Abstract
Outer membrane vesicles (OMVs) are spontaneously released by many gram-negative bacteria during their growth and constitute an important virulence factor for bacteria, helping them to survive through harsh environmental conditions. Native OMVs, naturally-released from bacteria, are produced at a level too low for vaccine manufacturing, requiring chemical treatment (detergent-extracted) or genetic manipulation, resulting in generalized modules for membrane antigens (GMMAs). Over the years, the nature and properties of OMVs have made them a viable platform for vaccine development. There are a few licensed OMV vaccines mainly for the prevention of meningitis caused by Neisseria meningitidis serogroup B (MenB) and Haemophilus influenzae type b (Hib). There are several candidates in clinical development against other gram-negative organisms from which the OMVs are derived, but also against heterologous targets in which the OMVs are used as carriers (e.g. coronavirus disease 2019 [COVID-19]). The use of OMVs for targets other than those from which they are derived is a major advancement in OMV technology, improving its versatility by being able to deliver protein or polysaccharide antigens. Other advances include the range of genetic modifications that can be made to improve their safety, reduce reactogenicity, and increase immunogenicity and protective efficacy. However, significant challenges remain, such as identification of general tools for high-content surface expression of heterologous proteins on the OMV surface. Here, we outline the progress of OMV vaccines to date, particularly discussing licensed OMV-based vaccines and candidates in clinical development. Recent trends in preclinical research are described, mainly focused on genetic manipulation and chemical conjugation for the use of OMVs as carriers for heterologous protein and polysaccharide antigens. Remaining challenges with the use of OMVs and directions for future research are also discussed.
Key Points
| Outer membrane vesicles (OMVs) represent a viable platform for vaccine development, and some OMV-based vaccines are currently licensed and are in clinical use. |
| OMVs have a long and rich history of use, particularly in vaccines against Neisseria meningitidis and Haemophilus influenzae type b, and a large body of preclinical data has been generated supporting the use of this platform to fight many other pathogens. |
| Recent trends see the use of OMVs as carriers for heterologous protein and polysaccharide antigens, supporting the development of multicomponent vaccines. |
Introduction
Many Gram-negative bacteria spontaneously release outer membrane vesicles (OMVs) during growth [1], very likely as a result of an imbalance between cell growth and outer membrane biosynthesis, with the excess membrane material released as OMVs [2]. A number of studies have reported the role of OMVs in enhancing the survival of bacteria in harsh environments and delivering virulence factors and DNA to host cells [3–5]. OMVs appear involved in bacterial pathogenesis [6], help biofilm formation, and increase survival in hosts [7] or in soil [8]. Furthermore, OMVs support bacterial defense against antibiotics [9] and contribute to transfer of antibiotic resistance between bacteria [10].
OMVs mimic the outside of bacteria, resembling a pathogen without the ability to cause disease, and containing multiple surface-exposed antigens. In addition, OMVs hold pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides (LPS), peptidoglycans, lipoproteins, and flagella, that confer self-adjuvanticity [11]. Furthermore, the particle size of OMVs facilitates uptake by antigen-presenting cells (APCs), aiding presentation to cognate T cells, and by follicular dendritic cells (FDCs) that activate antigen-specific B cells, with the induction of adaptive immune response. For these reasons, over the last years, OMVs have been regarded as a versatile platform for vaccine development [12, 13].
OMVs naturally released from bacteria are designated as native OMVs (nOMVs), but for many bacteria this occurs at levels too low for vaccine manufacturing. To increase yields, OMVs have been chemically extracted from whole bacteria using detergents (e.g. deoxycholate), resulting in vesicle-like aggregates of insoluble outer membrane proteins called detergent-extracted OMVs (dOMVs). The supernatant of the fermentation containing spontaneous vesicles is usually discarded and extraction of the OMV is then performed. A sonication step can be introduced to obtain dOMVs of smaller and more homogeneous size, also to simplify sterile filtration [12]. The use of detergents largely reduces LPS and lipoprotein content, consequently reducing reactogenicity and improving tolerability of the OMV [2, 14, 15]. However, this approach also results in loss of important protective lipoprotein antigens and compromises vesicle integrity, with contamination of the resulting preparation with cytoplasmic proteins [16, 17].
Bacteria can be genetically manipulated to alter OMV properties (e.g., yield, endotoxicity, protein content) and classified as mutant-derived OMVs (mdOMVs) or generalized modules for membrane antigens (GMMAs). Mutations are often introduced to destabilize the linkage of the outer membrane with the inner membrane and the peptidoglycan layer in the periplasm. At this scope, different strategies have been pursued. A common one is deletion of the tolR gene of the Tol-Pal system present in most Gram-negative bacteria [18–20]. Deletion of nlpI, with changes in peptidoglycan dynamics, has been used in Escherichia coli [21]; disruption of the VacJ/Yrb ABC (ATP-binding cassette) transport system, resulting in accumulation of phospholipids in the outer leaflet of the outer membrane, has been used in Haemophilus influenzae, Vibrio cholerae, and E. coli [22, 23]. Deletion of lpp [24], mltA (gna33) [16], rmpM [25], ompT [26], pagL [27], enterobacterial common antigen [22, 28], degP [1], and virk [29] also results in overblebbing of the bacterial outer membrane.
Because the LPS content is high in naturally released OMVs, additional mutations are introduced to reduce endotoxicity. This is usually achieved through modification of the lipid A structure, in particular reducing the number of acyl chains and phosphate groups of lipid A, impacting its ability to recognize/trigger toll-like receptor (TLR) 4 [30–32] and decreasing the inflammatory response associated with lipid A [11]. Deletion of acyltransferases such as MsbB (lpxM), htrB (lpxL) and pagP (adding a myristoyl, lauroyl or palmitoyl chain, respectively, to the lipid A) has been used in E. coli, Shigella and Salmonella [33–35]; deletion of lpxL1 and lpxL2 has been used in Neisseria meningitidis [36–38].
Compared with dOMVs, the purification of nOMVs and GMMAs is simpler and requires less steps [12]. OMVs can be separated from whole bacteria by a first tangential flow filtration (TFF), and a second TFF retains OMVs while removing soluble proteins and other low molecular weight impurities [39, 40].
In this review, we provide an overview of OMVs and their genetically modified version, GMMAs, as a vaccine platform to present protein or glycan antigens, key achievements at clinical level, trends emerging in preclinical studies, and future perspectives of the field.
Licensed Outer Membrane Vesicle (OMV)-Based Vaccines
A few OMV-based vaccines are currently licensed and in use. These are vaccines against meningitidis, and target N. meningitidis serogroup B (MenB) and H. influenzae type b (Hib) infections (Table 1).
Table 1.
