Abstract
Objective
Temporomandibular disorders (TMD) comprise a cluster of conditions with a wide range of etiological factors that causes pain and discomfort in the masticatory muscles (TMD-M) and temporomandibular joints (TMD-J). More than 50% of the patients with TMD report regular usage of drugs. However, there is still no consensus, nor is there any evidence-based support for clinicians when choosing between different drugs. Therefore, this systematic review, including a network meta-analysis (NMA), aimed to evaluate the scientific evidence and discuss the pharmacological treatment options available to treat painful TMD.
Method
An electronic search was undertaken to identify randomized controlled trials (RCTs) investigating pharmacological treatments for TMD-M and/or TMD-J, published until 6 April 2023. Since only 11 articles could be used for an NMA regarding TMD-M, a narrative synthesis was also performed for all 40 included RCTs. The quality of evidence was rated according to Cochrane’s tool for assessing risk of bias, while the certainty of evidence was rated according to Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Results
When it comes to TMD-M, evidence arises for wet needling therapies with BTX-A, granisetron, and PRP as well as muscle relaxants. For TMD-J, evidence points toward pharmacological treatment approaches including non-steroidal antiinflammatory drugs (NSAIDs) and glucocorticosteriods (for inflammatory conditions) as well as hyaluronic acid and dextrose.
Conclusions
The evidence clearly indicates that the pharmacological treatment approaches differ between TMD-M and TMD-J. Therefore, it is of great importance to first try to uncover each patient’s individual and multifactorial etiology and then employ a multifaceted treatment strategy, including pharmacological treatment approaches.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-023-01971-9.
Key Points
| Evidence clearly indicates that the pharmacological treatment approaches differ between temporomandibular disorders of muscular and arthrogenous origin. |
| For temporomandibular disorders of muscular origin, evidence arises for wet needling therapies with BTX-A, granisetron, and PRP as well as muscle relaxants. |
| For temporomandibular disorders of arthrogenous origin, evidence arises for non-steroidal antiinflammatory drugs (NSAIDs) and glucocorticosteriods (for inflammatory conditions) as well as hyaluronic acid and dextrose. |
Introduction
Temporomandibular disorders (TMD) comprise a cluster of conditions that causes pain and discomfort in the masticatory muscles, temporomandibular joints (TMJ), and surrounding structures [1, 2]. TMD encompasses a range of painful symptoms, such as ear and facial pain, headache in the temporal region, and tooth sensitivity, as well as non-painful symptoms such as clicking, popping, or crepitus of the TMJ, limited jaw-movements, and muscle fatigue or stiffness [2, 3].
TMD impacts approximately 10–15% of the adult population [4, 5] and seems to be three times more frequent in women [6]. Painful TMD of musculoskeletal origin, e.g., TMD-M, is the most common diagnosis with a frequency of 42–70% [7, 8]. It is often characterized as a persistent dull, mild-to-moderate muscle pain, which can be intensified into a sharper pain sensation and radiate to adjacent structures when provoked by jaw function [9]. Another typical sign of TMD-M is tenderness or pain on palpation of the masticatory muscles [2]. TMD-M has been shown to negatively affect quality of life [10] and is often associated with psychological distress such as depression and anxiety [11]. The underlying etiology is complex and likely to be both multifactorial and biopsychosocial [1, 12].
Painful TMD of arthrogenous origin, e.g., TMD-J, can manifest within a healthy TMJ or in a TMJ affected by inflammation, such as arthritis [2]. In a healthy TMJ, pain arises from nociceptors located in the surrounding soft tissue of the joint. This pain occurs mainly during mandibular movements and ceases as soon as the jaw returns to its natural resting position [13]. However, in an inflamed TMJ, pain also emerges from nociceptors in the subarticular bone, exposed by the inflammatory processes [13, 14]. In this case, pain is constantly throbbing and worsens with jaw function. TMJ arthritis can generally be categorized on the basis of its underlying causes as local arthritis or arthritis associated with systemic disease [15]. Examples of local arthritis include synovitis, traumatic arthritis, or capsulitis. While the etiology remains largely unknown, there are some reported contributing factors. Mechanical overload of the TMJ [16], a perforation of the TMJ disc [17], or a disc displacement in the TMJ [18–20] have been reported as potential contributing factors. Further, systemic arthritis stems from underlying systemic diseases such as rheumatoid arthritis, juvenile arthritis, and psoriatic arthritis, representing a localized manifestation of a broader systemic inflammatory process. As with local arthritis, the etiology remains largely unknown for systemic arthritis [21].
On the basis of the multifactorial etiology of painful TMD [22], and since painful TMD exhibit a range of intricate symptoms and underlying causes, it is advisable to employ a multifaceted treatment strategy [23–25]. This strategy encompasses a diverse range of therapeutic modalities, including occlusal splints [26], physiotherapy [27] and/or jaw exercises [28], behavioral medicine [24], TMJ surgery [29], and pharmacological interventions [30]. While reversible non-pharmacological interventions are often recommended as first-line treatments [24], pharmacological options can play a crucial role in pain management and enhancement of overall life quality in patients with painful TMD. Further, as many as 50% of patients with painful TMD have reported usage of drugs [31]. Numerous studies have investigated the efficacy of various pharmacological treatment in painful orofacial pain conditions, including TMD [32]. Non-steroidal antiinflammatory drugs (NSAIDs), opioids, corticosteroids, anxiolytics, antidepressant, muscle relaxants, and anticonvulsants are the most frequently used pharmacological agents prescribed by clinicians [33]. Nonetheless, the best pharmacological treatment modality with predictable outcomes based on solid evidence is still largely unknown. Therefore, this systematic review, including a network meta-analysis (NMA), aimed to evaluate the scientific evidence and discuss the pharmacological treatment options available to treat painful TMD.
Materials and Methods
Protocol
This systematic review, including an NMA, followed the protocol that was registered a priori in PROSPERO (the International Prospective Register of Systematic Reviews, registration no. CRD42023406861). Further, the included NMA of randomized controlled trials (RCTs) was conducted according to the Preferred Reporting Items for the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions (the PRISMA-P checklist) (Supplementary information 1) [34].
Selection Criteria
The following inclusion criteria were adopted on the basis of the PICOTS approach:
Population (P), Intervention (I), Comparator (C), Outcome (O), Time (T), Study (S)
The population (P) was adult patients having painful temporomandibular disorders. In the analysis they were subgrouped according to the location of pain to either (a) TMD-M; i.e., pain of myogenous origin, and (b) TMD-J; i.e., pain of arthrogenous origin. However, there were not enough studies on TMD-J, so this is only presented in the narrative part of the results.
The intervention (I) was any type of pharmacological treatment for painful TMD.
The comparators (C) were other pharmacological treatments, no treatment, placebo, or on waiting list.
The primary outcome (O) was pain reduction using a visual analogue scale (VAS; 0–100) or a numeric rating scale (NRS; 0–10). For the analysis the NRS was transformed to a 0–100 scale. The secondary outcome was changes in maximum mouth opening (MMO). However, there too few studies to do an NMA, so this is only presented in the narrative part of the results.
The follow-up time (T) was either short term ≤ 3 months, intermediate term 3–5 months, or long term ≥ 6 months
The study design (S) was composed only of randomized controlled trials that reported the outcomes of interest.
The following exclusion criteria were used: (1) studies presented in languages other than English and Scandinavian languages; (2) editorials, letters, legal cases, interviews, case-series, duplicates, observational studies, cross-sectional studies and case-control studies, non-randomized clinical trials, cohort studies, and review articles; (3) publications using duplicated data; (4) studies not investigating pharmacological treatments for painful TMD; and an additional criterion for the NMA (5) studies with missing data required to perform a meta-analysis, such as the post-treatment mean and standard deviation for the outcomes of interest.
Search Strategy
In collaboration with the librarians Lovisa Liljegren (LL) and Narcisa Hannerz (NH) at the Karolinska Institutet University Library, we designed a search strategy that identified randomized controlled studies reporting data on pharmacological treatments in a patient population with painful TMD. The electronic search was performed on 6 April 2023 and included all relevant RCTs, in any language and with any publication date, from the databases MEDLINE, EMBASE, CINAHL, the Cochrane Central Registry of Controlled Trials (CENTRAL), and Web of Science from the inception of each database to 6 April 2023.
The search strategy was developed in MEDLINE (Ovid) in collaboration LL and NH. The search strategies were peer-reviewed by NH before LL performed the search. For each search concept medical subject headings (MeSH-terms) and free-text terms were identified. The search was then translated, in part using Polyglot Search Translator [35], into the other databases. Finally, de-duplication was performed with the method presented by Bramer et al. in 2016 [36]. One final extra step was added to compare DOIs. Grey literature was not included. The complete search strategies for all databases are available in Supplementary information 2.
The Rayyan tool was used to assist with screening of titles and abstracts [37]. Two of the authors (MC and GB) independently and blinded screened the titles and the abstracts. When there was a conflict regarding potentially eligible articles for inclusion, a third author (NC) solved this disagreement by discussion, thus having the role of judge. All potentially eligible studies were then retrieved, and the full-text articles were reviewed by the same authors (MC and GB) to determine whether they met the inclusion criteria. Any disagreement was again resolved by discussion with the third author (NC).
Data Extraction
A data extraction form was developed for this review and pilot tested independently on two randomly selected studies by two of the authors (MC and GB) to ensure consistency in extraction. The extraction form was refined accordingly. Any disagreement in data extraction was resolved by discussion with a third author having the role of judge (NC). The extracted information included the characteristics of the studies and participants, i.e., authors, title, study design, subgroup diagnoses, diagnostic criteria used, age of patients, male–female ratio, treatment groups (number), duration of treatments/frequency, and outcome measures.
Assessment of Risk of Bias and Certainty of Evidence
Risk of bias was determined by two authors (MCh and JS) independently, using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB2) [38]. Any disagreement was resolved by discussion, with a third author having the role of judge (NC). The tool is structured into a fixed set of five domains of bias evaluating different aspects of the article including design, conduct, and reporting. Through an algorithm, judgment about the risk of bias is generated. The judgment can be that the article has either a low or a high risk of bias or that it can express some concerns.
Certainty of evidence was assessed (by EA) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach of meta-analysis [39] GRADE was implemented to identify the certainty of effect estimates from the meta-analysis for the outcomes of interest, i.e., pain intensity, in the present NMA. The certainty of evidence for RCTs, as assessed by the GRADE system, includes four levels of quality of evidence: (1) high quality of evidence—i.e., the real effect is close to that of the estimated effect; (2) moderate quality evidence—i.e., the real effect is likely to be close to the estimated effect, but there is a possibility that there is a substantial difference; (3) low-quality evidence—i.e., the real effect may be significantly different from the estimated effect; and finally (4) very low-quality evidence—i.e., the real effect is likely to be significantly different from the estimate of the effect. Thus, the certainty of evidence for RCTs, as assessed by the GRADE system, begins as high-quality evidence and is then down-rated due to limitations in study design (risk of bias), inconsistency, imprecision, indirectness, and publication bias.
