-based learned behavior
Mosquito host attraction and avoidance can also be tuned by skin odor-based learning behavior [55,56]. Exposure of Ae. aegypti to individual odorants, followed by a reward (heated blood meal) can change the valence of neutral odorants, such as L-(+)-lactic acid and 1-octen-3-ol, inducing attractive behavior [55]. Even training with the aversive odorants (Z)-hex-3-en-1-ol or the repellent DEET combined with a heated blood meal reward results in a partial to complete valence switch, inducing Ae. aegypti’s attractive behavior towards known repellents [55]. Conversely, exposure of Ae. aegypti to an aversive stimulus, along with human skin odor, changes the odor’s valence, inducing aversive behavior towards skin odor [56]. The degree of aversiveness in this instance was different amongst skin odors from different individuals [56], and this phenomenon is also observed against rat scent, but not with chicken scent. Altogether, these findings indicate that odor-based mosquito learning behavior is a property of certain chemicals in an odor scent [56]. Whereas kairomones like 1-octen-3-ol evoked aversive learned behavior upon previous exposure to an aversive stimulus, nonanal was neutral, and L-(+)-lactic acid showed even stronger attractive behavior [56]. Hence, mosquitoes can learn to avoid, or be attracted to, specific compounds in the host skin odor based on previous negative (host defense behavior) or positive (blood meal) experiences with those compounds [56]. Understanding mosquito host preference has uncovered important clues about the olfactory signals that lead to human attractiveness [33,51,53,56].
Molecular basis of mosquito attraction to humans
The mosquito peripheral olfactory system perceives chemical volatiles via ORNs housed in the olfactory sensilla present in the antennae, maxillary palps, and proboscis’ labella (Figure 2B) [35,57–59]. Such sensilla are innervated by two or three ORNs that express members of the odorant receptor gene families (Figure 2C) [60]. These encompass the odorant receptor (Or) [57,58,61] and the ionotropic receptor (Ir) gene families (Figure 2D,E) [62–64]. Whereas the tuning receptors of the former gene family form heteromeric tetramers with the coreceptor Orco [57,58,61,65], members of the later family pair with any one or two of the three coreceptors, named Ir25a, Ir8a, and Ir76b [66]. Both gene families encode ligand-gated ion channels [62–64,67]. The Ors perceive mostly aromatic and heterocyclic odorants in Anopheles mosquitoes [68]; the Irs are mostly tuned to amines and carboxylic acids as seen in fruit flies [62–64,66]. By contrast, CO2 is detected by a specific class of neurons (cpA) housed in the maxillary palp capitate peg sensilla (Figure 2B,C) [35]. In mosquitoes, the cpA neurons express three members of the gustatory receptor gene families (Grs), named Gr1–3 in Culicinae and Gr22–24 in Anophelinae (Figure 2D,E) [35]. These proteins assemble into a trimeric receptor that detects mostly CO2 but also skin odors [35–39].
Most of the axons of the ORNs extend to the primary olfactory center of the deutocerebrum called the antennal lobe (Figure 2B) [69]. The axons of multiple neurons of the same class converge to specific regions of the antennal lobe called the olfactory glomeruli (Figure 2B) [69]. The antennal lobes of mosquitoes bear between 65 and 81 glomeruli in Ae. aegypti [70,71] and 67–70 in An. gambiae females [60]. Since males and females exhibit the same number of glomeruli (67–70 in Anopheles mosquitoes), then the host-seeking ability of females is likely related to differences in antennal lobe volume (2:1 female over male; [60]) as well as sexual dimorphism in the expression of the transcription factor fruitless in the brain and peripheral ORNs [72]. As male Ae. aegypti are also attracted to humans in the field [73], further investigation is needed to unveil the factors triggering host finding. By 24 h after adult emergence, the mosquito peripheral olfactory system exhibits complete activation of olfactory receptor gene expression, with olfactory related gene expression increasing as female mosquitoes mature and develop host-seeking abilities [74].
In contrast to the one receptor to neuron dogma postulated based on observations of the Drosophila melanogaster olfactory system [69], fluorescent labeling of Ae. aegypti olfactory glomeruli using genetic drivers for the odorant receptor coreceptors, Orco, Ir25a, Ir8a, and Ir76b, and the CO2 receptor subunit Gr3, identified significant neuronal overlap in the expression of the different odorant receptor gene families [75], as observed in fruit flies [76]. These findings agreed with the greater number of odorant receptors in the Ae. aegypti genome [77] as compared to the number of olfactory glomeruli, breaking the one-receptor-to-neuron dogma in mosquitoes [75]. The CO2 receptor neurons also express the Ir25a receptor (Figure 2E), which is activated by amines [75], and the glomeruli innervated by CO2 receptor neurons respond to trimethylamine in Gr3−/− mosquitoes [75]. Interestingly, trimethylamine can replace CO2 and attract Gr3−/− Ae. aegypti mosquitoes when associated with L-(+)-lactic acid [75]. Furthermore, wild-type Ae. aegypti females are only weakly attracted to a blend of trimethylamine and L-(+)-lactic acid [75]. As the correspondent olfactory neurons of Orco and Gr3 knockout mosquitoes are devoid of spontaneous activities [28,78,79], and CO2 receptor knockouts are unresponsive to multiple skin odors [79], the expression of ionotropic receptors in Orco and CO2 receptor neurons might play a modulatory role, fine-tuning the neuronal responses of such neurons.
