Abstract
Background/Aims
Ultrasonography has a low sensitivity for detecting early-stage hepatocellular carcinoma (HCC) in cirrhotic patients. Non-contrast abbreviated magnetic resonance imaging (aMRI) demonstrated a comparable performance to that of magnetic resonance imaging without the risk of contrast media exposure and at a lower cost than that of full diagnostic MRI. We aimed to investigate the cost-effectiveness of non-contrast aMRI for HCC surveillance in cirrhotic patients, using ultrasonography with alpha-fetoprotein (AFP) as a reference.
Methods
Cost-utility analysis was performed using a Markov model in Thailand and the United States. Incremental cost-effectiveness ratios were calculated using the total costs and quality-adjusted life years (QALYs) gained in each strategy. Surveillance protocols were considered cost-effective based on a willingness-to-pay value of $4,665 (160,000 Thai Baht) in Thailand and $50,000 in the United States.
Results
aMRI was cost-effective in both countries with incremental cost-effectiveness ratios of $3,667/QALY in Thailand and $37,062/QALY in the United States. Patient-level microsimulations showed consistent findings that aMRI was cost-effective in both countries. By probabilistic sensitivity analysis, aMRI was found to be more cost-effective than combined ultrasonography and AFP with a probability of 0.77 in Thailand and 0.98 in the United States. By sensitivity analyses, annual HCC incidence was revealed as the most influential factor affecting cost-effectiveness. The cost-effectiveness of aMRI increased in settings with a higher HCC incidence. At a higher HCC incidence, aMRI would remain cost-effective at a higher aMRI-to-ultrasonography with AFP cost ratio.
Conclusions
Compared to ultrasonography with AFP, non-contrast aMRI is a cost-effective strategy for HCC surveillance and may be useful for such surveillance in cirrhotic patients, especially in those with high HCC risks.
Keywords: Cost-effectiveness analysis, Liver neoplasms, Chronic liver disease, Early detection of cancer
INTRODUCTION
Primary liver cancer is the 6th most common cancer and the 3rd leading cause of cancer-related death worldwide.1 The most common primary liver cancer is hepatocellular carcinoma (HCC), accounting for 75% to 85% of all cases. Because most patients with early HCC are asymptomatic, regular surveillance are critical for detection of early HCC, leading to more successful curative treatments with an expected survival of over 5 years, compared to only 2 to 2.5 years when detected at more advanced stages.2
Surveillance for HCC using combined ultrasonography with serum alpha-fetoprotein (AFP) testing every 6 months is recommended in high-risk individuals, including patients with cirrhosis, chronic hepatitis B infection, and chronic hepatitis with advanced liver fibrosis.3 Ultrasonography is less invasive and has lower costs than computed tomography and magnetic resonance imaging (MRI). However, the sensitivity of ultrasonography for detecting HCC dramatically decreased in cirrhotic patients with a sensitivity of only 0.21 to 0.47 for early-stage HCC, due to the irregularities and coarseness of liver parenchyma, hindering the visualization of small HCC nodules.4,5 MRI and computed tomography had a higher sensitivity of 0.81 to 0.86 and 0.65 to 0.68, with a relatively similar specificity of 0.91 to 0.92 for early HCC detection among cirrhotic patients.5,6 Nevertheless, both imaging modalities are not commonly used for large-scale HCC surveillance due to their high costs, the potential adverse effect on renal function and allergic reactions of contrast media as well as much longer acquisition time for full diagnostic MRI.
Abbreviated MRI (aMRI) is a new MRI protocol consisting of a limited number of sequences, thus, requiring shorter acquisition time than full diagnostic MRI.7 Due to a better sensitivity than ultrasonography for early HCC detection and a lower cost than full diagnostic MRI, several aMRI protocols have been investigated for HCC surveillance.8,9 aMRI is categorized into non-contrast and contrast-enhanced aMRI. Non-contrast aMRI protocols commonly include T2-weighted imaging and diffusion-weighted imaging and/or T1-weighted imaging. Contrast-enhanced aMRI protocols are divided into dynamic-aMRI protocol, and hepatobiliary phase-aMRI. Regarding early HCC detection (<2 cm), a recent meta-analyses reported a pooled sensitivity of 0.77 for non-contrast aMRI which was relatively similar to a sensitivity of 0.76 for full diagnostic MRI.10 Accordingly, aMRI has recently been proposed as an alternative method for HCC surveillance, especially in cirrhotic patients.8
Cost-effectiveness analyses have been conducted to determine optimal surveillance protocol in cirrhotic patients.11,12 A previous study assessed the cost-effectiveness of surveillance using contrast-enhanced aMRI in high and intermediate-risk cirrhotic patients, characterized by a biomarker-based HCC risk score. aMRI provided a lower incremental cost-effectiveness ratio (ICER) of $2,100 per quality-adjusted life-year (QALY) gained than the usual biannual ultrasound in all cirrhotic patients.12 Whether non-contrast aMRI is cost-effectiveness for HCC surveillance in cirrhotic patients remains uninvestigated. Furthermore, studies comparing the implementation of aMRI in different settings, such as high- and low-income countries, are warranted.
Due to the high performance, increasing availability, good safety profiles and relatively lower costs of non-contrast aMRI, we aimed to investigate the cost-effectiveness of using non-contrast aMRI as a surveillance tool for HCC detection in cirrhotic patients, compared to ultrasonography with AFP.