Data synthesis
Before conducting the NMA in this review, a network plot was performed to present the network geometry. This was performed to assess whether the included RCTs were connected [40]. NMA was connected for the outcome of post-treatment pain intensity for TMD-M, while the other outcomes of post-treatment pain intensity for TMD-J as well as MMO with and without pain were not analyzed using NMA, but instead reported narratively.
As presented previously by our group [24, 41–43], the post-treatment pain intensity values, which were the outcome of interest, were used to calculate the standardized mean difference (SMD). For each possible pair of treatments, the results from the NMA are presented as a summary of relative effect sizes (SMD). The statistical unit used was number of patients.
The method used in this review follows our previous publications step by step and has been previously described by our group [24, 41–43]. Statistical models used in this NMA were according to multiple published statistics and assumptions [44–48].
To conduct the NMA, using the mvmeta command, the software STATA (StataCorp. 2011. Stata Statistical Software: Release 15. College Station, TX) was used [49–51]. To identify any local inconsistency, the loop-specific approach was performed separately in each closed loop of the network. The inconsistency factor was analyzed by analyzing the difference between direct and indirect estimates for a defined comparison in the loop of the network. The amount of the inconsistency factors and their 95% confidence intervals (CI) were used to infer the detection of inconsistency in each loop. In addition, a common heterogeneity estimate, within each loop, was assumed [47]. By using the ifplot command in STATA, the results of this approach were presented in a forest plot [46]. To control for the assumption of consistency in the entire network the design-by treatment model using STATA and the mvmeta command were performed, as described by Higgins and colleagues [52–54]. A meta-regression analysis of the mean of pain reduction based on VAS and follow-up time was used to assess whether the duration of follow-up influenced the post-treatment pain intensity. RCTs with a high risk of bias were excluded, and the analysis was then repeated to assess the robustness of the results. Ranking probabilities for all treatments at each possible rank for each intervention was then estimated. The treatment hierarchy was analyzed using the surface under the cumulative ranking (SUCRA) curve and mean ranks [54, 55]. SUCRA can also be presented as a percentage of treatment that can be ranked first without uncertainty [55].
Results
Literature Search Outcome
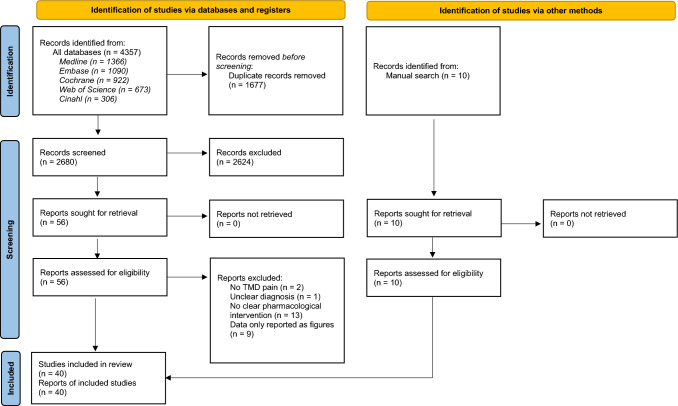
The full electronic search resulted in 4357 articles from all databases, but after removal of 1677 duplicates, a total of 2680 article titles and abstracts were screened. Out of the 2680 articles, 2527 were excluded after reading the titles and abstracts, resulting in 153 articles sought for retrieval. An additional ten were found from other sources. Finally, after reading the 163 full-text articles, 123 did not meet the inclusion criteria and were excluded, resulting in a total of 40 RCTs [56–95] included in this systematic review, out of which 11 were used in the NMA [56, 59, 69, 70, 72, 75, 76, 81, 83, 85, 93]. Figure 1 shows the PRISMA flow diagram with the process of evaluating RCTs for inclusion.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the database search strategy. TMD temporomandibular disorders
Presentation of Network Geometry
Nine interventions (botulinum toxin-A, clonazepam, morphine 5 mg, morphine 1.5 mg, magnesium sulfate, lidocaine, melatonin, cyclobenzaprine, and placebo) were included in the network diagrams for the outcome of post-treatment pain intensity via VAS, as shown in Fig. 2.
Fig. 2.
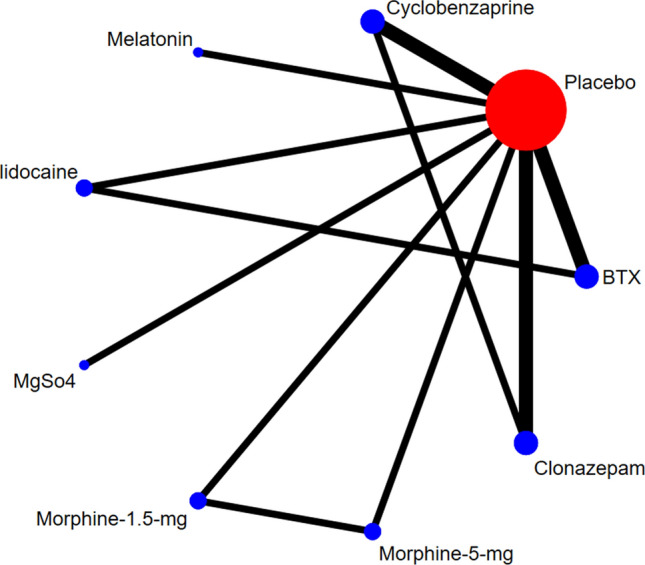
Network geometry for the outcome of post-treatment pain intensity. BTX botulinum toxin-A, MgSo4 magnesium sulphate
Study Characteristics, Individual Data, and Certainty of Evidence
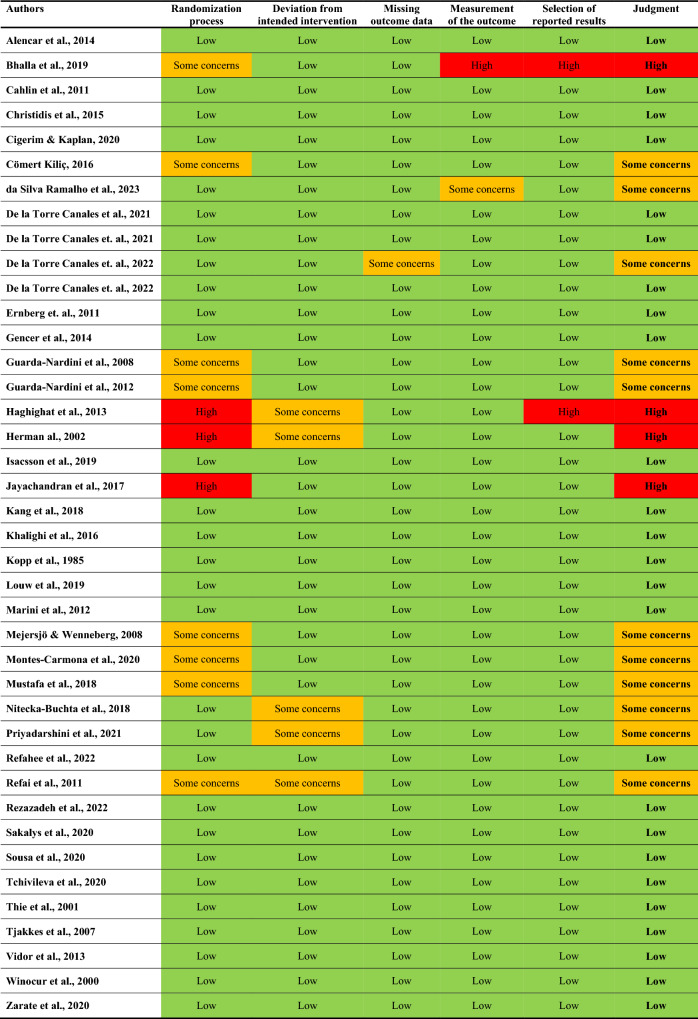
The extracted study characteristics of the included RCTs are presented in Table 1. In total, 25 of the included studies showed a low risk of bias (green), 11 some concerns (orange), and 4 a high risk of bias (red), as presented with color and text in Table 2. For the NMA estimates, the certainty of evidence for all comparisons ranged from low (cyclobenzaprine) to very low (botulinum toxin-A, clonazepam, morphine 5 mg, morphine 1.5 mg, magnesium sulfate, lidocaine, melatonin, and placebo).
Table 1.