Some of the genes encoding odorant coreceptors have been edited in mosquitoes, unveiling their roles in mosquito host-seeking behavior [28,42,78–81]. The first odorant receptor knocked out was the odorant receptor coreceptor Orco in Ae. aegypti [78]. Both male and female Orco knockouts lose attraction to honey [78]. In the absence of CO2, female Orco knockouts lose attraction to human skin odor, which is mildly attractive to wild-type mosquitoes [78]. By contrast, activation by CO2 strongly enhances attraction to human skin odor in both wild-type and Orco knockout female mosquitoes, demonstrating that CO2 does not gate Orco-driven behavior, and other olfactory pathways can only be activated in the presence of CO2 [78]. Similarly, An. gambiae (Anopheles coluzzii) female Orco knockouts partially lose attraction to human hands [81].
Gene knockout of the Orco receptor in Ae. aegypti demonstrated that Or-expressing neurons play an important role in host discrimination [78]. Whereas wild-type mosquitoes showed strong attraction to human subjects, as opposed to guinea pigs, Orco mutants partially lose such preferred human attraction [78]. Comparisons of the olfactory systems between (sub)species of anthropophilic (anthropophagic) and zoophilic (zoophagic) mosquitoes reveal both sequence and expression differences in the olfactory system [51,82,83]. Although the anthropophagic An. gambiae and the zoophagic Anopheles quadriannulatus display very similar chemosensory gene repertoires, divergences at sequence and transcriptional levels are evident [82]. Such divergences encompass a few receptors evolving under positive selection, most of the Irs genes showing differential expression, and a subset of the Ors genes of An. quadriannulatus predominantly expressed in the antennae of An. gambiae [82]. Between the anthropophilic Ae. aegypti aegypti and the zoophilic Ae. aegypti formosus, Or4 plays a major role in host preferences [51,83]. Or4 is among the highest expressed odorant receptor genes in anthropophilic mosquitoes [51]. Also, the alleles of the Or4 receptor show clear genetic structuring between anthropophilic and zoophilic mosquitoes [51,83], and these differences parallel the strength of Or4 neuronal activation by the human skin odorant sulcatone [51].
Knocking out the ortholog genes encoding the subunits of the heteromeric CO2 receptor in An. gambiae [79], or expression of different combinations of these subunits of Ae. aegypti [84] and Cx. quinquefasciatus [85] using heterologous expression systems, demonstrated a conserved role of each subunit in CO2 sensing [79,84,85]. Whereas knockouts of the An. gambiae Gr23 and Gr24 subunits demonstrated that these subunits are essential for CO2 detection, knockouts of the Gr22 displayed only reduced sensitivity [79]. Further, Ae. aegypti knockouts of a CO2 receptor subunit (Gr3) bore a nonfunctional CO2 receptor and lost their attraction to CO2 in cage experiments [28]. The synergy in attraction evoked by the combination of CO2 with L-(+)-lactic acid or human skin odor or heat were also lost in Gr3 knockouts [28]. Interestingly, the Gr3 knockout females exhibited similar attraction to human skin odor alone and to a human arm as wild-type mosquitoes [28]. Thus, CO2 seems to be essential for long-range host attraction, yet mosquitoes can find human hosts by sensing and integrating other cues such as heat, skin odor, and humidity [28].
When the ionotropic receptor coreceptor Ir8a, which is predominantly expressed in the antennae, is knocked out in female Ae. aegypti, these mosquitoes lose the ability to detect odor cues abundant on the human skin, such as L-(+)-lactic acid and other carboxylic acids [80]. In the absence of CO2, Ir8a mutants showed quite reduced attraction to human skin odors [80], as observed for the Orco mutants, indicating that both receptors contribute to skin odor attraction [80]. Upon CO2 activation, Orco knockouts display similar attraction to human skin odors as wild-type mosquitoes [28,80] whereas Ir8a knockouts exhibit reduced attraction to the same cues [80], indicating that CO2 is necessary to gate skin odor attraction through the Ir8a neuronal pathways but not through the Orco pathways [80]. Interestingly, double knockouts of Orco/Ir8a do not completely abrogate attraction to human skin and skin odors in the presence or absence of CO2, pointing to the existence of still other receptors and neural pathways responsible for skin odor detection [80].