Outer membrane vesicle-based vaccines licensed and in clinical development
| Status of development | Vaccine | Pathogen | Developer |
|---|---|---|---|
| Phase I | Avacc 10 | COVID-19 | Intravacc (Netherlands) |
| iNTS-GMMA | Invasive non-typhoidal Salmonella | GSK (Italy) | |
| – | Neisseria gonorrhoea | GSK (Italy) | |
| Phase II | altSonflex1-2-3 | Shigella | GSK (Italy) |
| Registered | PedvaxHib (PRP-OMPC) | Haemophilus influenzae type b | Merck Co. |
| Procomvax/Comvax (PRP-OMPC and hepatitis B) | H. influenzae type b and hepatitis B | Merck Co.a | |
| Vaxelis (diphtheria and tetanus toxoids, acellular pertussis, inactivated poliovirus, PRP-OMPC and hepatitis B) | Diphtheria, tetanus, pertussis, poliomyelitis, H. influenzae type b and hepatitis b | Merck Co. and Sanofi Pasteur | |
| Bexsero (4CMenB) | N. meningitidis B | GSK (Italy) | |
| MenZB (NZ dOMV) | N. meningitidis B | Novartis Vaccine and Diagnostics (Italy)b and National Institute of Public Health (Norway) | |
| VA-MENGOC-BC | N. meningitidis B | Finlay Institute (Cuba) | |
| Norway MenBVAC | N. meningitidis B | Norwegian Institute of Public Health (Norway)a |
aMarketing authorization was not renewed by the market authorization holder (Procomvax)
bNo longer licensed, NVD was taken over by GSK
Formulated with three recombinant proteins in Bexsero (Bexsero is a trademark owned by or licensed to the GSK group of companies), the OMV detergent extracted from N. meningitidis (from strain NZ98/254) is indicated for prevention of meningitis and meningococcemia caused by N. meningitidis serogroup B. The vaccine works by stimulating the production of bactericidal antibodies that recognize the vaccine antigens (NHBA, NadA, fHbp) and the PorA, as a major component of the OMV. A review of the Meningococcal Antigen Typing System (MATS) by Muzzi et al. suggests that the vaccine covers between 81 and 84% of MenB isolates [41]. It was tested in several clinical trials showing a good immune response, as measured by human complement serum bactericidal activity (hSBA). In a study in infants [42], when the OMV component was increased while keeping the recombinant proteins the same, there was a corresponding augmentation of the immune response measured as proportions of infants with hSBA ≥ 5 and geometric mean titers (GMTs), which increased in a dose-dependent manner, lowest in infants receiving 6.25 μg and highest in those receiving the full dose of OMV in Bexsero (i.e. 25 μg). However, there was no similar trend for systemic reactogenicity, with a similar reactogenicity profile observed for recipients of one-quarter, half, and full doses of OMV. For local reactions, this was much higher among recipients of 4-component MenB vaccine containing OMVs compared with individuals who received a vaccine without OMV. Furthermore, the vaccine formulated with OMV, when compared with the recombinant proteins alone, gave a much higher frequency of fever. The vaccine was licensed in 2013 and is indicated for active immunization of individuals from 2 months of age against invasive meningococcal disease (IMD) caused by MenB [43]. The safety of the vaccine has been evaluated in 17 studies, including 10 randomized controlled clinical trials with infants, children, adolescents and adults, with a higher frequency of fever when coadministered with routine vaccines (69–79% of subjects), compared with those receiving routine vaccinations alone (44–59%), although most events were of mild to moderate severity and short-lived (lasting about 1 day) [43]. Although the vaccine was licensed using immunogenicity endpoints, within 3 years of introducing Bexsero in the UK there was a 75% reduction of MenB IMD cases in vaccine-eligible infants [44].
The same OMVs were formulated alone in the New Zealand MenB vaccine, used to fight an outbreak of meningitidis due to N. meningitidis serogroup B in that country. The OMV vaccine was tested in several clinical trials where it showed an acceptable safety profile and a strong immune response after three doses of the vaccine. The vaccination program was staggered throughout the different regions of New Zealand, delivered to different age groups at a time between 2004 and 2006, and used for infant routine vaccination until 2008 [45]. The vaccine was estimated to be 77% effective and prevented an estimated 210 cases of MenB IMD with its attendant sequelae between July 2004 and December 2008 [46]. Similarly, in Norway, a dOMV vaccine developed in 1983 against MenB was used to control outbreaks of MenB disease. The vaccine was administered to 171,800 adolescents from 1988 to 1991, with an estimated efficacy of 87% after 10 months and with a rapid decline thereafter, aligned with a reduction in serum bacterial activity [47]. The vaccine was also used between 2006 and 2009 to control an outbreak in Normandy, France, due to the same B14:P1.7,16 strain, with a decrease in the incidence of cases from 31.6 to 5.9 per 100,000 between 2006 and 2009 [48]. Another dOMV-based vaccine (VA-MENGOC-BC, Finlay Institute, Havana, Cuba) was licensed for use in Cuba against MenB in 1987 (VA-MENGOC-BC is a registered trademark of Finlay Institute of Vaccines, Cuba) [49], succeeding in reducing the incidence of MenB disease by 93–98% in the following 20 years, eventually resulting in MenB no longer being a public health problem in Cuba [50]. In 2014, the vaccine was acquired by Abivax for distribution outside Cuba in countries in Asia and Latin America.
Hib-OMPC was the first OMV-based vaccine to be licensed widely. It is a highly purified capsular polysaccharide of H. influenzae type b (polyribosylribitol phosphate [PRP]) covalently bound to outer membrane protein complex (OMPC), obtained by detergent extraction of OMVs from the B11 strain of N. meningitidis serogroup B. For licensure, PRP-OMPC was shown to be 93–100% efficacious in a pivotal trial that enrolled 3486 Navajo infants (a population at particularly high risk of disease) vaccinated at 2 and 4 months of age [51]. Licensed as PedvaxHib (PedvaxHIB is a licensed trademark of Merck Sharp and Dohme Corp.), the vaccine induced anti-PRP levels > 0.15 μg/mL in 88% and > 1.0 μg/mL in 52% of the infants, with a GMT of 0.95 μg/mL 1–3 months after the first dose; rising to 91% and 60%, respectively, after the second and third doses [51].
PRP-OMPC stimulates a strong immune response after one dose in children with a high risk of Hib infection in early infancy [52]. However, the American Advisory Committee on Immunization Practices (ACIP) recommends that previously unvaccinated infants aged 2–6 months should receive two doses of PRP-OMPC administered at least 2 months apart, to ensure longevity of protection. They also recommend one or two doses in unvaccinated children depending on their age [53].
Eventually, PedvaxHib was combined with hepatitis B surface antigen (HbsAg) and licensed as Comvax (Procomvax in the EU). The combination resulted in anti-PRP antibody levels lower than the previously reported levels in the stand-alone vaccine (Procomvax–EU/Comvax–US) [54]. Procomvax and Comvax are registered trademarks of Merck and Co./MSD in Europe.
The immunogenicity of Comvax (7.5 μg H. influenzae type b PRP, 5 μg HBsAg) was assessed in 1602 infants and children 6 weeks–15 months of age in five clinical studies. In these studies, the immune response of Comvax was compared with that obtained using the monovalent vaccines, PedvaxHib (7.5 μg PRP) and RECOMBIVAX HB (5 μg HBsAg) administered at separate sites, either concurrently or 1 month apart. In infants not previously vaccinated with Hib or Hepatitis B vaccine, antibody responses to Comvax showed proportions achieving anti-PRP levels > 1.0 μg/mL after the second dose, similar to those of subjects receiving the monovalent vaccine (72.4%) [55]. The vaccine is indicated for vaccination against invasive Hib disease and HBV infection in infants born from HbsAg-negative women with three doses at 2, 4, and 12–15 months (MMR weekly, CDC). Procomvax was taken off the European market by the manufacturer in 2009, who opted against renewing the market authorization for commercial reasons. There was no safety concern linked to that decision.