Extracted study characteristics of the 40 included RCTs
| Authors, year | Study design | Subgroup diagnosis | Criteria used | Age of patients (years) | Male:female ratio | Treatment groups (no. of participants) | Duration of treatments/frequency | Outcomes measure | Follow-up time | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Alencar et al. (2014) [56] | RCT | Myofascial jaw pain | AAOP |
G1: 37 G2: 36.5 G3: 36.9 |
G1: 1:15 G2: 0:15 G3: 1:15 |
G1: placebo (15) G2: cyclobenzaprine (15) G3: tizanidine (15) |
G1: every day for 3 weeks G2: every day for 3 weeks G3: every day for 3 weeks |
Pain VAS | 3 weeks | Low |
| Bhalla et al. (2019) [57] | RCT | Myofascial pain | Clinical examination |
G4: NM G5: NM G6: NM |
G4: NM G5: NM G6: NM |
G4: ibuprofen + chlorzoxazone or carbamazepinen (10) G5: ibuprofen + chlorzoxazone or carbamazepine + holistic treatment (10) G6: holistic treatment (10) |
G4: every day for 2 weeks G5: every day for 2 weeks, including 60 min of holistic treatment the first day G6: 60 min of holistic treatment the first day |
Pain VAS | 3 months | High |
| Cahlin et al. (2011) [58] | RCT | Osteoarthritis | RDC/TMD |
G1: 54 G7: 61 |
G1: 1:10 G7: 1:6 |
G1: placebo (29) G7: glucosamine sulphate (30) |
G1: every day for 6 weeks G7: every day for 6 weeks |
Pain VAS MMO |
6 weeks | Low |
| Christidis et al. (2015) [59] | RCT | Myofascial pain | DC/TMD |
G1: 39.1 G8: 38.3 |
G1: 1:10 G8: 1:10 |
G1: placebo (20) G8: granisetron (20) |
G1: once a week for 3 weeks G8: once a week for 3 weeks |
Pain VAS MMO |
6 months | Low |
| Cigerim and Kaplan (2020) [60] | RCT | Myofascial pain | DC/TMD |
G1: 27.0 G9: 27.0 G10: 27.0 G11: 27.0 |
G1: 1:4 G9: 1:4 G10: 1:4 G11: 1:4 |
G1: placebo/control = paracetamol (47) G9: naproxen (42) G10: naproxen + codeine (40) G11: naproxen + single-dose dexamethasone (40) |
G1: every day for 1 week G9: every day for 1 week G10: every day for 1 week G11: every day for 1 week |
Pain VAS | 4 weeks | Low |
| Cömert Kiliç (2016) [61] | RCT | Osteoarthritis | DC/TMD |
G12: 32 G13: 28 |
G12: 1:8 G13: 1:3 |
G12: arthrocentesis + PRP injections (18) G13: arthrocentesis plus HA injection (13) |
G12: arthrocentesis + 1 mL PRP injection initially, and then four consecutive 1 mL PRP injection in TMJ at monthly intervals G13: arthrocentesis + a single intraarticular injection of HA |
Pain VAS |
G12:11.6 months G13: 12.8 months |
Some concerns |
| Da Silva Ramalho et al. (2023) [62] | RCT | Myofascial pain | Not specified |
G14: 39 G15: 37 |
G14: 1:4 G15: 1:4 |
G14: BTX-A in masseter (10) G15: BTX-A in masseter and temporalis (10) |
G14: single-session BTX-A injection in masseter, bilaterally (1 mL into every facial point, three points in each muscle) G15: single-session BTX-A injection in masseter and temporal muscles (1 mL into every facial point, three points in each masseter and two points in each temporal muscle) |
Pain VAS | 6 months | Some concerns |
| De la Torre Canales et al. (2021) [64] | RCT | Myofascial pain | RDC/TMD |
G1: 31 G16: 30 G17: 35 |
0:54 |
G1: placebo (n = 18) G16: acupuncture (n = 18) G17: BTX-A low (n = 18) |
G1: single-session bilateral injection in masseter and anterior temporalis NaCl 0.9% was bilaterally injected into the same muscles and sit G16: four sessions of traditional acupuncture, one 20-min session/week for 1 month G17: single-session bilateral injection in masseter and anterior temporalis using 30 U and 10 U of BoNT-A, distributed in five sites/muscles |
Pain VAS | 1 month | Low |
| De la Torre Canales et al. (2021) [65] | RCT | Myofascial pain | RDC/TMD |
G1: 37 G17: 37 G18: 37 G19: 37 G20: 37 |
0:100 |
G1: placebo G18: OA (n = 20) G17: BTX-A low (n = 20) G19: BTX-A medium (n = 20) G20: BTX-A high (n = 20) |
G1: single-session saline (0.9%) intramuscular injection (0.4 mL in m temporalis and 0.6 mL in masseter) G18: OA every night for 6 months G17: single-session intramuscular injection BTX-A low 10 U in m temporalis and 30 U in masseter G19: single-session intramuscular injection BTX-A medium 20 U in m temporalis and 50 U in masseter G20: single-session intramuscular injection BTX-A high 25 U in m temoralis and 75 U in masseter |
Pain VAS | 6 months | Low |
| De la Torre Canales et al. (2022) [63] | RCT | Myofascial pain | RDC/TMD | – | – |
G17: BTX-A-low (n = MD) G19: BTX-A-medium (n = MD) G20: BTX-A-high (n = MD) |
G17: BTX-A-low (masseter 30 U, temporalis 10 U), G19: BTX-A-medium (temporalis 20 U, masseter 50 U) G20: BTX-A-high temporalis 25 U, masseter 75 U) |
Pain VAS | 6 years | Some concerns |
| De la Torre Canales et al. (2022) [66] | RCT | Myofascial pain | RDC/TMD |
G1: 18–45 G17: 18–45 G19: 18–45 G20: 18–45 |
0:80 |
G1: placebo (n = 20) G17: BTX-A low (n = 20) G19: BTX-A medium (n = 20) G20: BTX-A high (n = 20) |
G1: saline solution 0.9%. 1–4: bilateral injection G17: 10 U in each temporalis and 30 U in each masseter G19: 20 U in each temporalis and 50 U in each masseter G20: 25 U in each temporalis and 75 U in each masseter |
Pain VAS MMO |
6 months | Low |
| Ernberg et al. (2011) [67] | RCT | Myofascial pain | RDC/TMD |
G1: 38 G14: 38 |
G1: 1:10 G14: 1:10 |
G1: placebo (n = 21) G14: BTX-A intramuscular (masseter) (n = 21) |
G1: single-session intramuscular isotonic saline masseter injection G14: single-session intramuscular BTX-a (50 U) injection |
Pain VAS MMO |
7 months | Low |
| Gencer et al. (2014) [68] | RCT | Internal derangement of the TMJ |
Wilke’s classification G1: Control groups selected from early-stage (I) patients G21–G23: Late-intermediate (IV) and late-stage (V) patients included in the study groups |
G1 40 G21: 36 G22: 38 G23: 40 |
G1: 12/13 G21: 11/14 G22: 11/14 G23:11/14 |
G1: intraarticular injection saline solution (n = 25) G21: HA intraarticular injection (n = 25) G22: betamethasone intraarticular injection (n = 25) G23: tenoxicam intraarticular injection (n = 25) |
G1 intraarticular injection saline solution G21: HA G22: betamethasone G23: tenoxicam |
Pain VAS | 6 weeks | Low |
| Guarda-Nardini et al. (2008) [69] | RCT | Myofascial pain | RDC/TMD |
G1: 25–45 G24: 25–45 |
1:1 |
G1: placebo (n = 10) G24: BTX-A (n = 10) |
G1: single-session intramuscular injection G24: single-session with 4 BTX-A intramuscular injections (masseter 30 U, temporalis anterior 20 U) |
Pain VAS MMO |
6 months | Some concerns |
| Guarda-Nardini et al. (2012) [70] | RCT | Myofascial pain | RDC/TMD | G25: 48 G26: 43 | 1:3 |
G25: BTX-A masseter and temporalis muscle (n = 15) G26: three sessions with fascial manipulation treatment (n = 15) |
G25: single session of multiple botulin toxin injections in the temporalis and masseter muscles (150 U) G26: 3× (±1) 50-min sessions on a weekly basis, for a total of 150 (±50) min over a 2–4-week span |
Pain VAS MMO |
3 months | Some concerns |
| Haghighat et al. (2013) [71] | RCT | Symptomatic osteoarthrosis | RDC/TMD |
G27: 27 G28: 27 |
1:3 1:3 |
G27: ibuprofen 400 mg (n = 30) G28: glucosamine sulphate 1500 mg (n = 30). |
G27: twice a day for 3 months G28: once a day for 3 months |
Pain VAS MMO |
3 months | High |
| Herman et al. (2002) [72] | RCT | Myofascial pain | RDC/TMD |
G1: 30 G29: 27 G30: 24 |
G1: 1:2 G29: 1:5 G30:: 1:8 |
G1: placebo (n = 15) G29: clonazepam (n = 13) G30: cyclobenzaprine (n = 13) |
G1: placebo capsule G29: capsule 1 h before bedtime for 3 weeks G30: capsule 1 h before bedtime for 3 weeks |
Pain VAS | 3 weeks | High |
| Isacsson et al. (2019) [73] | RCT | Unilateral TMJ arthralgia | DC/TMD |
G1: 56 G31: 48 |
G1: 1:3 G31: 1:6 |
G1: placebo (n = 27) G31: methylprednisolone (n = 27) |
G1: single-session intraarticular injection G31: single-session intraarticular injection |
Pain VAS MMO |
6 weeks | Low |
| Jayachandran et al. (2017) [74] | RCT | TMJ osteoarthritis | Clinical examination |
G32: 49 G33: 49 G34: 49 |
G32: 13:17 G33: 13:17 G34: 13:17 |
G32: diclofenac sodium 50 mg (n = 10) G33: diclofenac sodium + oral enzymes [bromelain, trypsin, rutoside trihydrate] (n = 10) G34: oral enzyme ([romelain, trypsin, rutoside trihydrate] (n = 10) |
G32: twice a day for 10 days G33: twice a day for 10 days G34: twice a day for 10 days |
Pain VAS | 10 days | High |
| Kang et al. (2018) [75] | RCT | Myalgia | DC/TMD |
G1: M: 30, F: 29 G35: M: 30, F: 29 G36: M: 30, F: 29 G37: M: 30, F: 29 G38: M: 30, F: 29 |
G1: 6:5 G35: 8:5 G36: 5:6 G37: 6:5 G38: 2:3 |
G1: saline in masseter (n = 11) G35: morphine 1.5 mg in masseter (n = 13) G36: morphine 5 mg masseter (n = 11) G37: lidocaine masseter (n = 11) G38: morphine 5 mg trapezius (n = 5) |
G1: single bolus intramuscular injection G35: single bolus intramuscular injection G36: single bolus intramuscular injection G37: single bolus intramuscular injection G38: single bolus intramuscular injection |
Pain VAS | 48 h | Low |
| Khalighi et al. (2016) [76] | RCT | Myofascial pain | RDC/TMD |
G39: 36 G40: 36 |
1:3 |
G39: naproxen 500 mg + placebo laser sessions (n = 20) G40: active laser (diode 810 nm CW) as treatment + placebo drug (n = 20) |
G39: naproxen daily for 3 weeks + 12 placebo laser sessions G40: 12 active low-level laser sessions + daily placebo drug for 3 weeks |
Pain VAS MMO |
2 months | Low |
| Kopp et al. (1985) [76] | RCT | Arthralgia | Clinical examination |
G1: 46 G41: 46 |
G1: 1:7 G41: 1:7 |
G1: intraarticular injection of 0.5 mL sodium hyaluronate (10 mg/mL) (n = 18) G41: intraarticular injection of 0.5 mL of corticosteroid betamethasone (n = 15) |
G1: single intraarticular injection G41: single intraarticular injection |
Helkimos index | 4 weeks | Low |
| Louw et al. (2019) [78] | RCT | TMJ arthralgia | RDC/TMD |
G42: 44 G43: 50 |
G42: 1:3 G43: 1:23 |