Besides odorants, the peripheral sensory system of mosquitoes also perceives physical cues, such as heat and humidity [86,87]. Even though humidity and dryness receptors are yet to be pinpointed for mosquitoes, heat is perceived by the Ir21a receptor [88] and via transient receptor potential (TRPA1) channels [86]. A thermoregulator mediating mosquito heat-seeking was identified in An. gambiae female mosquito antennae [88]. Female Ir21a-receptor knockouts lose most of their attractiveness to a surface warmed at the human body temperature as well as preference for a warmed blood meal [88]. Conversely, female Ae. aegypti knockouts for the trpa1 gene, which encodes a thermosensor and receptor of noxious compounds, are still attracted to warmed objects, yet lose their normal avoidance to stimuli at temperatures higher than the human body [42]. These mosquitoes also lose their avoidance to feed on noxious compounds detected by the TRPA1 channel [42] and to the smell of the mosquito repellent herb catnip [89].
Integration of olfactory information in the olfactory, taste, and visual brain centers
The mosquito olfactory glomerulus is innervated by neurons coexpressing different odorant coreceptors [75] but it also can receive innervations from different classes of olfactory neuron [75]. In addition, specific glomeruli innervated by Ir8a-expressing neurons are activated exclusively by exposure to a combination of CO2 and L-(+)-lactic acid, but not by either of these kairomones alone [90]. As such neurons display very reduced expression of other odorant receptor coreceptors [75] it is possible that activation of the glomerulus innervated by CO2 receptor neurons disinhibits olfactory neurons innervating other glomeruli [90]. A similar phenomenon is observed in floral scent discrimination by Ae. aegypti [91]. The release of higher amounts of the attractive odor nonanal by the preferred orchid leads to activation of the LC2 glomerulus and inhibition of the AM2 glomerulus via GABAergic (γ-aminobutyric acid) interneurons [91]. The latter glomerulus detects odors that are aversive to Ae. aegypti [91].
The signals detected by such olfactory and thermal receptors responsible for sensing human chemical and physical cues are transduced to the primary sensory centers in the mosquito brain [92], where the information is integrated and decoded by the higher brain centers to trigger the behavioral responses [56,60,93,94]. CO2 modulates visual responses in the optical lobe, potentially coupling host identification and host location [94]. Host attraction and avoidance can also be modulated by odor-based mosquito learning behavior, which involves a food-based reward and an aversive stimulus, respectively [56]. Physical evidence for this sort of phenomenon, by contrast, was demonstrated by the axon projections of olfactory neurons on the proboscis’ labella to the subesophageal zone, which is the brain region responsible for taste coding (Figure 2B). These axonal connections potentially integrate taste and smell into the perception of flavor [60]. Together, these findings highlight the initial steps in the elucidation of the complex organization of the primary sensory centers in the mosquito antennal lobes and the subesophageal ganglion, and the complexity of neuronal coding used by mosquitoes to decipher host-choice and drive host-seeking behavior.
Concluding remarks
Disrupting the neural coding that drives mosquito attraction to humans can lead to a reduction in mosquito-borne disease transmission. As only a handful of odorants (DEET, picaridin, eucalyptol, and N-acylpiperidines) have been shown to effectively repel mosquitoes [95–98], the quest for novel effective mosquito repellents has taken the search of huge databases using computational tools involving chemoinformatics [99] and machine learning [100]. Testing DEET substitutes found through chemoinformatics [99] across fruit fly species showed conserved repellency [101], suggesting that such novel repellents might also repel mosquitoes. In addition, previous findings associating odor volatiles with manipulation of mosquito behavior support the potential application of novel chemical ecology approaches in mosquito control (Figure 3):
Figure 3. Odorant-based applications to reduce mosquito attraction.