The monovalent vaccine was also combined with diphtheria and tetanus toxoids, Bordetella pertussis antigens, HbsAg and inactivated polio virus as a hexavalent pediatric vaccine (VAXELIS). VAXELIS is a registered trademark of MSP Vaccine Company. This combination is indicated for primary vaccination and booster vaccination in infants and toddlers from 6 weeks of age, with a primary vaccination schedule of two to three doses, at an interval of at least 1 month between the doses. The safety profile of the vaccine is comparable with other multivalent vaccines for the same indication; however, a coadministration booster study with pneumococcal vaccine (Prevnar 13) revealed a frequency of fever of up to 52.5% among recipients of both vaccines, although the fevers were of mild to moderate intensity and short-lived (< 48 h) [56]. Overall, the safety of Hib PRP-OMPC vaccines is comparable with those of other Hib vaccines, with non-clinically significant differences between them [57].
OMV Vaccines in Clinical Development
Considering the advantages of OMVs in presenting multiple antigens in their natural configuration with self-adjuvanticity, there are a number of OMV-based vaccines in clinical development to fight pathogens from which they derive (Table 1).
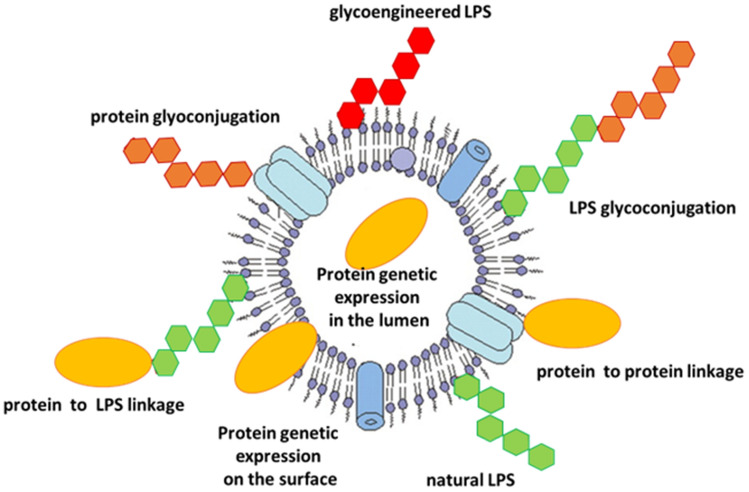
GMMAs naturally display O-polysaccharides on their surface (Fig. 1) and have been exploited as vaccine candidates to prevent non-typhoidal Salmonella and Shigella, now in clinical trials [39, 58–60].
Fig. 1.
Main strategies adopted to display protein and glycan antigens on OMVs or GMMAs. GMMAs generalized modules for membrane antigens, LPS lipopolysaccharide, OMVs outer membrane vesicles
In mice, O-polysaccharides displayed on Salmonella typhimurium and Salmonella enteritidis GMMAs have been shown to elicit a strong anti-O-polysaccharide immunoglobulin (Ig) G antibody response, at levels comparable with those induced by the corresponding CRM197 conjugates formulated with alum [61]. Interestingly, GMMAs enhanced the IgG antibody isotype profile and resulted in greater serum bactericidal activity as compared with protein conjugates. The bivalent formulation of S. typhimurium and S. enteritidis GMMAs adsorbed on Alhydrogel is now being tested in a phase I trial in European adults (NCT05480800). This formulation has also been combined with a glycoconjugate vaccine against S. typhi (Typhibev), for which a phase I trial in healthy adults has also been started (NCT 05480800).
The first GMMA-based vaccine to be tested in clinical trials was the Shigella sonnei GMMA vaccine 1790 GAHB. GMMAs were purified from an S. sonnei strain genetically engineered to increase blebbing (ΔtolR), produce less reactogenic penta-acylated lipid A (ΔhtrB), and stably express the virulence plasmid encoding for the O-polysaccharide [39]. S. sonnei GMMAs, adsorbed on Alhydrogel, were tested in two phase I trials, one extension booster trial, one phase IIa clinical trial, and eventually in a phase IIb human challenge infection model (CHIM) trial [60]. O-Polysaccharide-based S. sonnei GMMAs have been demonstrated to be well tolerated and immunogenic in healthy adults in European and disease-endemic countries, eliciting bactericidal anti-O-polysaccharide IgG response after one dose of vaccination [62–65]. Noteworthy, revaccination 3 years apart from the primary immunization showed the ability of the vaccine to induce a robust anamnestic memory response [66].
1790GAHB failed to show clinical efficacy in the CHIM trial, likely due to inadequate immune response linked to the low O-polysaccharide dose tested. However, based on those results, a new generation S. sonnei construct has been developed, allowing to increase 10 times the O-polysaccharide dose. Such GMMAs have been combined in a 4-component formulation with Shigella flexneri 1b, 2a, and 3a GMMAs that is currently being tested in a phase I/II trial (NCT05073003) [60].
There is also epidemiologic evidence of a moderate level of effectiveness of N. meningitidis B OMV vaccines against Neisseria gonorrhoeae [67–69], leading to suggestions that an OMV-based vaccine could be suitable for prevention of gonococcal infection, both through a vaccine against meningococcal B (NCT04297436 and NCT04415424) and with GMMAs specific for N. gonorrhoeae, with clinical trials underway (NCT05630859).
Vaccines with heterologous targets, in which the OMVs are conjugated to other antigens, are also in early clinical development, such as the intranasally delivered vaccine against coronavirus disease 2019 (COVID-19; Avacc 10) developed by Intravacc BV (NCT05604690).
Trends Emerging in Preclinical Studies
Traditionally, OMVs have been proposed to fight diseases caused by pathogens from which they derive [12], with some of them licensed or in clinical development and many others at the preclinical stage [2, 12]. The immune response can be directed to the LPS rather than protein components, as shown in early studies with naturally released V. cholerae OMVs, which elicited an antibody response by mucosal immunization protecting mice offspring from oral V. cholerae challenge [70] and ensured cross protection against the two variants Inaba or Ogawa of the most epidemiologically relevant serotype O1. OMVs failed to cross-react with O139 [71].
Recent studies have shown how genetic manipulations can be easily performed with the aim to further improve OMV safety, immunogenicity, and protective efficiency. For example, it has been shown that disruption of small noncoding RNA improves the protective efficacy of OMVs against Helicobacter pylori infection in a mouse model [72]. Both intranasal and intraperitoneal immunization with flagellin-deficient S. typhimurium OMVs resulted in efficient protection against heterologous S. choleraesuis and S. enteritidis challenge [73] and immunization with OMVs from major outer membrane protein-deficient S. typhimurium mutants enhanced cross-protection [74].
Recently, OMVs have also been proposed as delivery systems for heterologous antigens. Indeed, OMVs can be modified to display either proteins or polysaccharides (Fig. 1) derived from different (even phylogenetically distant) pathogens (viral, bacterial, parasitic).
OMVs as Carrier for Heterologous Proteins
In particular, OMVs produced by E. coli strains have been used as a delivery system for recombinant proteins. Fusion of the antigen to secretion signals or periplasmic proteins have been used for expression of recombinant antigens in the lumen of the vesicles [75–80] (Fig. 1). GFP was expressed in the lumen of E. coli OMVs through the twin-arginine (Tat) signal sequence [77]. Luminal expression in E. coli OMVs by fusion to the periplasmic side of the abundant outer membrane protein OmpA has been tested for Group A Streptococcus (i.e., Slo, SpyCEP, SpyAD), Group B Streptococcus (i.e., SAM_1372), and chlamydia protein antigens [75, 76, 78]. The pneumococcal surface adhesin A (PspA) was expressed in the lumen of S. typhimurium OMVs by fusion to the N-terminal β-lactamase signal sequence [79].