G42: intraarticular injections 20% dextrose + 0.2% lidocaine (n = 30) G43: intraarticular injections 0.2% lidocaine (n = 24) |
G42: intraarticular injections at 0, 1, and 2 months G43: 0.2% lidocaine; intraarticular injections at 0, 1, and 2 months At 3-month follow-up, allocation groups were revealed; participants in both groups were offered open-label injection of 20% dextrose/0.2% lidocaine monthly on a by-request basis |
Pain VAS MMO |
1 year | Low |
| Marini et al. (2012) [79] | RCT | TMJ osteoarthritis or arthralgia | RDC/TMD |
G44: 14–54 G45: 14–54 |
G44: 1:2 G45: 1:2 |
G44: palmitoylethanolamide (n = 12) G45: ibuprofen (n = 12) |
G44: palmitoylethanolamide 300 mg (morning) + 600 mg (evening) for 7 days and then 300 mg twice a day for an additional 7 days G45: ibuprofen 600 mg |
Pain VAS MMO |
2 weeks | Low |
| Mejersjö and Wenneberg (2008) [80] | RCT | TMJ osteoarthritis | RDC/TMD |
G18 : 62 G32: 62 |
G18: 1:14 G32: 1:14 |
G18: OA (n = 15) G32: diclofenac 50 mg (n = 14) |
G18: OA every night for 3 months G32: 2–3 times a day for 3 months |
Pain VAS MMO |
1 year | Some concerns |
| Montes-Carmona et al. (2020) [81] | RCT | Localized myalgia, myofascial pain, referred pain | DC/TMD | G1: 43 G37: 45 G46: 42 |
G1: 6:1 G37: 4:1 G46: 4:1 |
G1: placebo (n = 20) G37: lidocaine (n = 20) G46: BTX-A (n = 20) |
G1: single-session injection of 0.9% saline solution in masseter and temporalis mucles G37: single-session injection of 2% lidocaine with vasoconstrictor in masseter and temporalis mucles G46: single-session injection of onabotulinumtoxin A |
Pain VAS MMO |
6 months | Some concerns |
| Mustafa et al. (2018) [82] | RCT | TMJ hypermobility | Patient history and clinical examination |
G47: 25 G48: 24 G49: 27 G50: 25 |
G47: 4:5 G48: 1:2 G49: 1:8 G50: 1:2 |
G47: saline solution + lidocaine (n = 9) G48: 10% dextrose (n = 10) G49: 20% dextrose (n = 9) G50: 30% dextrose (n = 9) |
G47: injections into four different areas of each TMJ in four sessions at monthly intervals G48: injections into four different areas of each TMJ in four sessions at monthly intervals G49: injections into four different areas of each TMJ in four sessions at monthly intervals G50: injections into four different areas of each TMJ in four sessions at monthly intervals |
Pain VAS MMO |
4 months | Some concerns |
| Nitecka-Buchta et al. (2018) [83] | RCT | Myalgia | DC/TMD |
G1: 40 G51: 37 G52: 43 |
G1: 7:8 G51: 1:2 G52: 5:8 |
G1: placebo/saline (n = 15) G51: collagen injections (MD Muscle [Guna]) (n = 15) G52: 2% lidocaine without a vasoconstrictor injection (n = 13) |
G1: masseter triggerpoint injections at day 0 and day 7 G51: masseter triggerpoint injections at day 0 and day 7 G52: masseter triggerpoint injections at day 0 and day 7 |
Pain VAS MMO |
14 days | Some concerns |
| Priyadarshini et al. (2021) [84] | RCT | TMJ internal derangement | Wilkes stages II or III |
G18: 28 G53: 32 |
G18: 1:2 G53: 1:2 |
G18: OA (n = 17) G53: prolotherapy 50% dextrose (n = 17) |
G18: 12 hours/day for 3 months G53: four sessions of intraarticular injection: day 1, day 14, day 42, and day 82 |
Pain VAS MMO |
1 year | Some concerns |
| Refahee et al. (2022) [85] | RCT | Myofascial pain | DC/TMD |
G1: 31 G54: 36 |
G1: 1:5 G54: 1:5 |
G1: saline master trigger point injection (n = 90) G54: MgSo4 masseter trigger point injection (n = 90) |
G1: single intramuscular injection G54: single intramuscular injection |
Pain VAS MMO |
6 months | Low |
| Refai et al. (2011) [86] | RCT | Painful TMJ subluxation or dislocation | Patient history and clinical examination |
G55: 30 G56: 23 |
G55: 1:2 G56: 0:6 |
G55: saline solution + 2% mepivacaine (n = 6) G56: dextrose 10% + 2% mepivacaine (n = 6) |
G55: four intraarticular injections each 6 weeks apart G56: four intraarticular injections each 6 weeks apart |
MMO | 8 months | Some concerns |
| Rezazadeh et al. (2022) [87] | RCT | Painful TMJ clicking and tender lateral pterygoid muscle | RDC/TMD |
G1: 25 G57: 28 |
G1, G57: 17:19 |
G1: placebo/saline (n = 18) G57: BTX-A 300 U (n = 18) |
G1: single-session intramuscular injection m pterygoidies lateralis G57: single-session intramuscular injection m pterygoidies lateralis |
MMO Helkimos index |
3 months | Low |
| Sakalys et al. (2020) [88] | RCT | Masseter myofascial pain | Simons D, Travell J, Simons L. Travell and Simons’ Myofascial Pain | G43: 47 G58: 49 |
G43: 3:22 G58: 6:19 |
G43: lidocaine masseter injection (n = 25) G58: PRP injection (n = 25) |
G43: single-session intramuscular injection m masseter G58: single-session intramuscular injection m masseter |
Pain VAS | 4 weeks | Low |
| Sousa et al. (2020) [89] | RCT | Arthralgia | DC/TMD |
G18: 41 G59: 41 G60: 37 G61: 37 |
G18: 1:4 G59: 1:4 G60: 1:4 G61: 1:4 |
G18: OA (n = 20) G59: OA + intraarticular betamethasone (n = 20) G60: OA + intraarticular sodium hyaluronate (n = 20) G61: OA + intraarticular PRP (n = 20) |
G18: OA every night for 6 months G59: OA every night for 6 months + single-session intraarticular injection G60: OA every night for 6 months + single-session intraarticular injection G61: OA every night for 6 months + single-session intraarticular injection |
Pain VAS MMO |
6 months | Low |
| Tchivileva et al. (2020) [90] | RCT | Myalgia | DC/TMD |
G1: 34 G62: 34 |
G1: 1:4 G62: 1:2 |
G1: placebo (n = 99) G62: extended-release propranolol hydrochloride [60 mg, twice a day] (n = 100) |
G1: Twice a day for 10 weeks G62: Twice a day for 10 weeks |
MMO | 10 weeks | Low |
| Thie et al. (2001) [91] | RCT | Osteoarthritis | Confirmed on computed tomography |
G27: 39 G28: 37 |
G27: 1:8 G28: 1:8 |
G27: ibuprofen (n = 28) G28: glucosamine sulphate (n = 21) |
G27: once a day for 90 days G28: once a day for 90 days |
MMO | 90 days | Low |
| Tjakkes et al. (2007) [92] | RCT | Arthralgia, osteoarthrosis, and osteoarthritis | RDC/TMD |
G1: 33 G63: 33 |
G1: 1:18 G63: 1:18 |
G1: placebo (n = 19) G63: ultracain (n = 19) |
G1: single-session intraarticular injection G63: single-session intraarticular injection |
Pain VAS | 3 weeks | Low |
| Vidor et al. (2013) [93] | RCT | Myofascial pain | RDC/TMD |
G1: 30 G64: 32 |
G1: 0:32 G64: 0:32 |
G1: placebo (n = 16) G64: melatonin (n = 16) |
G1: at bedtime for 28 days G64: at bedtime for 28 days |
Pain VAS | 28 days | Low |
| Winocur et al. (2000) [94] | RCT | Unilateral TMJ pain | Patient history and clinical examination |
G1: 38 G65: 36 |
G1: 1:3 G65: 1:5 |
G1: Placebo (n = 13) G65: 0.025% capcacin cream (n = 17) |
G1: vehicle cream applied to the painful area four times/day G65: capcacin cream applied to the painful area four times/day |
MMO | 4 weeks | Low |
| Zarate et al. (2020) [95] | RCT | Arthralgia | RDC/TMD |
G42: 45 G43: 50 |
G42: 1:5 G43: 1:5 |
G42: dextrose prolotherapy (20% dextrose/0.2% lidocaine) G43: 0.2% lidocaine (n = 15) |
G42: intraarticular injection at 0, 1, and 2 months (n = 14) G43: intraarticular injection at 0, 1, and 2 months (n = 15) |
MMO | 3 months | Low |
G1: placebo, G2: cyclobenzaprine, G3: tizanidine, G4: ibuprofen + chlorzoxazone or carbamazepine, G5: ibuprofen + chlorzoxazone or carbamazepine + holistic treatment, G6: holistic treatment, G7: glucosamine sulfate, G8: granisetron, G9: naproxen, G10: naproxen + codeine, G11: naproxen + single-dose dexamethasone, G12 arthrocentesis + PRP injections, G13: arthrocentesis plus HA injection, G14: BTX-A in masseter, G15: BTX-A in masseter and temporalis, G16: acupuncture, G17: BTX-A Low, G18: OA, G19: BTX-A medium, G20: BTX-A high, G21: HA intraarticular injection, G22: betamethasone intraarticular injection, G23: tenoxicam intraarticular injection, G24: Botox in masseter and temporalis, G25: BTX-A injections in masseter and temporalis muscle, G26: three sessions with fascial manipulation treatment, G27: ibuprofen, G28: glucosamine, G29: clonazepam, G30: cyclobenzaprine, G31: intraarticular injection of methylprednisolone, G32: diclofenac sodium, G33: diclofenac sodium + oral enzymes (bromelain, trypsin, rutoside trihydrate), G34: oral enzyme (bromelain, trypsin, rutoside trihydrate), G35: morphine 1.5 mg masseter, G36: morphine 5 mg masseter, G37: lidocaine masseter, G38: morphine 5 mg trapezius, G39: naproxen + 12 placebo laser sessions, G40: low-level laser treatment + placebo drug, G41: intraarticular injection corticosteroid betamethasone, G42: intraarticular injections 20% dextrose + 0.2% lidocaine, G43: intraarticular injections 0.2% lidocaine, G46: BTX-A, G47: intraarticular injection saline solution + lidocaine, G48: intraarticular injection 10% dextrose, G49: intraarticular injection 20% dextrose, G50: intraarticular injection 30% dextrose, G51: collagen muscle injections, G52: 2% lidocaine without vasoconstrictor muscle injections, G53 intraarticular injection 50% dextrose, G54: magnesium sulfate masseter trigger point injection, G55: intraarticular saline + 2% mepivacaine, G56: intraarticular dextrose 10% + 2% mepivacaine, G57: BTX-A single-session intramuscular (m pterygoideus lateralis) injection, G58: PRP intramuscular injection, G59: OA + intraarticular betamethasone, G60: OA + intraarticular sodium hyaluronate, G61: OA + intraarticular PRP, G62: extended-release propranolol hydrochloride, G63: intraarticular ultracain, G64: melatonin, G65: 0.025% capcacin cream
AAOP American Academy of Orofacial Pain, BTX-A botulinum toxin-A, CW continuous wave, DC/TMD diagnostic criteria for temporomandibular disorders, HA hyaluronic acid, MgSo4 magnesium sulfate, MD missing data, DMMO maximal mouth opening, NM not mentioned, OA occlusal appliance, PRP platelet-rich plasma, RCT randomized controlled trial, RDC/TMD Research Diagnostic Criteria for Temporomandibular Disorders, TMJ temporomandibular joint, VAS visual analogue scale
Table 2.