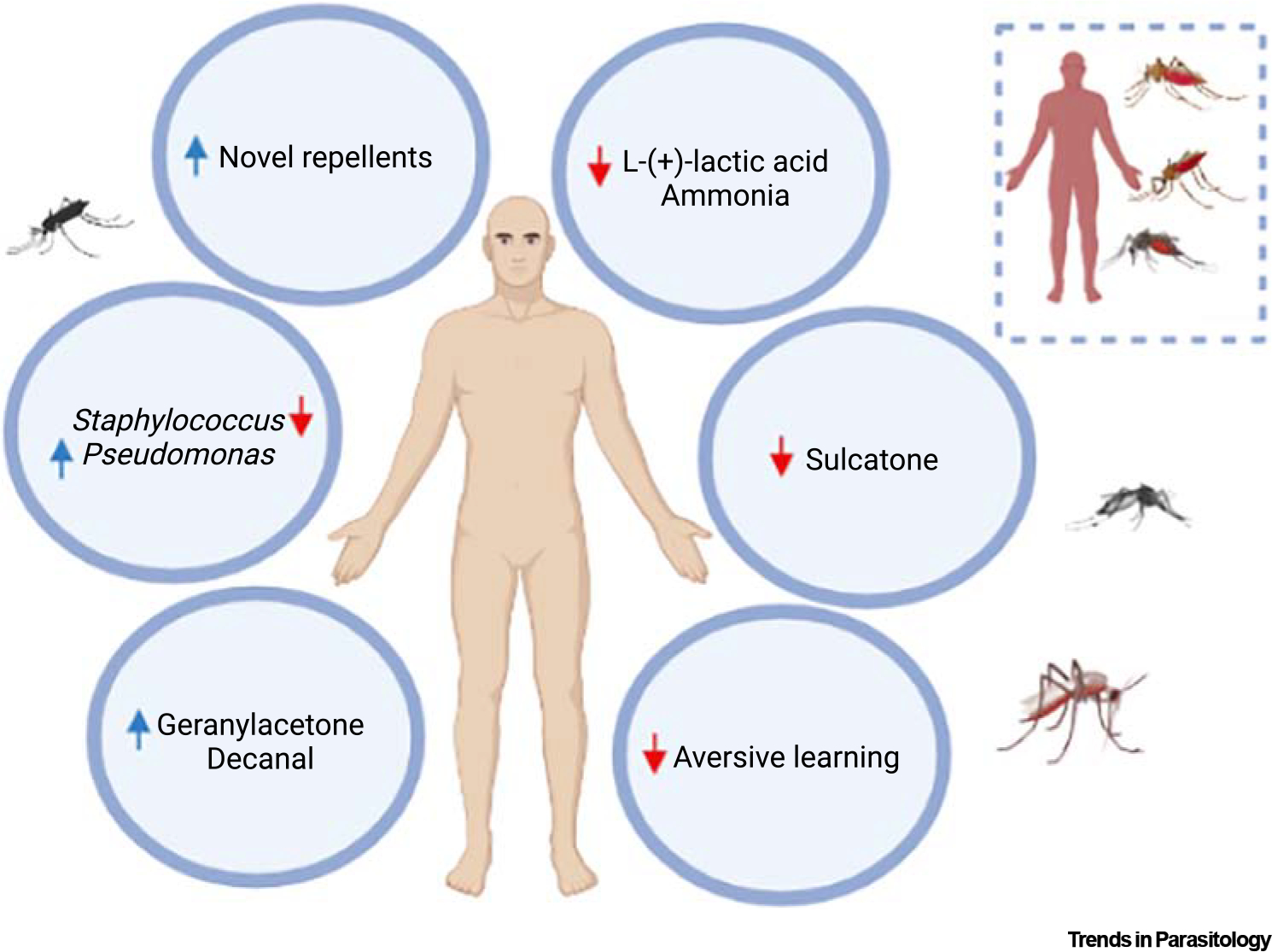
Reducing mosquito attraction to a human host may be achieved by the development of novel repellents. Also, applying an attractive odorant, such as decanal and geranylacetone, on the human skin can also result in mosquito aversive behavior. Alternative strategies to reduce mosquito attraction include training mosquitoes to avoid a human host by integrating an odorant to host-aversive behavior, changing the human skin microbiota profile to a less attractive one, reducing the emission of odorants that ignite mosquito attraction, for example, L-(+)-lactic acid and ammonia, as well as odorants linked to human attractiveness, for instance sulcatone. Inset. Without any intervention, mosquitoes are highly attracted to humans, on which they feed and transmit pathogens. Blue and red arrows point to increasing or decreasing, respectively, the chemical volatiles released or the densities of specific skin resident bacteria. Figure created with BioRender.
Differences in skin microbiota composition are associated with human attractiveness [54]. So, inducing shifts in the human skin microbiota composition might reduce mosquito bites without affecting the skin homeostasis.
Aedes mosquitoes are only attracted to other human body odors and CO2 in the presence of L-(+)-lactic acid [28]. The same role is played by the combination of ammonia and L-(+)-lactic acid in Anopheles mosquitoes [13,24]. Hence, reducing production of both ammonia and L-(+)-lactic acid might reduce mosquito attraction and bites.
Increasing the quantities of attractive odors on the human skin can evoke repellency behavior in mosquitoes and even reduce probing activity, as shown for the skin odorants decanal and geranylacetone [53].
The skin odorant sulcatone drives the mosquito’s preference for human odor [51]; so, reducing sulcatone on the human skin might deflect anthropophilic mosquitoes from biting humans.
Mosquitoes can also learn to avoid hosts by associating skin odors with the host aversive behavior [56]. Applying such odors on the human skin might also cause a deficit in mosquito learning, reducing human bites.
Designing strategies to modify the profile of human skin odor volatiles appears to be pivotal to reduce mosquito bites. Even though odorant coreceptor and heat-sensing knockout mosquitoes are yet attracted to human hosts [28,78,88], re-engineering the native human skin microbiota with bacteria secreting lower amounts of attractive and higher quantities of aversive odorants might completely abrogate mosquito attraction to human hosts (see Outstanding questions). If successful, this strategy opens the doors to a variety of ways in which mosquito host-seeking behavior can be manipulated, leading potentially to a reduction in mosquito bites and preventing the transmission of mosquito-borne diseases.