However, protein localization on the OMV surface should be preferable for a stronger immune response [76, 79, 80]. Only a few studies have reported a direct comparison between OMVs presenting the same antigen on the surface or in the lumen of the vesicles. E. coli alkaline phosphatase PhoA, loaded in V. cholerae OMVs, elicited a low immune response after intranasal immunization of mice, likely because of the location of the enzyme in the lumen of the OMV as opposed to the surface [80]. In the study by Fantappiè et al., OMVs induced high functional antibody titers against GAS and GBS proteins loaded into the lumen of E. coli OMVs and immunization with Slo-OMV- and SpyCEP-OMV-protected mice against GAS lethal challenge [76]. Similarly, mice immunized intranasally with PspA in the lumen of S. typhimurium OMVs developed antigen-specific serum antibody response, while no detectable response was developed by an equivalent dose of recombinant PspA [79]. Mice immunized with the recombinant OMV were protected against challenge with Streptococcus pneumoniae. However, additional studies were suggested to assess whether the anti-PspA immune response could have been enhanced by localizing the antigen at the surface of the OMV [79]. Salverda and collaborators demonstrated that the expression of the Borrelial surface-exposed lipoprotein OspA on the surface of N. meningitidis OMVs resulted in a strong anti-OspA antibody response compared with the construct with luminal expression of OspA, where no antigen-specific antibody response was observed [81]. Similar results were obtained by Necchi et al., where a direct comparison between N. meningitidis fHbp inside S. typhimurium GMMAs or chemically linked on the GMMA surface was performed. The immune response elicited by fHbp expressed in the lumen of GMMAs was extremely low and much lower compared with the same antigen displayed on the GMMA surface [82]. Also the authors clearly showed the need to have fHbp on GMMAs (through genetic manipulation or chemical conjugation), resulting in a much stronger bactericidal response compared with fHbp simply physically mixed to GMMAs [83].
However, the expression of heterologous proteins on the OMV surface is quite challenging, often characterized by low expression levels and being antigen-dependent. Different strategies have been proposed for surface protein expression. Proteins that are normally exported beyond the cell surface by proteolytic processing can be retained on the OMV surface by preventing the proteolysis [84]. The protein of interest can be fused to membrane-associated proteins such as the β-barrel domain of autotransporters (e.g. Hbp, AIDA). The autotransporter hemoglobin protease (Hbp) of E. coli was used to express Mycobacterium tuberculosis proteins and epitopes of the major outer membrane protein MOMP from Chlamydia thrachomatis on S. typhimurium OMVs [85, 86]. The same system was used to display high density of two S. pneumoniae protein antigens on Salmonella OMVs. Intranasal immunization with resulting OMVs induced strong protection in a murine model of pneumococcal colonization, without the need for a mucosal adjuvant [87]. This method of antigen display is highly efficient, but it seems limited to small proteins. Kim et al. fused several heterologous proteins to the C-terminus of the pore-forming cytotoxin ClyA [88]. Engineered E. coli OMVs displaying green fluorescent protein (GFP), genetically fused with ClyA, elicited stronger anti-GFP antibody titers in immunized mice compared with GFP alone [89]. Through the same expression system, E. coli OMVs expressing Acinetobacter baumannii Omp22 induced significantly higher Omp22-specific antibodies than immunization with higher amounts of recombinant Omp22 protein formulated with alum [90]. The surface expression of OspA in meningococcal OMVs was achieved by fusion to a membrane anchoring second lipoprotein fHbp [81].
A COVID-19 subunit vaccine based on a recombinant, six-proline-stabilized, D614G spike protein (mC-Spike) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fused to the LPS-binding peptide sequence mCramp (mC) of meningococcal OMVs [91] elicited high levels of neutralizing antibodies in the golden Syrian hamster model, after intranasal immunization, together with a detectable mucosal response. The candidate vaccine was also protective in a hamster challenge model. OMVs engineered to incorporate peptides derived from the receptor binding motif (RBM) of the spike protein from SARS-CoV-2 have also been made and shown to elicit neutralizing antibodies in mice [92].
A chimeric fusion protein of the H1-type hemagglutinin (HA) of the pandemic influenza A virus (H1N1) strain from 2009 (H1N1pdm09) and the receptor binding domain (RBD) of the Middle East respiratory syndrome coronavirus (MERS-CoV) has also been expressed on OMVs from E. coli DH10ß, inducing an immune response in mice that protected from influenza challenge [93].
Alternative ways to decorate the surface of OMVs with heterologous proteins have also been proposed, where antigens are produced separately and added post OMV production (Fig. 1). Among these strategies, the SpyTag-SpyCatcher system uses the SpyCatcher domain from a Streptococcus pyogenes surface protein, which recognizes a cognate 13-amino-acid peptide (SpyTag). After recognition, a covalent isopeptide bond is formed between the side chains of a lysine in SpyCatcher and an aspartate in SpyTag [94]. The SpyTag is expressed on OMVs and used for coupling to a SpyCatcher fused to any protein [95, 96].
This approach has recently been used to couple the RBD domain of SARS-CoV2-Spike harboring SpyTag to OMVs from S. typhimurium displaying Hbp modified with the SpyCatcher peptide [97]. The vaccine candidate was immunogenic and protective in a hamster model.
More recently, a method for avidin-based vaccine antigen crosslinking was proposed where biotinylated proteins are linked to the exterior of OMVs whose surfaces are remodeled with biotin-binding proteins. The resulting OMVs, when tested in mice, elicited strong antigen-specific antibody responses [98].
Furthermore, chemical conjugation of proteins to OMVs has been used with the scope to decorate OMVs with heterologous protein antigens and the potential to result in multicomponent vaccines. Chemical conjugation is a rapid method to exploit OMVs as carrier, and it allows, within a certain range, to better control the amount and density of antigen displayed on the vesicles. OMVs from different pathogens (N. meningitidis, Salmonella, Shigella) have been linked to different proteins (N. meningitidis fHbp, E. coli SslE and FdeC, malaria proteins Pfs25, Pfs230 and CSP, etc.), showing ability of the antigens on OMVs to elicit much stronger functional antibody responses compared with protein antigens alone [83, 99–102]. The chemical approach has very recently been extended to viral antigens (e.g. influenza A virus hemagglutinin and rabies glycoprotein), confirming the ability of OMVs to significantly increase antigen-specific humoral and cellular responses [103].
Glycoconjugated OMVs
OMVs have been also proposed as carriers for polysaccharides, starting from the above-mentioned licensed Hib-OMPC conjugate vaccine that was initially shown effective in inducing antibody response in animals [104] and triggering cytokine-mediated responses by engaging TLR2 [105]. Conjugation of Hib polysaccharide to dOMVs from B. pertussis has also been shown as a viable modality to induce responses against both pertussis and H. influenzae [106].
Considering their ease of production and purification, and the potential for an increased immune response, recent numerous examples have been reported about the use of GMMAs as carrier for polysaccharides [61, 100, 107–110].