Summary of risk of bias assessed by the revised Cochrane risk-of-bias tool for randomized trials (RoB2)
Results of Individual Studies
Individual results of every included RCT such as means, standard deviations, and sample size for overall post-treatment pain intensity, short to intermediate term (i.e., ranging from 2 days to ≤ 6 months) and long term (≥ 6 months), are reported in Supplementary information 3.
Narrative Synthesis of Pharmacological Treatment Outcomes for TMD of Muscular Origin
Non-steroidal Antiinflammatory Drugs
The electronic search revealed two RCT-studies that investigated the effect of naproxen as pharmacological treatment of TMD-M. On the basis of these studies, naproxen alone does not seem to have a pain-reducing effect. In the study by Cigerim and Kaplan (2023), treatment of TMD-M was more effective when naproxen was combined with codeine than when used alone or in combination with dexamethasone [60]. In the study by Khalighi et al. (2016), naproxen alone did not show any pain-reducing effect nor any increase in MMO [76].
Muscle Relaxants
When it comes to muscle relaxants, three RCT studies were found in the databases. These studies used skeletal muscle relaxants in addition to self-care management. However, there were diverging results concerning the treatment outcome regarding reduction in TMD-M pain. For example, cyclobenzaprine, a serotonin type 2 (5-HT2) receptor antagonist, was shown to have a significantly better pain-reducing effect when compared with the benzodiazepine clonazepam and with placebo [72]. On the contrary, when tizanidine, an alpha-2 adrenergic receptor agonist, was compared with placebo, no significant pain-reducing effect was found and there were no differences between substances [56]. Finally, in the third study, 10 weeks of treatment with propranolol, a nonselective beta-adrenergic receptor antagonist, reduced TMD-M to a slightly higher degree than placebo. However, in this study, propranolol was more prone to adverse effects [90].
Melatonin
Only one RCT study was found investigating the pain-reducing effect of melatonin on TMD-M. This study showed that melatonin reduces pain scores in a significantly higher degree than placebo and that this effect was independent of the effect on sleep quality [93].
Wet Needling Therapies
There are several different types of wet needling therapies for TMD-M, and the focus seems to be on botulinum toxin-A (BTX-A) [62–67, 69, 81], but other wet needling therapies such as lidocaine [75, 81, 83], magnesium sulfate [85], granisetron (5-HT3 receptor antagonist) [59], platelet-rich plasma (PRP) [88], and morphine [75] have been investigated in RCTs as well.
Several studies investigating which effect BTX-A has on pain intensity in patients with TMD-M were found. BTX-A has been shown to be more effective than placebo in reducing local pain of muscular origin in bruxers and patients with TMD-M [62, 63, 69, 81]. Two studies also reported a long-lasting pain-reducing effect of BTX-A in patients with localized TMD-M [63, 81]. The pain-reducing effect in patients with localized TMD-M does not seem to depend on dosage [63]. Further, it does not depend on whether only the masseter muscle is treated or both the temporal and masseter muscles are treated [62]. However, when it comes to the patient group with persistent TMD-M, it was shown in a study by Ernberg et al. (2011) that BTX-A had no effect on pain and that the number needed to treat (NNT) at the 1-month follow-up was 11 [67]. When BTX-A has been compared with other non-pharmacological treatments, it has been shown that the pain-reducing effect was equivalent to physiotherapy [70], acupuncture [64], and occlusal appliances [65]. Similar findings have been shown when it comes to MMO. BTX-A has been shown to improve MMO in a greater extent when compared with placebo in bruxers [69] and patients with localized TMD-M [81], regardless of dosage [66].
When it comes to other wet needling therapies, there are several one-of-a-kind studies showing promising results included in this review. First, in a study by Christidis et al. (2015), the 5-HT3 receptor antagonist granisetron was shown to have a 30–50% pain-reducing effect, which also was significantly higher than placebo, lasting for more than 6 months, with a NNT of 4. Further, granisetron also increased the MMO significantly [59]. Second, in a study by Kang et al. (2018), a single dose of morphine had an analgesic effect for 48 h and was significantly more effective than placebo. The same study also indicated that a higher dose, 5 mg, is more effective than a dose of 1.5 mg [75]. The third study by Refahee et al. (2022) indicates that a single injection with magnesium sulfate significantly reduced pain and increased MMO up to 3 months in patients with TMD-M [85]. In the fourth study, Nitecka-Buchta et al. (2018) showed that repeated intramuscular injections with collagen were significantly more efficient in pain reduction than injections with lidocaine [83]. In the fifth study by Sakalys et al. (2020), a single injection with PRP resulted in a significantly greater pain reduction after 4 weeks than a single injection with lidocaine [88]. In the sixth and final study on wet needling therapies (except for BTX-A), four different treatment strategies for painful TMD-M were compared. The treatments were either an occlusal splint alone or in combination with either betamethasone, sodium hyaluronate, or PRP. Even though all four treatment approaches were effective, the one with the combination of occlusal splint and PRP was the only that achieved long-term success [89].
Network Meta-analysis and Treatment Ranking of Pharmacological Treatment Outcomes for TMD of Muscular Origin, Post-treatment Pain Intensity, Other Comparisons versus Placebo, SMD
Altogether, 11 RCTs with a total of 457 patients were identified [56, 59, 69, 70, 72, 75, 76, 81, 83, 85, 93]. All these reported pain reductions using the VAS after pharmacological treatment of TMD-M. The post-treatment pain intensity compared post-treatment pain intensity in eight comparisons versus placebo. The eight comparisons included intramuscular injections of BTX-A [69, 70, 81], intramuscular injection of lidocaine [81], cyclobenzaprine [56, 72], melatonin [93], magnesium sulfate [85], morphine 1.5 mg [75], morphine 5 mg [75], and clonazepam [72, 83].
The follow-up times ranged from 2 days [75], 2 weeks [83], 3 weeks [56, 72], 4 weeks [93], 3 months [70], and 6 months [69, 81, 85].
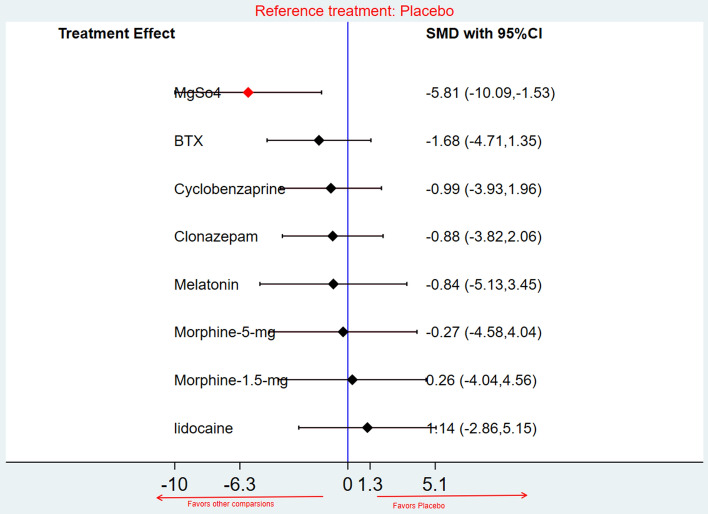
The NMA revealed a significant pain reduction after pharmacological treatment with magnesium sulfate when compared with placebo (SMD = −5.81; CI −10.09 to −1.53; very low-quality evidence). However, there were no statistically significant differences between the other comparisons and placebo as shown in Fig. 3.
Fig. 3.
Forest plot, network meta-analysis, post-treatment pain intensity and placebo versus other pharmacological treatments. BTX botulinum toxin-A, CI confidence interval, MgSo4 magnesium sulphate, SMD standardized mean difference
Treatment Ranking
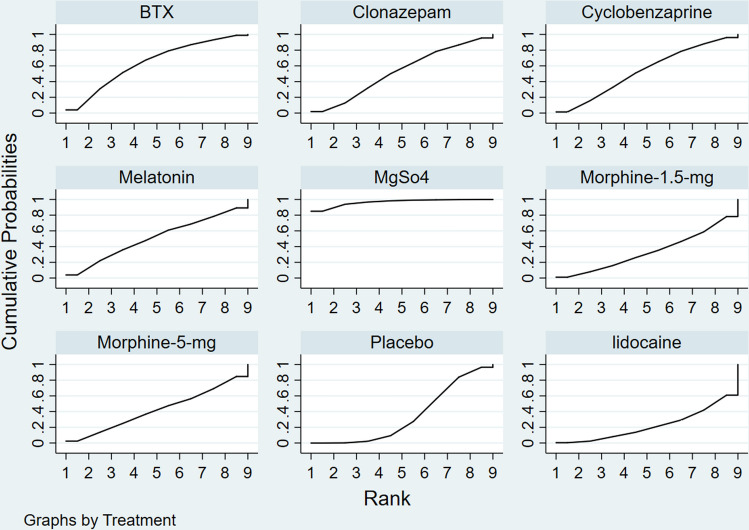
The most effective pharmacological treatment option in reducing TMD-M pain at follow-up times ranging from 2 days to 6 months was magnesium sulfate (96.9%; very low-quality evidence), which was followed by BTX-A (64%; very low-quality evidence), cyclobenzaprine (53.6%; low-quality evidence), clonazepam (52.6%; very low-quality evidence), melatonin (50.9%; very low-quality evidence), morphine 5 mg (42%; very low-quality evidence), placebo (34.4%; very low-quality evidence), morphine 1.5 mg (33.7%; very low-quality evidence), and lastly, lidocaine (22%; very low-quality evidence), as illustrated in Fig. 4 and Table 3.
Fig. 4.
Surface under the cumulative ranking curve, overall and subgroup analyses based on pharmacological treatment types. BTX botulinum toxin-A, MgSo4 magnesium sulfate
Table 3.
Pharmacological treatment of patients with temporomandibular disorders of a muscular origin ranked using the SUCRA
| Treatment | SUCRA | PrBest | Mean rank |
|---|---|---|---|
| Botulinum toxin-A | 64.0 | 4.0 | 3.9 |
| Placebo | 34.4 | 0.0 | 6.2 |
| Cyclobenzaprine | 53.6 | 1.4 | 4.7 |
| Melatonin | 50.9 | 3.9 | 4.9 |
| Lidocaine | 22.3 | 0.4 | 7.2 |
| Magnesium sulfate | 96.5 | 85.0 | 1.3 |
| Morphine 1.5 mg | 33.7 | 1.1 | 6.3 |
| Morphine 5 mg | 42.0 | 2.4 | 5.6 |
| Clonazepam | 52.6 | 1.8 | 4.8 |
PRBest probability of being the best, SUCRA surface under the cumulative ranking curve
Narrative Synthesis of Pharmacological Treatment Outcomes for TMD of Arthrogenous Origin
Non-steroidal Antiinflammatory Drugs
The electronic search revealed four RCT studies investigating the effect of NSAIDs as pharmacological treatment for TMD-J. In one study investigating patients with TMD-J, diclofenac sodium was shown to have a significant pain-reducing effect, but that the effect is even better if it is combined with bromelain, rutoside, trihydrate, and trypsin [74]. In another study, ibuprofen was compared with the endocannabinoid-like lipid mediator palmitoylethanolamide (PEA). Even though ibuprofen was shown to have a significant pain-reducing effect and increased MMO, PEA was more effective [79]. Two studies comparing the effect of ibuprofen and glucosamine sulfate showed that ibuprofen resulted in similar or less pain reduction than glucosamine [71, 91].