Outstanding questions.
What are the main odors igniting Culex quinquefasciatus human attraction? Does it follow similar patterns as Anopheles gambiae and Aedes aegypti? How about the other mosquito vectors?
Are there other skin volatiles defining human discrimination by mosquitoes besides sulcatone? Is sulcatone the key odorant driving human attractiveness in other mosquito vectors?
Can mosquito learning be used as a strategy to induce effective mosquito repellency behavior?
Can we completely abrogate mosquito host-seeking behavior by shutting down all olfactory pathways?
Will re-engineering the human skin microbiota be a strategy effective against all mosquito species? Or should this strategy be designed for each main mosquito species?
Highlights.
Mosquito host-seeking is mediated by attraction to physical and chemical cues released in the breath and by the skin.
Most of the skin volatiles are produced by the host skin microbiota, and differences in microbiota composition correlate with human attractiveness.
Quantitative and qualitative discrimination of skin volatiles may be an important driver of host preference for anthropogenic mosquito species.
Differences in skin odor composition are detected by the peripheral olfactory system and discriminated by sequence and expression polymorphisms of olfactory receptors.
Designing new repellents and re-engineering the human skin microbiota to alter the composition of the human scent may possibly reduce mosquito bites and prevent the spread of mosquito-borne diseases.
Acknowledgments
This work was supported in part by a DARPA ReVector Program Grant (HR00112020030) awarded to O.S.A and a National Institute of Health RO1 grant awarded to O.S.A and J.A.R. (R01AI148300). The views, opinions, and/or findings expressed should not be interpreted as representing the official views or policies of the Department of Defense or the US Government.
Glossary
- Anthropophagic
feeding on humans
- Anthropophilic
attraction to and/or preference for feeding on humans
- Anthropo(zoo)philic
attraction to and/or preference for humans and other animals
- Coreceptor
a protein subunit that pairs with other receptors’ subunits, assembling into fully functional receptors
- Edited (gene editing)
a molecular technique that modifies the genetic material by means of deletions (knockouts) and insertions of specific DNA sequences (knockins)
- Genetic drivers
genetic components responsible for controlling gene expression, such as promoters and enhancers. Promoters can be inserted into the genetic material (gene editing) to control the expression of exogenous genes (knockins)
- Glomeruli (olfactory)
structures of the antennal lobes where synapses form between olfactory receptor neurons, projection neurons, and local interneurons. Whereas local interneurons have modulatory roles, projection neurons transmit the signals to the higher brain centers
- Habituation
weakening of behavioral responses due to repeated stimulation
- Heated blood meal
blood warmed at the human body temperature (37°C) and offered to mosquitoes using feeding devices
- Higher brain centers
regions of the insect brain responsible for learning and memory (mushroom bodies) and innate behavior (lateral horn)
- Kairomones
chemical cues released by an animal species that evoke mosquito behavioral responses
- Mosquito host choice (host preference)
the range of host species a mosquito species is typically attracted to and/or feeds on
- Mosquito human discrimination (host specificity)
the degree of selectivity exhibited by a mosquito species in choosing a host
- Odor-based learning behavior
the association of an odorant with a reward, leading to increased behavioral responses towards such an odorant
- Olfactory receptor neurons (ORNs)
the set of sensory neurons that perceive odorants in the antennae’ sensilla and transmit the information to other brain centers
- One receptor to neuron dogma
each olfactory receptor neuron expresses an individual tuning receptor along with a coreceptor, which is also shared with many other ORNs
- Peripheral olfactory system
the set of sensilla and their housed ORNs that mediate the detection of kairomones and pheromones
- Primary sensory centers
the brain structures where input sensory information is received
- Skin microbiota
the microbes (viruses, fungi, and bacteria) covering the skin surface and associated structures (hair follicles, sebaceous glands, and sweat glands)
- Skin odor
the human emanations extracted from skin (volatile bouquet) by different sampling techniques
- Synergism
when responses to two stimuli are stronger than the sum of the responses to each individual stimulus
- Tuning receptors
unique receptor subunits that pair with a shared coreceptor, forming the functional odor receptor
- Valence (odor)
odor valance refers to the spectrum of behavioral responses evoked by an odorant-based stimulus
- Zoophilic
attraction to and/or preference for feeding on (nonhuman) species
- Zoophagic
feeding on (non-human) species
Footnotes
Declaration of interests
O.S.A is a founder of Agragene, Inc. with equity. All other authors declare no competing interests.