GMMAs can serve as carrier for chemically linked polysaccharides, offering the possibility to direct the polysaccharide conjugation either to the LPS/lipooligosaccharides (LOS) or to the proteins exposed onto the vesicles (Fig. 1) [107]. A variety of structurally diverse polysaccharides from different pathogens (N. meningitidis serogroups A and C, H. influenzae type b, Streptococcus group A carbohydrate and Salmonella typhi Vi) have been successfully covalently bound to GMMAs, generating strong antipolysaccharide immune responses in animals [100, 109, 110]. The level and functionality of raised antibodies was not greatly impacted by the number of glycans linked per GMMA, although lower saccharide loading was shown to better ensure preservation of the immunogenicity of GMMA-exposed proteins. On the other hand, the glycan length needed case-by-case optimization to obtain a robust immune response. Compared with linkage to proteins, LOS/LPS-directed conjugation was also efficient in inducing a strong functional immune response against the polysaccharides [107].
OMV and GMMA combine display of multiple copies of carbohydrates, favoring B-cell activation, with their presentation in the native bacterial context. Moreover, their size is optimal for immune stimulation and they promote intrinsic adjuvant properties due to the presence of TLRs such as TLR2 and 4 [13]. Interestingly, using GMMAs from S. sonnei and S. typhimurium as a model, it has been observed that the induced immune response is mediated by antigen presentation by FDC to cognate B cells [111]. Engagement of TLR4 was seen to be critical to induce strong antibody production, whereas TLR2 activation did not appear to play any role in GMMA immunogenicity.
In addition to chemical conjugation, GMMAs and OMVs can also be engineered to express heterologous glycans resulting in the so-called glycoengineered OMV (glyOMV) [112]. E. coli strains not expressing polymeric O-polysaccharides have been genetically modified to insert operons for biosynthesis of the heterologous polysaccharide into the wbbL gene, while maintaining the lipid A-core production as acceptor. Through this strategy, the heterologous glycan structure is synthesized on the cytoplasmic side of the inner membrane, assembled on the native undecaprenyl pyrophosphate carrier (Und-PP), and translocated to the periplasm side by the action of endogenous flippase Wzx. Finally, the endogenous O-antigen ligase ‘WaaL’ transfers the assembled polysaccharide en bloc to the lipid A-core structure. Alternatively, engineered glycans can be assembled directly, one residue at a time, starting from the terminal sugars of the truncated lipid A-core expressed on the cytoplasmic side of the inner membrane, and then flipped to the periplasm in an MsbA-dependent manner [14]. The resulting LPS molecules are transported to the outer membrane and flipped to the extracellular space by the Lpt protein complex, such that the glycoengineered lipid A-core structures are being transferred to the outer membrane and incorporated into the vesicles. Since various plasmid-encoded O-polysaccharide biosynthetic pathways can be incorporated in E. coli, this approach renders OMV a ‘plug-and-play’ platform to display glycotopes from different pathogenic bacteria.
Using this tactic, Chen et al. [113] generated a panel of glyOMVs containing O-polysaccharides from eight different strains of pathogenic bacteria, including the highly virulent Francisella tularensis subsp. tularensis type A strain Schu S4 (creating ft-glyOMVs). Two weeks post-immunization, ft-glyOMVs induced in mice two- to threefold higher levels of O-polysaccharide-specific IgG compared with the native ftLPS. Mice were protected from lethal challenge with F. tularensis Schu S4, as well as with F. tularensis subsp. holoarctica type B strains that display O-polysaccharide with same structure. Ft-glyOMVs also elicited a protective IgA-mediated mucosal immune response, when subcutaneously administered.
Price et al. [114] successfully engineered E. coli OMVs to express capsular polysaccharides from S. pneumoniae. GlyOMVs induced serum IgG opsonophagocytic titers comparable with the corresponding chemical conjugates contained in PCV13. E. coli OMVs were also glycoengineered to express a heptasaccharide from C. jejuni, showing reduced bacterial colonization upon challenge of vaccinated chickens [114].
Stevenson et al. achieved high-level surface expression of PNAG polysaccharide on the OMV surface [115], using a hypervesiculating JC8031 strain of E. coli. S. aureus PNAG deacetylase IcaB was expressed in PNAG-positive JC8031 cells (dPNAG-glyOMV) to enrich glyOMVs with deacetylated PNAG. Immunization of mice with both engineered glyOMVs resulted in the production of high levels of PNAG-specific antibodies, but only antibodies elicited by dPNAG-OMVs were able to mediate in vitro killing of two distinct PNAG-producing bacterial species (i.e., the Gram-positive S. aureus and the Gram-negative F. tularensis holoartica). The vaccine candidate also induced protection of mice challenged with lethal doses of S. aureus and F. tularensis.
Tian et al. biosynthesized S. flexneri 2a O-polysaccharide in Salmonella OMVs, showing that immunization of mice, both intranasally and intraperitoneally, with the OMV vaccine induced significant specific anti-Shigella LPS antibodies in the serum and IgA in vaginal secretions and fluid from bronchopulmonary lavage, and provided significant protection against virulent S. flexneri 2a infection [116].
Conclusions
OMVs have a long and rich history of use, particularly in vaccines against N. meningitidis and H. influenzae type b. A large body of preclinical data has been generated to fight many other pathogens, supporting the use of OMVs and their genetically modified version (GMMAs) as a promising affordable platform to deliver both homologous and heterologous protein and carbohydrate antigens.
The nanosize of OMVs is thought to enhance uptake by APCs, whose activation occurs through recognition of multiple PAMPs on OMVs by TLRs. B-cell activation is facilitated by the repetition of epitopes on the OMV surface and by the size of OMVs, which allows them direct access to the lymphatic system. T-cell/B-cell cooperation is essential for the generation of high affinity antibody-producing plasma cells and memory B cells. More studies on the OMV mode of action will allow to better understand how immunity to this class of vaccines is induced, and will help elucidating whether the mechanisms identified are platform-related or pathogen-specific.
The COVID-19 pandemic, as well as the emergence of antimicrobial resistance, are catalyzing the progression of novel vesicle-based candidate vaccines into clinical development (Table 1). Homologous meningococcal OMVs are included in licensed vaccines, and GMMAs to fight homologous pathogens from which they are derived are being tested in clinical studies. Data from current trials, both in terms of safety and immunogenicity, will be key to further support the use of this platform for novel vaccine development.
Current advancements in the field focus on OMVs and GMMAs as carriers for heterologous protein and carbohydrate antigens (Fig. 1). Vaccines against COVID-19 developed using this technology (Avacc 10) are undergoing clinical testing. Generation of this type of multicomponent vesicles has been shown to be feasible by different new technologies, including chemical conjugation, molecular engineering and Spy-tag/Spy-capture. These new approaches will favor simplification of vaccines targeting a variety of different pathogenic mechanisms or even different pathogens. We expect that in the future, this class of vaccines will advance significantly, and more vesicle-based vaccines will become available to help fight emerging diseases and unmet medical needs.
Declarations
Funding
No funding was used to assist in the preparation of this article.
Conflict of interest
Francesca Micoli, Roberto Adamo, and Usman Nakakana are employees of the GSK group of companies. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
All authors wrote the main manuscript text, prepared figures, and reviewed and approved the manuscript.