Glucosamine Sulfate
When it comes to glucosamine sulfate, the findings are diverging. One study could not show any difference between treatment with glucose amine sulfate and placebo, neither regarding pain intensity nor MMO in patients with TMD-J [58]. However, when glucosamine sulfate was compared with ibuprofen it showed similar [91] and better [71] effects on both TMD-J pain and MMO. In the case with similar effect immediately after 90-day treatment, there was a long-term difference after 120 days where glucosamine sulfate was superior to ibuprofen [91].
Topical Treatment—Capsaicin
One study investigating topical treatment of painful TMD-J fulfilled the inclusion criteria. That study showed that both placebo and capsaicin cream resulted in a significant improvement of unilateral TMD-J. There was, however, no statistically significant difference in either pain intensity or MMO when capsaicin was compared with placebo [94].
Wet Needling Therapies
There are several different types of wet needling therapies for TMD-J, some are antiinflammatory [68, 73] since TMD-J can be a painful condition assumed to be associated with local inflammation, and others focus on pain reduction with local anesthetics [78, 92, 95].
When it comes to antiinflammatory needling therapies, no difference in TMD-J pain was found after a single dose of methylprednisolone when compared with placebo (saline), however, the adverse effects were twice as common after treatment with methylprednisolone [73]. Similar findings were also reported in another study with the unselective NSAIDs betamethasone and tenoxicam, which were found to have less pain-reducing effect when compared with hyaluronic acid. However, all three pharmacological treatments were significantly superior when compared with placebo [68].
Further, for anesthetics, a combination of dextrose and lidocaine is superior to just lidocaine for reducing TMD-J pain intensity and increasing MMO [78, 95]. Likewise, the local anesthetic ultracain shows a short-term reduction in pain intensity, but no effect on MMO [92].
In regard to internal derangements of the TMJ with pain, dextrose prolotherapy has been found to have a significant long-term pain relief [82, 84, 95] and improved MMO [80, 83, 86, 95].
Pharmacological Treatment in Combination with Arthrocentesis
Pharmacological treatment can be used in combination with surgical procedures such as arthrocentesis of TMJs in patients with osteoarthritis and/or osteoarthrosis to alleviate joint pain. Only one study was found to have investigated this matter. When comparing the effect of postoperative injections with either hyaluronic acid or PRP, there were no differences in pain reduction or MMO [61].
Network Meta-analysis and Treatment Ranking of Pharmacological Treatment Outcomes for TMD of Arthrogenous Origin
There were not enough studies included to be able perform an NMA for this patient group.
Discussion
Currently there is no consensus regarding pharmacological treatments for painful TMD. The main findings of this systematic review provide some support for pharmacological treatment approaches for TMD of both muscular and arthrogenous origin. However, due to the small number of present and included RCTs on pharmacological treatments, in combination with the results presented in the narrative synthesis, one cannot generalize nor rank the pharmacological treatment options. Thus, there must be an individual assessment considering the multifactorial etiology of painful TMD with a range of intricate symptoms and causes. Thus, it is advisable to employ a multifaceted treatment strategy, including pharmacological treatment approaches [22–25].
For the large patient group with TMD-M [5], i.e., the TMD-M group, the NMA could only show a significant pain-reducing effect by the use of magnesium sulfate when compared with placebo, however, with very low-quality evidence. When the treatment alternatives were ranked, magnesium sulfate was placed first, followed by BTX-A, cyclobenzaprine, and clonazepam. Surprisingly, local anesthetics were ranked last, after placebo. On the basis of the narrative synthesis of this review, muscle relaxants, BTX-A, and some other wet needling agents such as granisetron, morphine, and PRP seem to be promising both when it comes to reduction of TMD-M pain and the increasing of MMO. These findings were not surprising and are in consistency with a previous NMA that also suggested that there might be a possible pain-reducing effect by BTX-A, and the muscle relaxant cyclobenzaprine could be a promising pharmacological treatment approach although lacking long-term follow-ups [32]. Further, two other NMAs also concluded that BTX-A, granisetron, and muscle relaxants could have a possible pain-reducing effect, thus ranking them high [24, 41]. When considering treatment with muscle relaxants, the reported side effects (drowsiness, dizziness, weakness, and ataxia) limits the usability [96] . In contrast to this NMA, the local anesthetic lidocaine was ranked among the highest in those NMAs [24, 41]. When it comes to non-opioid analgesic drugs, this systematic review could not provide any scientific evidence. However, they can be considered as a good complement to other treatment modalities for painful TMD-M. This and other studies suggest that non-opioid analgesic drugs can be recommended for patients with mild-to-moderate TMD-M pain, mainly as a complement to other treatment approaches [97, 98].
For the patient group with TMD-J, no NMA could be performed. Therefore, the discussion is based on the narrative synthesis of this review. The results from the synthesis of the included RCTs support pharmacological treatments of TMD-J with NSAIDs, glucocorticosteroids, and hyaluronic acid, which is in accordance with the findings from previous systematic reviews [25, 32, 99]. However, this review also indicates that the effect of prolotherapy with dextrose is promising, showing long-term pain-relieving effects and increased MMO. However, more studies are necessary to draw any conclusions. As for TMD-M, local anesthetics do not seem to provide any significant pain-reducing effects. Just like TMD-M, painful conditions arising from TMJ can be mechanical, inflammatory, due to mechanical overload, systemic diseases, etc., i.e., having a divergent, multifactorial etiology and individualized treatment plans including pharmacological treatment approaches [25, 32, 99].
Study Strengths and Limitations
The strengths of the present review encompass the following key aspects: (1) this research marks the inaugural publication of a network meta-analysis (NMA) that systematically compares the effectiveness of 39 distinct pharmacotherapeutic treatment options for TMD of muscular origin, according to the authors’ knowledge; (2) by exclusively incorporating RCTs within the NMA, the researchers ensured the presence of high-quality evidence while mitigating potential biases stemming from selection and performance factors; and (3) the integration of the GRADE-system into the analytical process effectively assigned accurate grades to all findings, preventing both overestimation and underestimation of outcomes.
Nevertheless, there exist several limitations associated with this study that warrant consideration: (1) owing to unavailable data, NMA was not executed for every comparison group, and not for TMD of articular origin either; (2) certain NMA-groups were characterized by a restricted number of RCTs and participants, highlighting the imperative need for additional RCTs with larger sample sizes to comprehensively assess effectiveness before arriving at definitive conclusions; (3) variations in follow-up durations across RCTs and inadequate follow-up periods in specific instances exerted additional influence on the analysis. Moreover, the absence of essential mean and standard deviation information hindered the execution of subgroup analyses; (4) limited data availability precluded statistical analysis of outcomes such as MMO with and without pain, necessitating a narrative presentation of results. Finally, most evidence acquired exhibited notably low quality. It is also important to note that certain included RCTs had limited sample sizes for patients with disc displacement, potentially compromising the reliability and robustness of the conclusions drawn. Therefore, a cautious approach is recommended when interpreting these findings.
Conclusions
This systematic review presents the current knowledge and evidence regarding pharmacological treatment approaches for painful temporomandibular disorders. Although a limited number of RCTs were included, there is some evidence, though not sufficient, to generalize the results. The evidence clearly indicates that the pharmacological treatment approaches differ between TMD-M and TMD-J. Therefore, it is of great importance to first try to uncover each patient’s individual and multifactorial etiology and then employ a multifaceted treatment strategy, including pharmacological treatment. When it comes to TMD-M evidence, an increasing body of evidence points toward wet needling therapies with BTX-A, granisetron, and PRP as well as muscle relaxants. For TMD-J, the evidence points toward pharmacological treatment approaches including NSAIDs and glucocorticosteriods (for inflammatory conditions) as well as hyaluronic acid and dextrose.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the librarians Lovisa Liljegren (LL) and Narcisa Hannerz (NH) at the Karolinska Institutet University Library for the assistance with the electronic literature search.
Declarations
Author Contributions
Nikolaos Christidis had the main idea for the article. However, all authors contributed to the study conception and design. Nikolaos Christidis, Malin Collin, and Essam Ahmed Al-Moraissi performed the literature search with help from the university library at Karolinska Institutet. Selection of papers was performed by Malin Collin and Golnaz Barjandi. Analysis of risk of bias was performed by Johanna Svedenlöf and Maria Christidis. Maria Christidis is a senior lecturer and responsible for the course “Scientific theory and methods” and teaches specifically about the different methods existing for risk of bias and certainty of evidence. Further, both Nikolaos Christidis and Essam Al-Moraissi are very experienced in carrying out systematic reviews and have, together with Maria Christidis, double checked all parts of the assessment of risk of bias and certainty of evidence. Data were analyzed by Essam Al-Moraissi. Nikolaos Christidis, Maria Christidis, Malin Collin, and Hajer Jasim drafted the first manuscript that was critically revised by all authors, who commented on previous versions of the manuscript. All authors read and approved the final version manuscript.
Funding
Open access funding provided by Karolinska Institute.
Conflict of Interest
Authors Nikolaos Christidis, Essam Ahmed Al-Moraissi, Golnaz Barjandi, Johanna Svedenlöf, Hajer Jasim, Maria Christidis, and Malin Collin declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
Not applicable.
Consent (participation & publication)
Not applicable.