References
- 1.Cardé RT (2015) Multi-cue integration: how female mosquitoes locate a human host. Curr. Biol. 25, R793–R795 [DOI] [PubMed] [Google Scholar]
- 2.van Breugel F et al. (2015) Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 25, 2123–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiras AE and Jepson PC (1994) Responses of female Aedes aegypti (Diptera: Culicidae) to host odours and convection currents using an olfactometer bioassay. Bull. Entomol. Res. 84, 207–211 [Google Scholar]
- 4.Hinze A et al. (2021) Mosquito host seeking in 3D using a versatile climate-controlled wind tunnel system. Front. Behav. Neurosci. Published online March 11, 2021. 10.3389/fnbeh.2021.643693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker T et al. (2005) Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208, 2963–2972 [DOI] [PubMed] [Google Scholar]
- 6.Lacey ES and Cardé RT (2011) Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet: 3-D flight analysis in a wind tunnel. Med. Vet. Entomol. 25, 94–103 [DOI] [PubMed] [Google Scholar]
- 7.Bosch OJ et al. (2000) Contribution of fatty acids to olfactory host finding of female Aedes aegypti. Chem. Senses 25, 323–330 [DOI] [PubMed] [Google Scholar]
- 8.Qiu YT et al. (2004) Behavioural and electrophysiological responses of the malaria mosquito Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) to human skin emanations. Med. Vet. Entomol. 18, 429–438 [DOI] [PubMed] [Google Scholar]
- 9.Smallegange RC et al. (2011) Sweaty skin: an invitation to bite? Trends Parasitol. 27, 143–148 [DOI] [PubMed] [Google Scholar]
- 10.Acree F Jr et al. (1968) L-Lactic acid: a mosquito attractant isolated from humans. Science 161, 1346–1347. 10.1126/science.161.3848.1346 [DOI] [PubMed] [Google Scholar]
- 11.Braks MAH and Takken W (1999) Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J. Chem. Ecol. 25, 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaides N et al. (1968) The skin surface lipids of man compared with those of eighteen species of animals. J. Invest. Dermatol. 51, 83–89 [DOI] [PubMed] [Google Scholar]
- 13.Smallegange RC et al. (2009) The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J. Chem. Ecol. 35, 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N (2015) Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol. 60, 537–559 [DOI] [PubMed] [Google Scholar]
- 15.Conrad MD and Rosenthal PJ (2019) Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect. Dis. 19, e338–e351 [DOI] [PubMed] [Google Scholar]
- 16.Cobo F (2016) Determinants of parasite drug resistance in human lymphatic filariasis. Rev. Esp. Quimioter. 29, 288–295 [PubMed] [Google Scholar]
- 17.Okumu FO et al. (2010) Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS One 5, e8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernier UR et al. (2015) Laboratory studies of Aedes aegypti attraction to ketones, sulfides, and primary chloroalkanes tested alone and in combination with L-lactic acid. J. Am. Mosq. Control Assoc. 31, 63–70 [DOI] [PubMed] [Google Scholar]
- 19.Leal WS (2014) The enigmatic reception of DEET – the gold standard of insect repellents. Curr. Opin. Insect. Sci. 6, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antwi FB et al. (2008) Risk assessments for the insect repellents DEET and picaridin. Regul. Toxicol. Pharmacol. 51, 31–36 [DOI] [PubMed] [Google Scholar]
- 21.Konopka JK et al. (2021) Olfaction in Anopheles mosquitoes. Chem. Senses 46, bjab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitzen J et al. (2008) Effect of human odours and positioning of CO2 release point on trap catches of the malaria mosquito Anopheles gambiae sensu stricto in an olfactometer. Physiol. Entomol. 33, 116–122 [Google Scholar]
- 23.Webster B et al. (2015) Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J. Chem. Ecol. 41, 59–66 [DOI] [PubMed] [Google Scholar]
- 24.Smallegange RC et al. (2005) Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem. Senses 30, 145–152 [DOI] [PubMed] [Google Scholar]
- 25.Mukabana WR et al. (2012) A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J. Chem. Ecol. 38, 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Loon JJA et al. (2015) Mosquito attraction: crucial role of carbon dioxide in formulation of a five-component blend of human-derived volatiles. J. Chem. Ecol. 41, 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhulst NO et al. (2010) Differential attraction of malaria mosquitoes to volatile blends produced by human skin bacteria. PLoS One 5, e15829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMeniman CJ et al. (2014) Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernier UR et al. (2003) Synergistic attraction of Aedes aegypti (L.) to binary blends of L-lactic acid and acetone, dichloromethane, or dimethyl disulfide. J. Med. Entomol. 40, 653–656 [DOI] [PubMed] [Google Scholar]
- 30.Williams CR et al. (2006) Laboratory and field assessment of some kairomone blends for host-seeking Aedes aegypti. J. Am. Mosq. Control Assoc. 22, 641–647 [DOI] [PubMed] [Google Scholar]
- 31.Bernier UR et al. (2007) Laboratory comparison of Aedes aegypti attraction to human odors and to synthetic human odor compounds and blends. J. Am. Mosq. Control Assoc. 23, 288–293 [DOI] [PubMed] [Google Scholar]
- 32.Allan SA et al. (2006) Attraction of mosquitoes to volatiles associated with blood. J. Vector Ecol. 31, 71–78 [DOI] [PubMed] [Google Scholar]
- 33.Syed Z and Leal WS (2009) Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. U. S. A. 106, 18803–18808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri SN et al. (2006) Electroantennogram and behavioral responses of Culex quinquefasciatus (Diptera: Culicidae) females to chemicals found in human skin emanations. J. Med. Entomol. 43, 207–213 [DOI] [PubMed] [Google Scholar]