References
- 1.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188(15):5385–5392. doi: 10.1128/jb.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10(11):1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi: 10.1128/mmbr.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13–24. doi: 10.1038/s41579-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 6.DeVoe IW, Gilchrist JE. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol. 2006;188(16):5945–5957. doi: 10.1128/jb.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palsdottir H, Remis JP, Schaudinn C, O'Toole E, Lux R, Shi W, et al. Three-dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography. J Bacteriol. 2009;191(7):2077–2082. doi: 10.1128/jb.01333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177(14):3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rumbo C, Fernández-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55(7):3084–3090. doi: 10.1128/aac.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini F, Rossi O, Necchi F, Micoli F. OMV vaccines and the role of TLR agonists in immune response. Int J Mol Sci. 2020 doi: 10.3390/ijms21124416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- 13.Piccioli D, Bartolini E, Micoli F. GMMA as a 'plug and play' technology to tackle infectious disease to improve global health: context and perspectives for the future. Expert Rev Vaccines. 2022;21(2):163–172. doi: 10.1080/14760584.2022.2009803. [DOI] [PubMed] [Google Scholar]
- 14.Gnopo YMD, Watkins HC, Stevenson TC, DeLisa MP, Putnam D. Designer outer membrane vesicles as immunomodulatory systems—reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev. 2017;114:132–142. doi: 10.1016/j.addr.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Rappuoli R, Pizza M, Masignani V, Vadivelu K. Meningococcal B vaccine (4CMenB): the journey from research to real world experience. Expert Rev Vaccines. 2018;17(12):1111–1121. doi: 10.1080/14760584.2018.1547637. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, et al. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6(6):1856–1866. doi: 10.1002/pmic.200500164. [DOI] [PubMed] [Google Scholar]
- 17.van de Waterbeemd B, Mommen GP, Pennings JL, Eppink MH, Wijffels RH, van der Pol LA, et al. Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J Proteome Res. 2013;12(4):1898–1908. doi: 10.1021/pr301208g. [DOI] [PubMed] [Google Scholar]
- 18.Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubes R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180(18):4872–4878. doi: 10.1128/JB.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner L, Praszkier J, Hutton ML, Steer D, Ramm G, Kaparakis-Liaskos M, et al. Increased outer membrane vesicle formation in a Helicobacter pylori tolB mutant. Helicobacter. 2015;20(4):269–283. doi: 10.1111/hel.12196. [DOI] [PubMed] [Google Scholar]
- 20.Mitra S, Sinha R, Mitobe J, Koley H. Development of a cost-effective vaccine candidate with outer membrane vesicles of a tolA-disrupted Shigella boydii strain. Vaccine. 2016;34(15):1839–1846. doi: 10.1016/j.vaccine.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Schwechheimer C, Rodriguez DL, Kuehn MJ. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. MicrobiologyOpen. 2015;4(3):375–389. doi: 10.1002/mbo3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulp AJ, Sun B, Ai T, Manning AJ, Orench-Rivera N, Schmid AK, et al. Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PLoS ONE. 2015;10(9):e0139200. doi: 10.1371/journal.pone.0139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maharjan S, Saleem M, Feavers IM, Wheeler JX, Care R, Derrick JP. Dissection of the function of the RmpM periplasmic protein from Neisseria meningitidis. Microbiology (Reading, England). 2016;162(2):364–375. doi: 10.1099/mic.0.000227. [DOI] [PubMed] [Google Scholar]
- 26.Premjani V, Tilley D, Gruenheid S, Le Moual H, Samis JA. Enterohemorrhagic Escherichia coli OmpT regulates outer membrane vesicle biogenesis. FEMS Microbiol Lett. 2014;355(2):185–192. doi: 10.1111/1574-6968.12463. [DOI] [PubMed] [Google Scholar]
- 27.Elhenawy W, Bording-Jorgensen M, Valguarnera E, Haurat MF, Wine E, Feldman MF. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. MBio. 2016 doi: 10.1128/mBio.00940-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon KJ, Castelli ME, García Vescovi E, Feldman MF. Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J Bacteriol. 2012;194(12):3241–3249. doi: 10.1128/jb.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidik S, Kottwitz H, Benjamin J, Ryu J, Jarrar A, Garduno R, et al. A Shigella flexneri virulence plasmid encoded factor controls production of outer membrane vesicles. G3 (Bethesda). 2014;4(12):2493–2503. doi: 10.1534/g3.114.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wang Z, Chen J, Ernst RK, Wang X. Influence of lipid A acylation pattern on membrane permeability and innate immune stimulation. Mar Drugs. 2013;11(9):3197–3208. doi: 10.3390/md11093197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han Y, Li Y, Chen J, Tan Y, Guan F, Wang X. Construction of monophosphoryl lipid A producing Escherichia coli mutants and comparison of immuno-stimulatory activities of their lipopolysaccharides. Mar Drugs. 2013;11(2):363–376. doi: 10.3390/md11020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zariri A, van der Ley P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev Vaccines. 2015;14(6):861–876. doi: 10.1586/14760584.2015.1026808. [DOI] [PubMed] [Google Scholar]
- 33.Rossi O, Pesce I, Giannelli C, Aprea S, Caboni M, Citiulo F, et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants. J Biol Chem. 2014;289(36):24922–24935. doi: 10.1074/jbc.M114.566570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi O, Caboni M, Negrea A, Necchi F, Alfini R, Micoli F, et al. Toll-like receptor activation by generalized modules for membrane antigens from lipid a mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin Vaccine Immunol. 2016;23(4):304–314. doi: 10.1128/CVI.00023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tondi S, Clemente B, Esposito C, Sammicheli C, Tavarini S, Martin LB, et al. Dissecting in vitro the activation of human immune response induced by Shigella sonnei GMMA. Front Cell Infect Microbiol. 2022;12:767153. doi: 10.3389/fcimb.2022.767153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69(10):5981–5990. doi: 10.1128/iai.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisseha M, Chen P, Brandt B, Kijek T, Moran E, Zollinger W. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect Immun. 2005;73(7):4070–4080. doi: 10.1128/iai.73.7.4070-4080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, et al. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA) Vaccine. 2014;32(23):2688–2695. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 39.Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS ONE. 2015;10(8):e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerritzen MJH, Salverda MLM, Martens DE, Wijffels RH, Stork M. Spontaneously released Neisseria meningitidis outer membrane vesicles as vaccine platform: production and purification. Vaccine. 2019;37(47):6978–6986. doi: 10.1016/j.vaccine.2019.01.076. [DOI] [PubMed] [Google Scholar]
- 41.Muzzi A, Brozzi A, Serino L, Bodini M, Abad R, Caugant D, et al. Genetic meningococcal antigen typing system (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine. 2019;37(7):991–1000. doi: 10.1016/j.vaccine.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 42.Esposito S, Prymula R, Zuccotti GV, Xie F, Barone M, Dull PM, et al. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine, 4CMenB, in infants (II) Hum Vaccin Immunother. 2014;10(7):2005–2014. doi: 10.4161/hv.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bexsero summary of product characteristics. https://www.medicines.org.uk/emc/product/5168/smpc.