Code Availability
Not applicable.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Durham J, Newton-John TR, Zakrzewska JM. Temporomandibular disorders. BMJ (Clinical research ed). 2015;350:h1154. doi: 10.1136/bmj.h1154. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peck CC, Goulet JP, Lobbezoo F, Schiffman EL, Alstergren P, Anderson GC, et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J Oral Rehabil. 2014;41(1):2–23. doi: 10.1111/joor.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain. 2013;14(12 Suppl):T116–T124. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lövgren A, Häggman-Henrikson B, Visscher CM, Lobbezoo F, Marklund S, Wänman A. Temporomandibular pain and jaw dysfunction at different ages covering the lifespan–a population based study. Eur J Pain. 2016;20(4):532–540. doi: 10.1002/ejp.755. [DOI] [PubMed] [Google Scholar]
- 6.Häggman-Henrikson B, Liv P, Ilgunas A, Visscher CM, Lobbezoo F, Durham J, et al. Increasing gender differences in the prevalence and chronification of orofacial pain in the population. Pain. 2020 doi: 10.1097/j.pain.0000000000001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-de-las-Penas C, Svensson P. Myofascial temporomandibular disorder. Curr Rheumatol Rev. 2016;12(1):40–54. doi: 10.2174/1573397112666151231110947. [DOI] [PubMed] [Google Scholar]
- 8.Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T20–32.e1-3. doi: 10.1016/j.jpain.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc (1939) 2000;131(9):1307–1315. doi: 10.14219/jada.archive.2000.0384. [DOI] [PubMed] [Google Scholar]
- 10.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis PHF, Laxe LAC, Lacerda-Santos R, Münchow EA. Distribution of anxiety and depression among different subtypes of temporomandibular disorder: a systematic review and meta-analysis. J Oral Rehabil. 2022;49(7):754–767. doi: 10.1111/joor.13331. [DOI] [PubMed] [Google Scholar]
- 12.Kapos FP, Exposto FG, Oyarzo JF, Durham J. Temporomandibular disorders: a review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020;13(4):321–334. doi: 10.1111/ors.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okeson JP. Management of temporomandibular disorders and occlusion-E-book. Elsevier Health Sciences; 2014. [Google Scholar]
- 14.Ibi M. Inflammation and temporomandibular joint derangement. Biol Pharm Bull. 2019;42(4):538–542. doi: 10.1248/bpb.b18-00442. [DOI] [PubMed] [Google Scholar]
- 15.ICD-11 . International classification of diseases 11th revision. Zurich: World Health Organization; 2018. [Google Scholar]
- 16.Cui SJ, Yang FJ, Wang XD, Mao ZB, Gu Y. Mechanical overload induces TMJ disc degeneration via TRPV4 activation. Oral Dis. 2023;00:1–13. doi: 10.1111/odi.14595. [DOI] [PubMed] [Google Scholar]
- 17.Machon V, Levorova J, Hirjak D, Drahos M, Foltan R. Temporomandibular joint disc perforation: a retrospective study. Int J Oral Maxillofac Surg. 2017;46(11):1411–1416. doi: 10.1016/j.ijom.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Dimitroulis G. The prevalence of osteoarthrosis in cases of advanced internal derangement of the temporomandibular joint: a clinical, surgical and histological study. Int J Oral Maxillofac Surg. 2005;34(4):345–349. doi: 10.1016/j.ijom.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15(3):193–205. [PubMed] [Google Scholar]
- 20.Roh H-S, Kim W, Kim Y-K, Lee J-Y. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. J Cranio-Maxillofac Surg. 2012;40(3):283–286. doi: 10.1016/j.jcms.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Angelotti F, Parma A, Cafaro G, Capecchi R, Alunno A, Puxeddu I. One year in review 2017: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2017;35(3):368–378. [PubMed] [Google Scholar]
- 22.Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95(10):1084–1092. doi: 10.1177/0022034516653743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roldán-Barraza C, Janko S, Villanueva J, Araya I, Lauer HC. A systematic review and meta-analysis of usual treatment versus psychosocial interventions in the treatment of myofascial temporomandibular disorder pain. J Oral Facial Pain Headache. 2014;28(3):205–222. doi: 10.11607/ofph.. [DOI] [PubMed] [Google Scholar]
- 24.Al-Moraissi EA, Conti PCR, Alyahya A, Alkebsi K, Elsharkawy A, Christidis N. The hierarchy of different treatments for myogenous temporomandibular disorders: a systematic review and network meta-analysis of randomized clinical trials. Oral Maxillofac Surg. 2022;26(4):519–533. doi: 10.1007/s10006-021-01009-y. [DOI] [PubMed] [Google Scholar]
- 25.List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta-analyses. J Oral Rehabil. 2010;37(6):430–451. doi: 10.1111/j.1365-2842.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- 26.Alkhutari AS, Alyahya A, Rodrigues Conti PC, Christidis N, Al-Moraissi EA. Is the therapeutic effect of occlusal stabilization appliances more than just placebo effect in the management of painful temporomandibular disorders? A network meta-analysis of randomized clinical trials. J Prosthetic Dent. 2021;126(1):24–32. doi: 10.1016/j.prosdent.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database of Syst Rev. 2017;1(1):Cd011279. doi: 10.1002/14651858.CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medlicott MS, Harris SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. 2006;86(7):955–973. doi: 10.1093/ptj/86.7.955. [DOI] [PubMed] [Google Scholar]
- 29.Guarda-Nardini L, De Almeida AM, Manfredini D. Arthrocentesis of the temporomandibular joint: systematic review and clinical implications of research findings. J Oral Facial Pain Headache. 2021;35(1):17–29. doi: 10.11607/ofph.2606. [DOI] [PubMed] [Google Scholar]
- 30.Wu M, Cai J, Yu Y, Hu S, Wang Y, Wu M. Therapeutic agents for the treatment of temporomandibular joint disorders: progress and perspective. Front Pharmacol. 2020;11:596099. doi: 10.3389/fphar.2020.596099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson Cahlin B, Samuelsson N, Dahlstrom L. Utilization of pharmaceuticals among patients with temporomandibular disorders: a controlled study. Acta Odontol Scand. 2006;64(3):187–192. doi: 10.1080/00016350600573191. [DOI] [PubMed] [Google Scholar]
- 32.Häggman-Henrikson B, Alstergren P, Davidson T, Högestätt ED, Östlund P, Tranaeus S, et al. Pharmacological treatment of oro-facial pain-health technology assessment including a systematic review with network meta-analysis. J Oral Rehabil. 2017;44(10):800–826. doi: 10.1111/joor.12539. [DOI] [PubMed] [Google Scholar]
- 33.Ouanounou A, Goldberg M, Haas DA. Pharmacotherapy in temporomandibular disorders: a review. Journal (Canadian Dental Association). 2017;83:h7. [PubMed] [Google Scholar]
- 34.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 35.Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195–207. doi: 10.5195/jmla.2020.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. [DOI] [PMC free article] [PubMed]
- 39.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salanti G, Kavvoura FK, Ioannidis JP. Exploring the geometry of treatment networks. Ann Intern Med. 2008;148(7):544–553. doi: 10.7326/0003-4819-148-7-200804010-00011. [DOI] [PubMed] [Google Scholar]
- 41.Al-Moraissi EA, Alradom J, Aladashi O, Goddard G, Christidis N. Needling therapies in the management of myofascial pain of the masticatory muscles: a network meta-analysis of randomised clinical trials. J Oral Rehabil. 2020;47(7):910–922. doi: 10.1111/joor.12960. [DOI] [PubMed] [Google Scholar]
- 42.Al-Moraissi EA, Farea R, Qasem KA, Al-Wadeai MS, Al-Sabahi ME, Al-Iryani GM. Effectiveness of occlusal splint therapy in the management of temporomandibular disorders: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2020;49(8):1042–1056. doi: 10.1016/j.ijom.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Al-Moraissi EA, Goddard G, Christidis N. Are acupuncture and dry needling effective in the management of masticatory muscle pain: a network meta-analysis of randomised clinical trials. J Oral Rehabil. 2022 doi: 10.1111/joor.13382. [DOI] [PubMed] [Google Scholar]
- 44.Ahn E, Kang H. Concepts and emerging issues of network meta-analysis. Korean J Anesthesiol. 2021;74(5):371–382. doi: 10.4097/kja.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet. 2008;60:311–334. doi: 10.1016/s0065-2660(07)00413-0. [DOI] [PubMed] [Google Scholar]
- 46.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research synthesis methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roger M. Harbord JPTH. Stand Genomic Sci. 2008;8:493–519. [Google Scholar]
- 50.StataCorp . Stata statistical software: release 13. College Station: StataCorpLP; 2013. [Google Scholar]
- 51.White IR. Network meta-analysis. Stata Journal. 2015;15(4):951–985. doi: 10.1177/1536867X1501500403. [DOI] [Google Scholar]
- 52.Higgins JPT. Cochrane handbook for systematic reviews of interventions version 5.0. 1. 2008. The Cochrane Collaboration. http://www.cochrane-handbook.org. Accessed 30 Nov 2023.