- 35.Lu T et al. (2007) Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner SL et al. (2011) Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474, 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tauxe GM et al. (2013) Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell 155, 1365–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coutinho-Abreu IV et al. (2019) Odorant ligands for the CO2 receptor in two Anopheles vectors of malaria. Sci. Rep. 9, 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaninia M et al. (2019) Hold your breath – Differential behavioral and sensory acuity of mosquitoes to acetone and carbon dioxide. PLoS One 14, e0226815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mburu MM et al. (2017) 2-Butanone as a carbon dioxide mimic in attractant blends for the Afrotropical malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar. J. 16, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacWilliam D et al. (2018) Signaling mode of the broad-spectrum conserved CO2 receptor is one of the important determinants of odor valence in Drosophila. Neuron 97, 1153–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corfas RA and Vosshall LB (2015) The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife 4, e11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu MZ and Vosshall LB (2019) General visual and contingent thermal cues interact to elicit attraction in female Aedes aegypti mosquitoes. Curr. Biol. 29, 2250–2257 [DOI] [PubMed] [Google Scholar]
- 44.Alberto DAS et al. (2021) The olfactory gating of visual preferences to human skin and colors in mosquitoes. bioRxiv Published online July 27, 2021. 10.1101/2021.07.26.453916 [DOI] [Google Scholar]
- 45.Spitzen J et al. (2013) A 3D analysis of flight behavior of Anopheles gambiae sensu stricto malaria mosquitoes in response to human odor and heat. PLoS One 8, e62995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkes FM et al. (2017) Exploiting Anopheles responses to thermal, odour and visual stimuli to improve surveillance and control of malaria. Sci. Rep. 7, 17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neafsey DE et al. (2015) Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arensburger P et al. (2010) Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330, 86–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nene V et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansson BS and Stensmyr MC (2011) Evolution of insect olfaction. Neuron 72, 698–711 [DOI] [PubMed] [Google Scholar]
- 51.McBride CS et al. (2014) Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Z et al. (2020) Chemical signatures of human odour generate a unique neural code in the brain of Aedes aegypti mosquitoes. bioRxiv Published online November 2, 2020. 10.1101/2020.11.01.363861 [DOI] [Google Scholar]
- 53.Logan JG et al. (2008) Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 34, 308–322 [DOI] [PubMed] [Google Scholar]
- 54.Verhulst NO et al. (2011) Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One 6, e28991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinauger C et al. (2014) Olfactory learning and memory in the disease vector mosquito Aedes aegypti. J. Exp. Biol. 217, 2321–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinauger C et al. (2018) Modulation of host learning in Aedes aegypti mosquitoes. Curr. Biol. 28, 333–344.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohbot J et al. (2007) Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 16, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill CA et al. (2002) G protein-coupled receptors in Anopheles gambiae. Science 298, 176–178 [DOI] [PubMed] [Google Scholar]
- 59.Saveer AM et al. (2018) Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci. Rep. 8, 5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riabinina O et al. (2016) Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun. 7, 13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leal WS et al. (2013) Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc. Natl. Acad. Sci. U. S. A. 110, 18704–18709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rytz R et al. (2013) Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 43, 888–897 [DOI] [PubMed] [Google Scholar]
- 63.Benton R et al. (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silbering AF et al. (2011) Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13357–13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butterwick JA et al. (2018) Cryo-EM structure of the insect olfactory receptor Orco. Nature 560, 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abuin L et al. (2011) Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato K et al. (2008) Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 [DOI] [PubMed] [Google Scholar]
- 68.Carey AF et al. (2010) Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vosshall LB et al. (2000) An olfactory sensory map in the fly brain. Cell 102, 147–159 [DOI] [PubMed] [Google Scholar]
- 70.Shankar S and McMeniman CJ (2020) An updated antennal lobe atlas for the yellow fever mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 14, e0008729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ignell R et al. (2005) Neuronal architecture of the mosquito deutocerebrum. J. Comp. Neurol. 493, 207–240 [DOI] [PubMed] [Google Scholar]
- 72.Basrur NS et al. (2020) Fruitless mutant male mosquitoes gain attraction to human odor. eLife 9, e63982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amos BA et al. (2021) Comment on ‘Fruitless mutant male mosquitoes gain attraction to human odor’. bioRxiv Published online July 12, 2021. 10.1101/2021.05.24.445527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill SR and Ignell R (2021) Modulation of odour-guided behaviour in mosquitoes. Cell Tissue Res. 383, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Younger MA et al. (2020) Non-canonical odor coding ensures unbreakable mosquito attraction to humans. bioRxiv Published online November 8, 2020. 10.1101/2020.11.07.368720 [DOI] [Google Scholar]