- 44.Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–317. doi: 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- 45.Burton C, Best E, Broom M, Heffernan H, Briggs S, Webb R. Pediatric invasive meningococcal disease, Auckland, New Zealand (Aotearoa), 2004–2020. Emerg Infect Dis. 2023;29(4):686–695. doi: 10.3201/eid2904.221397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold R, Galloway Y, McNicholas A, O'Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29(40):7100–7106. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 47.Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338(8775):1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 48.Caron F, du Châtelet IP, Leroy JP, Ruckly C, Blanchard M, Bohic N, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11(6):455–463. doi: 10.1016/s1473-3099(11)70027-5. [DOI] [PubMed] [Google Scholar]
- 49.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14(2):195–207. [PubMed] [Google Scholar]
- 50.Sierra-González VG. Cuban meningococcal vaccine VA-MENGOC-BC: 30 years of use and future potential. MEDICC Rev. 2019;21(4):19–27. doi: 10.37757/mr2019.V21.N4.4. [DOI] [PubMed] [Google Scholar]
- 51.Santosham M, Wolff M, Reid R, Hohenboken M, Bateman M, Goepp J, et al. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med. 1991;324(25):1767–1772. doi: 10.1056/nejm199106203242503. [DOI] [PubMed] [Google Scholar]
- 52.Granoff DM, Anderson EL, Osterholm MT, Holmes SJ, McHugh JE, Belshe RB, et al. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr. 1992;121(2):187–194. doi: 10.1016/s0022-3476(05)81186-2. [DOI] [PubMed] [Google Scholar]
- 53.Recommendations for Use of Haemophilus b Conjugate Vaccines and a Combined Diphtheria T, Pertussis, and Haemophilus b Vaccine Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 42(RR-13). Publication date: 17 Sep 1993. [PubMed]
- 54.Procomvax summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/procomvax-epar-product-information_en.pdf.
- 55.Comvax summary of product characteristics. https://www.drugs.com/pro/comvax-vaccine.html.
- 56.Vaxelis summary of product characteristics. https://www.medicines.org.uk/emc/product/12264/smpc.
- 57.Gilsdorf JR. Hib vaccines: their impact on haemophilus influenzae type b disease. J Infect Dis. 2021;224(12 Suppl 2):S321–S330. doi: 10.1093/infdis/jiaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schager AE, Dominguez-Medina CC, Necchi F, Micoli F, Goh YS, Goodall M, et al. IgG responses to porins and lipopolysaccharide within an outer membrane-based vaccine against nontyphoidal Salmonella develop at discordant rates. MBio. 2018 doi: 10.1128/mBio.02379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Benedetto G, Alfini R, Cescutti P, Caboni M, Lanzilao L, Necchi F, et al. Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35(3):419–426. doi: 10.1016/j.vaccine.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 60.Micoli F, Nakakana UN, Berlanda SF. Towards a four-component GMMA-based vaccine against Shigella. Vaccines. 2022 doi: 10.3390/vaccines10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci USA. 2018;41(115):10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Micoli F, Rossi O, Conti V, Launay O, Sciré AS, Aruta MG, et al. Antibodies elicited by the Shigella sonnei GMMA vaccine in adults trigger complement-mediated serum bactericidal activity: results from a phase 1 dose escalation trial followed by a booster extension. Front Immunol. 2021;12:671325. doi: 10.3389/fimmu.2021.671325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obiero CW, Ndiaye AGW, Scire AS, Kaunyangi BM, Marchetti E, Gone AM, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Scire AS, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBioMedicine. 2017;22:164–172. doi: 10.1016/j.ebiom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapulu MC, Nakakana U, Sciré AS, Sarakinou E, Conti V, Rossi O, et al. Complement-mediated serum bactericidal activity of antibodies elicited by the Shigella sonnei GMMA vaccine in adults from a shigellosis-endemic country: exploratory analysis of a Phase 2a randomized study. Front Immunol. 2022;13:971866. doi: 10.3389/fimmu.2022.971866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Launay O, Ndiaye AGW, Conti V, Loulergue P, Scire AS, Landre AM, et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a phase 1 clinical trial. Front Immunol. 2019;10:335. doi: 10.3389/fimmu.2019.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390(10102):1603–1610. doi: 10.1016/s0140-6736(17)31449-6. [DOI] [PubMed] [Google Scholar]
- 68.Whelan J, Kløvstad H, Haugen IL, Holle MR, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection, Norway. Emerg Infect Dis. 2016;22(6):1137–1139. doi: 10.3201/eid2206.151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azze RFO. A meningococcal B vaccine induces cross-protection against gonorrhea. Clin Exp Vaccine Res. 2019;8(2):110–115. doi: 10.7774/cevr.2019.8.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun. 2010;78(10):4402–4420. doi: 10.1128/iai.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008;76(10):4554–4563. doi: 10.1128/iai.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li B, Xu Y, Xu T, Guo Z, Xu Q, Li Y, et al. Disruption of sncRNA improves the protective efficacy of outer membrane vesicles against Helicobacter pylori infection in a mouse model. Infect Immun. 2022;90(8):e0026722. doi: 10.1128/iai.00267-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Q, Liu Q, Yi J, Liang K, Hu B, Zhang X, et al. Outer membrane vesicles from flagellin-deficient Salmonella enterica serovar Typhimurium induce cross-reactive immunity and provide cross-protection against heterologous Salmonella challenge. Sci Rep. 2016;6:34776. doi: 10.1038/srep34776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Jie K, Li B, Yu H, Ruan H, Wu J, et al. Immunization with outer membrane vesicles derived from major outer membrane protein-deficient salmonella typhimurium mutants for cross protection against Salmonella Enteritidis and avian pathogenic Escherichia coli O78 infection in chickens. Front Microbiol. 2020;11:588952. doi: 10.3389/fmicb.2020.588952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartolini E, Ianni E, Frigimelica E, Petracca R, Galli G, Berlanda Scorza F, et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J Extracell Vesicles. 2013 doi: 10.3402/jev.v2i0.20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fantappiè L, de Santis M, Chiarot E, Carboni F, Bensi G, Jousson O, et al. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J Extracellular Vesicles. 2014 doi: 10.3402/jev.v3.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279(3):2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SH, Kim KS, Lee SR, Kim E, Kim MS, Lee EY, et al. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochem Biophys Acta. 2009;1788(10):2150–2159. doi: 10.1016/j.bbamem.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muralinath M, Kuehn MJ, Roland KL, Curtiss R., 3rd Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect Immun. 2011;79(2):887–894. doi: 10.1128/iai.00950-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77(1):472–484. doi: 10.1128/iai.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salverda ML, Meinderts SM, Hamstra HJ, Wagemakers A, Hovius JW, van der Ark A, et al. Surface display of a borrelial lipoprotein on meningococcal outer membrane vesicles. Vaccine. 2016;34(8):1025–1033. doi: 10.1016/j.vaccine.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 82.Necchi F, Stefanetti G, Alfini R, Palmieri E, Carducci M, Di Benedetto R, et al. Neisseria meningitidis factor H binding protein surface exposure on Salmonella typhimurium GMMA Is critical to induce an effective immune response against both diseases. Pathogens (Basel, Switzerland). 2021. 10.3390/pathogens10060726. [DOI] [PMC free article] [PubMed]