- 53.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol. 2016;76:193–199. doi: 10.1016/j.jclinepi.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Alencar FGP, Jr, Viana PG, Zamperini C, Becker A. Patient education and self-care for the management of jaw pain upon awakening: a randomized controlled clinical trial comparing the effectiveness of adding pharmacologic treatment with cyclobenzaprine or tizanidine. J Oral Facial Pain Headache. 2014;28(2):119–127. doi: 10.11607/ofph.963. [DOI] [PubMed] [Google Scholar]
- 57.Bhalla K, Kamarthi N, Malik SS, Goel S, Gupta S. Comparison of conventional pharmacological therapy and holistic approaches (Naturopathy and Yoga) in the management of chronic orofacial pain: a randomized controlled study. J Indian Acad Oral Med Radiol. 2019;31(1):29–35. doi: 10.4103/jiaomr.jiaomr_3_19. [DOI] [Google Scholar]
- 58.Cahlin BJ, Dahlstrom L. No effect of glucosamine sulfate on osteoarthritis in the temporomandibular joints—a randomized, controlled, short-term study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):760–766. doi: 10.1016/j.tripleo.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Christidis N, Omrani S, Fredriksson L, Gjelset M, Louca S, Hedenberg-Magnusson B, et al. Repeated tender point injections of granisetron alleviate chronic myofascial pain—a randomized, controlled, double-blinded trial. J Headache Pain. 2015;16:104. doi: 10.1186/s10194-015-0588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cigerim L, Kaplan V. Analgesic efficacy of naproxen-codeine, naproxen+dexamethasone, and naproxen on myofascial pain: a randomized double-blind controlled trial. Cranio J Craniomandib Pract. 2020 doi: 10.1080/08869634.2020.1824411. [DOI] [PubMed] [Google Scholar]
- 61.Cömert Kiliç S, Güngörmüş M. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis plus hyaluronic acid for the treatment of temporomandibular joint osteoarthritis: a randomized clinical trial. Int J Oral Maxillofac Surg. 2016;45(12):1538–1544. doi: 10.1016/j.ijom.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 62.da Silva Ramalho JA, Palma LF, Ramalho KM, Tedesco TK, Morimoto S. Effect of botulinum toxin A on pain, bite force, and satisfaction of patients with bruxism: a randomized single-blind clinical trial comparing two protocols. Saudi Dent J. 2023;35(1):53–60. doi: 10.1016/j.sdentj.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De la Torre Canales G, Camara-Souza MB, Poluha RL, de Figueredo OMC, Nobre BBDS, Ernberg M, et al. Long-term effects of a single application of botulinum toxin type A in temporomandibular myofascial pain patients: a controlled clinical trial. Toxins. 2022 doi: 10.3390/toxins14110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De La Torre Canales G, Camara-Souza MB, Poluha RL, Grillo CM, Conti PCR, Sousa MdLRd, et al. Botulinum toxin type A and acupuncture for masticatory myofascial pain: a randomized clinical trial. J Appl Oral Sci Rev FOB. 2021;29:e20201035. doi: 10.1590/1678-7757-2020-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De la Torre CG, Poluha RL, Alvarez Pinzon YN, Rodrigues Conti PC, Manfredini D, Sanchez-Ayala A, et al. Effects of botulinum toxin type A on the psychosocial features of myofascial pain TMD subjects: a randomized controlled trial. J Oral Facial Pain Headache. 2021;35(4):288–296. doi: 10.11607/ofph.2917. [DOI] [PubMed] [Google Scholar]
- 66.De la Torre Canales G, Poluha RL, Pinzon NA, Da Silva BR, Almeida AM, Ernberg M, et al. Efficacy of botulinum toxin type-A I in the improvement of mandibular motion and muscle sensibility in myofascial pain TMD subjects: a randomized controlled trial. Toxins. 2022 doi: 10.3390/toxins14070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ernberg M, Hedenberg-Magnusson B, List T, Svensson P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: a randomized, controlled, double-blind multicenter study. Pain. 2011;152(9):1988–1996. doi: 10.1016/j.pain.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Gencer ZK, Ozkiris M, Okur A, Korkmaz M, Saydam L. A comparative study on the impact of intra-articular injections of hyaluronic acid, tenoxicam and betametazon on the relief of temporomandibular joint disorder complaints. J Cranio-Maxillo-facial Surg. 2014;42(7):1117–1121. doi: 10.1016/j.jcms.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 69.Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio. 2008;26(2):126–135. doi: 10.1179/crn.2008.01710.1179/crn.2008.017. [DOI] [PubMed] [Google Scholar]
- 70.Guarda-Nardini L, Stecco A, Stecco C, Masiero S, Manfredini D. Myofascial pain of the jaw muscles: comparison of short-term effectiveness of botulinum toxin injections and fascial manipulation technique. Cranio. 2012;30(2):95–102. doi: 10.1179/crn.2012.01410.1179/crn.2012.014. [DOI] [PubMed] [Google Scholar]
- 71.Haghighat A, Behnia A, Kaviani N, Khorami B. Evaluation of glucosamine sulfate and ibuprofen effects in patients with temporomandibular joint osteoarthritis symptom. J Res Pharm Pract. 2013;2(1):34–39. doi: 10.4103/2279-042X.114087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herman CR, Schiffman EL, Look JO, Rindal DB. The effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trial. J Orofac Pain. 2002;16(1):64–70. [PubMed] [Google Scholar]
- 73.Isacsson G, Schumann M, Nohlert E, Mejersjo C, Tegelberg A. Pain relief following a single-dose intra-articular injection of methylprednisolone in the temporomandibular joint arthralgia-A multicentre randomised controlled trial. J Oral Rehabil. 2019;46(1):5–13. doi: 10.1111/joor.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jayachandran S, Khobre P. Efficacy of bromelain along with trypsin, rutoside trihydrate enzymes and diclofenac sodium combination therapy for the treatment of TMJ osteoarthritis—a randomised clinical trial. J Clin Diagn Res JCDR. 2017;11(6):ZC09–ZC111. doi: 10.7860/JCDR/2017/25771.9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang SK, Lee YH, Park H, Ro JY, Auh QS. Effects of intramuscular morphine in men and women with temporomandibular disorder with myofascial pain. Oral Dis. 2018;24(8):1591–1598. doi: 10.1111/odi.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khalighi HR, Mortazavi H, Mojahedi SM, Azari-Marhabi S, Moradi AF. Low level laser therapy versus pharmacotherapy in improving myofascial pain disorder syndrome. J Lasers Med Sci. 2016;7(1):45–50. doi: 10.15171/jlms.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kopp S, Wenneberg B, Haraldson T, Carlsson GE. The short-term effect of intra-articular injections of sodium hyaluronate and corticosteroid on temporomandibular joint pain and dysfunction. J Oral Maxillofac Surg. 1985;43(6):429–435. doi: 10.1016/s0278-2391(85)80050-110.1016/s0278-2391(85)80050-1. [DOI] [PubMed] [Google Scholar]
- 78.Louw WF, Reeves KD, Lam SKH, Cheng AL, Rabago D. Treatment of temporomandibular dysfunction with hypertonic dextrose injection (prolotherapy): a randomized controlled trial with long-term partial crossover. Mayo Clin Proc. 2019;94(5):820–832. doi: 10.1016/j.mayocp.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Marini I, Bartolucci ML, Bortolotti F, Gatto MR, Bonetti GA. Palmitoylethanolamide versus a nonsteroidal anti-inflammatory drug in the treatment of temporomandibular joint inflammatory pain. J Orofac Pain. 2012;26(2):99–104. [PubMed] [Google Scholar]
- 80.Mejersjo C, Wenneberg B. Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: a randomized controlled trial. J Oral Rehabil. 2008;35(10):729–738. doi: 10.1111/j.1365-2842.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- 81.Montes-Carmona JF, Gonzalez-Perez LM, Infante-Cossio P. Treatment of localized and referred masticatory myofascial pain with botulinum toxin injection. Toxins. 2020 doi: 10.3390/toxins13010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mustafa R, Gungormus M, Mollaoglu N. Evaluation of the efficacy of different concentrations of dextrose prolotherapy in temporomandibular joint hypermobility treatment. J Craniofac Surg. 2018;29(5):E461–E465. doi: 10.1097/SCS.0000000000004480. [DOI] [PubMed] [Google Scholar]
- 83.Nitecka-Buchta A, Walczynska-Dragon K, Batko-Kapustecka J, Wieckiewicz M. Comparison between collagen and lidocaine intramuscular injections in terms of their efficiency in decreasing myofascial pain within masseter muscles: a randomized single-blind, controlled trial. Pain Res Manag. 2018;2018:8261090. doi: 10.1155/2018/8261090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Priyadarshini S, Gnanam A, Sasikala B, Elavenil P, Raja Sethupathy Cheeman S, Mrunalini R, et al. Evaluation of prolotherapy in comparison with occlusal splints in treating internal derangement of the temporomandibular joint—a randomized controlled trial. J Cranio-Maxillofac Surg. 2021;49(1):24–28. doi: 10.1016/j.jcms.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Refahee SM, Mahrous AI, Shabaan AA. Clinical efficacy of magnesium sulfate injection in the treatment of masseter muscle trigger points: a randomized clinical study. BMC Oral Health. 2022;22(1):408. doi: 10.1186/s12903-022-02452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Refai H, Altahhan O, Elsharkawy R. The efficacy of dextrose prolotherapy for temporomandibular joint hypermobility: a preliminary prospective, randomized, double-blind, placebo-controlled clinical trial. J Oral Maxillofac Surg. 2011;69(12):2962–2970. doi: 10.1016/j.joms.2011.02.128. [DOI] [PubMed] [Google Scholar]
- 87.Rezazadeh F, Esnaashari N, Azad A, Emad S. The effects of botulinum toxin A injection on the lateral pterygoid muscle in patients with a painful temporomandibular joint click: a randomized clinical trial study. BMC Oral Health. 2022;22(1):217. doi: 10.1186/s12903-022-02220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakalys D, Rokicki JP, Januzis G, Kubilius R. Plasma rich in growth factors injection effectiveness for myofascial pain treatment in masticatory muscles. Randomised controlled trial. J Oral Rehabil. 2020;47(7):796–801. doi: 10.1111/joor.12973. [DOI] [PubMed] [Google Scholar]
- 89.Sousa BMD, Lopez-Valverde N, Lopez-Valverde A, Caramelo F, Fraile JF, Payo JH, et al. Different treatments in patients with temporomandibular joint disorders: a comparative randomized study. Medicina (Kaunas) 2020 doi: 10.3390/medicina56030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tchivileva IE, Hadgraft H, Lim PF, Di Giosia M, Ribeiro-Dasilva M, Campbell JH, et al. Efficacy and safety of propranolol for treatment of temporomandibular disorder pain: a randomized, placebo-controlled clinical trial. Pain. 2020;161(8):1755–1767. doi: 10.1097/j.pain.0000000000001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thie NM, Prasad NG, Major PW. Evaluation of glucosamine sulfate compared to ibuprofen for the treatment of temporomandibular joint osteoarthritis: a randomized double blind controlled 3 month clinical trial. J Rheumatol. 2001;28(6):1347–1355. [PubMed] [Google Scholar]
- 92.Tjakkes GHE, Tenvergert EM, de Bont LGM, Stegenga B. The effect of intra-articular injection of ultracain in the temporomandibular joint in patients with preauricular pain: a randomized prospective double-blind placebo-controlled crossover study. Clin J Pain. 2007;23(3):233–236. doi: 10.1097/AJP.0b013e31802f0950. [DOI] [PubMed] [Google Scholar]
- 93.Vidor LP, Torres ILS, Custodio de Souza IC, Fregni F, Caumo W. Analgesic and sedative effects of melatonin in temporomandibular disorders: a double-blind, randomized, parallel-group, placebo-controlled study. J Pain Symptom Manag. 2013;46(3):422–432. doi: 10.1016/j.jpainsymman.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 94.Winocur E, Gavish A, Halachmi M, Eli I, Gazit E. Topical application of capsaicin for the treatment of localized pain in the temporomandibular joint area. J Orofac Pain. 2000;14(1):31–36. [PubMed] [Google Scholar]
- 95.Zarate MA, Frusso RD, Reeves KD, Cheng A-L, Rabago D. Dextrose prolotherapy versus lidocaine injection for temporomandibular dysfunction: a pragmatic randomized controlled trial. J Altern Complement Med. 2020;26(11):1064–1073. doi: 10.1089/acm.2020.0207. [DOI] [PubMed] [Google Scholar]
- 96.Richards BL, Whittle SL, Buchbinder R, Richards BL. Muscle relaxants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD008922.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swedish-Academy-of-Temporomandibular-Disorders. Treatment protocol for general dentists for patients with orofacial pain and jaw dysfunction. 2021. https://mau.se/om-oss/fakulteter-och-institutioner/odontologiska-fakulteten/odontologiska-fakulteten/dctmd/#accordion-93126. Accessed 30 Nov 2023.
- 98.Montinaro F, Nucci L, d’Apuzzo F, Perillo L, Chiarenza MC, Grassia V. Oral nonsteroidal anti-inflammatory drugs as treatment of joint and muscle pain in temporomandibular disorders: a systematic review. CRANIO®. doi: 10.1080/08869634.2022.2031688. [DOI] [PubMed]
- 99.List T, Axelsson S, Leijon G. Pharmacologic interventions in the treatment of temporomandibular disorders, atypical facial pain, and burning mouth syndrome. A qualitative systematic review. J Orofac Pain. 2003;17(4):301–310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.