- 76.Task D et al. (2021) Chemoreceptor co-expression in Drosophila olfactory neurons. bioRxiv Published online July 27, 2021. 10.1101/2020.11.07.355651 [DOI] [Google Scholar]
- 77.Matthews BJ et al. (2018) Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeGennaro M et al. (2013) orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu F et al. (2020) Gene editing reveals obligate and modulatory components of the CO receptor complex in the malaria vector mosquito, Anopheles coluzzii. Insect Biochem. Mol. Biol. 127, 103470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raji JI et al. (2019) Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr. Biol. 29, 1253–1262.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun H et al. (2020) Mutagenesis of the orco odorant receptor co-receptor impairs olfactory function in the malaria vector Anopheles coluzzii. Insect Biochem. Mol. Biol. 127, 103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinker DC et al. (2013) Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics 14, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rose NH et al. (2020) Climate and urbanization drive mosquito preference for humans. Curr. Biol. 30, 3570–3579.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar A et al. (2020) Contributions of the conserved insect carbon dioxide receptor subunits to odor detection. Cell Rep. 31, 107510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu P et al. (2020) CO2 per se activates carbon dioxide receptors. Insect Biochem. Mol. Biol. 117, 103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang G et al. (2009) Anopheles gambiae TRPA1 is a heatactivated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 30, 967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gingl E et al. (2005) Sensory representation of temperature in mosquito warm and cold cells. J. Neurophysiol. 94, 176–185 [DOI] [PubMed] [Google Scholar]
- 88.Greppi C et al. (2020) Mosquito heat seeking is driven by an ancestral cooling receptor. Science 367, 681–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melo N et al. (2021) The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr. Biol. 31, 1988–1994.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shankar S et al. (2020) Synergistic coding of human odorants in the mosquito brain. bioRxiv Published online November 4, 2020. 10.1101/2020.11.02.365916 [DOI] [Google Scholar]
- 91.Lahondère C et al. (2020) The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 117, 708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson RI (2008) Neural and behavioral mechanisms of olfactory perception. Curr. Opin. Neurobiol. 18, 408–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vosshall LB and Stocker RF (2007) Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 [DOI] [PubMed] [Google Scholar]
- 94.Vinauger C et al. (2019) Visual-olfactory integration in the human disease vector mosquito Aedes aegypti. Curr. Biol. 29, 2509–2516.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Syed Z and Leal WS (2008) Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. U. S. A. 105, 13598–13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klocke JA et al. (1987) 1,8-Cineole (Eucalyptol), a mosquito feeding and ovipositional repellent from volatile oil of Hemizonia fitchii (Asteraceae). J. Chem. Ecol. 13, 2131–2141 [DOI] [PubMed] [Google Scholar]
- 97.Kline DL et al. (2003) Olfactometric evaluation of spatial repellents for Aedes aegypti. J. Med. Entomol. 40, 463–467 [DOI] [PubMed] [Google Scholar]
- 98.Katritzky AR et al. (2008) Synthesis and bioassay of improved mosquito repellents predicted from chemical structure. Proc. Natl. Acad. Sci. U. S. A. 105, 7359–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boyle SM et al. (2013) Expanding the olfactory code by in silico decoding of odor-receptor chemical space. eLife 2, e01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kepchia D et al. (2019) Use of machine learning to identify novel, behaviorally active antagonists of the insect odorant receptor co-receptor (Orco) subunit. Sci. Rep. 9, 4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krause Pham C and Ray A (2015) Conservation of olfactory avoidance in Drosophila species and identification of repellents for Drosophila suzukii. Sci. Rep. 5, 11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dormont L et al. (2013) Human skin volatiles: a review. J. Chem. Ecol. 39, 569–578 [DOI] [PubMed] [Google Scholar]
- 103.Verhulst NO et al. (2009) Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 8, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Council SE et al. (2016) Diversity and evolution of the primate skin microbiome. Proc. R. Soc. B 283, 20152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verhulst NO et al. (2018) Do apes smell like humans? The role of skin bacteria and volatiles of primates in mosquito host selection. J. Exp. Biol. 221, jeb185959. [DOI] [PubMed] [Google Scholar]
- 106.Ross AA et al. (2018) Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc. Natl. Acad. Sci. U. S. A. 115, E5786–E5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu YT et al. (2006) Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem. Senses 31, 845–863 [DOI] [PubMed] [Google Scholar]
- 108.Mweresa CK et al. (2016) Enhancing attraction of African malaria vectors to a synthetic odor blend. J. Chem. Ecol. 42, 508–516 [DOI] [PubMed] [Google Scholar]