- 83.Alfini R, Brunelli B, Bartolini E, Carducci M, Luzzi E, Ferlicca F, et al. Investigating the role of antigen orientation on the immune response elicited by Neisseria meningitidis factor H binding protein on GMMA. Vaccines. 2022 doi: 10.3390/vaccines10081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Georgiou G, Segatori L. Preparative expression of secreted proteins in bacteria: status report and future prospects. Curr Opin Biotechnol. 2005;16(5):538–545. doi: 10.1016/j.copbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Jong WS, Daleke-Schermerhorn MH, Vikström D, Ten Hagen-Jongman CM, de Punder K, van der Wel NN, et al. An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb Cell Fact. 2014;13:162. doi: 10.1186/s12934-014-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daleke-Schermerhorn MH, Felix T, Soprova Z, Ten Hagen-Jongman CM, Vikstrom D, Majlessi L, et al. Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach. Appl Environ Microbiol. 2014;80(18):5854–5865. doi: 10.1128/aem.01941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuipers K, Daleke-Schermerhorn MH, Jong WS, ten Hagen-Jongman CM, van Opzeeland F, Simonetti E, et al. Salmonella outer membrane vesicles displaying high densities of pneumococcal antigen at the surface offer protection against colonization. Vaccine. 2015;33(17):2022–2029. doi: 10.1016/j.vaccine.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 88.Kim JY, Doody AM, Chen DJ, Cremona GH, Shuler ML, Putnam D, et al. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380(1):51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, et al. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci USA. 2010;107(7):3099–3104. doi: 10.1073/pnas.0805532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang W, Wang S, Yao Y, Xia Y, Yang X, Li K, et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci Rep. 2016;6:37242. doi: 10.1038/srep37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Ley PA, Zariri A, van Riet E, Oosterhoff D, Kruiswijk CP. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front Immunol. 2021;12:781280. doi: 10.3389/fimmu.2021.781280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grandi A, Tomasi M, Ullah I, Bertelli C, Vanzo T, Accordini S, et al. Immunogenicity and pre-clinical efficacy of an OMV-based SARS-CoV-2 vaccine. Res Square. 2023 doi: 10.21203/rs.3.rs-2788726/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shehata MM, Mostafa A, Teubner L, Mahmoud SH, Kandeil A, Elshesheny R, et al. Bacterial outer membrane vesicles (OMVs)-based dual vaccine for Influenza A H1N1 virus and MERS-CoV. Vaccines. 2019 doi: 10.3390/vaccines7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA. 2012;109(12):E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alves NJ, Turner KB, Daniele MA, Oh E, Medintz IL, Walper SA. Bacterial nanobioreactors-directing enzyme packaging into bacterial outer membrane vesicles. ACS Appl Mater Interfaces. 2015;7(44):24963–24972. doi: 10.1021/acsami.5b08811. [DOI] [PubMed] [Google Scholar]
- 96.van den Berg van Saparoea HB, Houben D, Kuijl C, Luirink J, Jong WSP. Combining protein ligation systems to expand the functionality of semi-synthetic outer membrane vesicle nanoparticles. Front Microbiol. 2020;11:890. 10.3389/fmicb.2020.00890. [DOI] [PMC free article] [PubMed]
- 97.Jiang L, Driedonks TAP, Jong WSP, Dhakal S, Bart van den Berg van Saparoea H, Sitaras I, et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J Extracellular Vesicles. 2022;11(3):e12192. 10.1002/jev2.12192. [DOI] [PMC free article] [PubMed]
- 98.Weyant KB, Oloyede A, Pal S, Liao J, Jesus MR, Jaroentomeechai T, et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat Commun. 2023;14(1):464. doi: 10.1038/s41467-023-36101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Benedetto R, Alfini R, Carducci M, Aruta MG, Lanzilao L, Acquaviva A, et al. Novel simple conjugation chemistries for decoration of GMMA with heterologous antigens. Int J Mol Sci. 2021 doi: 10.3390/ijms221910180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Micoli F, Alfini R, Di Benedetto R, Necchi F, Schiavo F, Mancini F, et al. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccines. 2020 doi: 10.3390/vaccines8030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scaria PV, Rowe CG, Chen BB, Muratova OV, Fischer ER, Barnafo EK, et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines. 2019;4:24. doi: 10.1038/s41541-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci USA. 2006;103(48):18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu K, Palmieri E, Samnuan K, Ricchetti B, Oldrini D, McKay PF, et al. Generalized modules for membrane antigens (GMMA), an outer membrane vesicle-based vaccine platform, for efficient viral antigen delivery. J Extracellular Vesicles. 2022;11(11):e12247. doi: 10.1002/jev2.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Immunol. 1990;145(9):3071–3079. doi: 10.4049/jimmunol.145.9.3071. [DOI] [PubMed] [Google Scholar]
- 105.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172(4):2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 106.Adamo R, Feron C. Conjugated haemophilus influenzae vaccine using Bordetella outer membrane vesicle. Immunogenic conjugates. WIPO Patent Application WO2020043874. Application no. PCT/EP2019/073195 (2019).
- 107.Micoli F, Alfini R, Di Benedetto R, Necchi F, Schiavo F, Mancini F, et al. Generalized modules for membrane antigens as carrier for polysaccharides: impact of sugar length, density, and attachment site on the immune response elicited in animal models. Front Immunol. 2021;12:719315. doi: 10.3389/fimmu.2021.719315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raso MM, Gasperini G, Alfini R, Schiavo F, Aruta MG, Carducci M, et al. GMMA and glycoconjugate approaches compared in mice for the development of a vaccine against Shigella flexneri serotype 6. Vaccines. 2020 doi: 10.3390/vaccines8020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palmieri E, Kis Z, Ozanne J, Di Benedetto R, Ricchetti B, Massai L, et al. GMMA as an alternative carrier for a glycoconjugate vaccine against Group A Streptococcus. Vaccines. 2022 doi: 10.3390/vaccines10071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gasperini G, Alfini R, Arato V, Mancini F, Aruta MG, Kanvatirth P, et al. Salmonella paratyphi a outer membrane vesicles displaying vi polysaccharide as a multivalent vaccine against enteric fever. Infect Immunity. 2021 doi: 10.1128/iai.00699-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piccioli D, Alfini R, Monaci V, Arato V, Carducci M, Aruta MG, et al. Antigen presentation by follicular dendritic cells to cognate b cells is pivotal for generalised modules for membrane antigens (GMMA) immunogenicity. Vaccine. 2022;40(44):6305–6314. doi: 10.1016/j.vaccine.2022.09.034. [DOI] [PubMed] [Google Scholar]
- 112.Valguarnera E, Feldman MF. Glycoengineered outer membrane vesicles as a platform for vaccine development. Meth Enzymol. 2017;597:285–310. doi: 10.1016/bs.mie.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 113.Chen L, Valentine JL, Huang C-J, Endicott CE, Moeller TD, Rasmussen JA, et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies. Proceed Natl Acad Sci USA. 2016:E3609–E18. 10.1073/pnas.1518311113. [DOI] [PMC free article] [PubMed]
- 114.Price NL, Goyette-Desjardins G, Nothaft H, Valguarnera E, Szymanski CM, Segura M, et al. Glycoengineered outer membrane vesicles: a novel platform for bacterial vaccines. Sci Rep. 2016;6:24931. doi: 10.1038/srep24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stevenson TC, Cywes-Bentley C, Moeller TD, Weyant KB, Putnam D, Chang Y-F, et al. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc Natl Acad Sci USA. 2018;115(8):E3106–E3115. doi: 10.1073/pnas.1718341115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tian H, Li B, Xu T, Yu H, Chen J, Yu H, et al. Outer membrane vesicles derived from salmonella enterica serotype typhimurium can deliver Shigella flexneri 2a O-polysaccharide antigen to prevent Shigella flexneri 2a infection in mice. Appl Environ Microbiol. 2021;87(19):e0096821. doi: 10.1128/aem.00968-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.