MATERIALS AND METHODS
The study was conducted based on the Consolidated Health Economic Evaluation Reporting Standards 2022.13 The study was approved by Chulalongkorn University’s Institutional Review Board Committee (IRB number: 806/63). Informed consent was waived due to the use of existing anonymous clinical data.
1. Overview of the Markov model
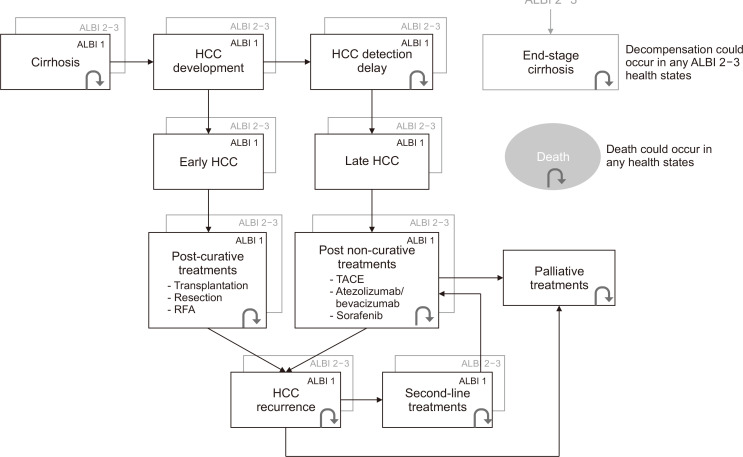
A cost-utility analysis was performed to compare the cost-effectiveness of biannual non-contrast aMRI, compared to the combination of ultrasonography with AFP which is the recommended surveillance strategy (Supplementary Fig. 1).3,14 A Markov model, representing the natural course of disease from cirrhosis to the HCC development and HCC treatments, was constructed using TreeAge Pro software (version 2022 R2; Williamstown, MA, USA). A surveillance starting age of 40 years old was selected for base-case analysis due to the substantially increased incidence of liver cancer in population aged above 40 years old.1,15 Especially in Asia and Oceania population, 10% to 12% of HCC patients had an age of disease onset of <50 years old.16 The starting age of 40 years old also coincided with the earliest age for surveillance in chronic hepatitis B patients stated in the recommendations by the American Association for the Study of Liver Diseases and the Asian Pacific Association for the Study of the Liver.3,17 An average annual HCC incidence in cirrhosis of 3% was used throughout the surveillance horizon in the base-case analysis. The 3% incidence represented the average of HCC incidences occurred in cirrhosis from common etiologies, including 3.6% in HBV-related cirrhosis,18,19 2% to 4% in HCV-related cirrhosis,20 3.8% in nonalcoholic fatty liver disease-related cirrhosis,21 and 2.9% in alcohol-related cirrhosis.22 Therefore, a simulated cohort of 100,000 cirrhotic patients aged 40 years with a 3% incidence rate of HCC was selected to undergo HCC surveillance with a cycle-length of 6 months, using ultrasonography plus AFP or aMRI, followed by confirmatory full diagnostic MRI to identify early-stage HCC (i.e., HCC within Milan criteria). Treatment options and outcomes were determined by HCC tumor stage and liver reserve (albumin-bilirubin [ALBI] grade). Early-stage HCC, defined by tumors within the Milan criteria, would undergo curative treatments, i.e., liver transplantation, surgical resection and local ablative therapy. Late-stage HCC would be limited to non-curative treatments, i.e., transarterial chemoembolization (TACE) and systemic treatment (atezolizumab plus bevacizumab or sorafenib). It was assumed that all patients diagnosed with HCC immediately received treatments within the same 6-month period. To emphasize on the importance of detecting early-stage HCC, cases of detected HCC and missed HCC were separated into different health states.23 False positive diagnoses of HCC after confirmatory full diagnostic MRI were transferred to a false positive health state, follow-up with full diagnostic MRI and transferred back to the corresponding cirrhotic states within the next cycle. Cirrhosis states were categorized by ALBI score into ALBI grade 1 (early-stage cirrhosis), ALBI grade 2-3 (late-stage cirrhosis), and end-stage cirrhosis. In all states, apart from the state-specific mortality, the patients could also die from cirrhosis-related causes or age-related mortality. The screening horizon was until all patients in the cohort transitioned to the death state. Hepatologists, radiologists and associated hospital personnel were consulted on the feasibility and verification of the model. The simplified Markov model is shown in Fig. 1.
Fig. 1.
Simplified Markov model. ALBI, albumin-bilirubin score; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
The model estimated the costs and QALYs throughout the surveillance and treatment process. We investigated the cost-effectiveness of each surveillance protocol in two separate settings: Thailand and the United States. Thailand, where most authors reside, was selected to represent a country with low medical costs, while the United States was selected as an example of a country with high medical costs and a higher proportion of liver transplantation.12,23,24 For Thailand setting, all costs were estimated in Thai Baht and converted to US dollars ($) with an exchange rate of 34.3 Thai Baht for $1 (September 2022). The interpretation of cost-effectiveness was based on a willingness-to-pay (WTP) of 160,000 Thai Baht/QALY ($4,665/QALY) according to the Thai Health Economic Working Group,25 and a WTP of $50,000 in the United States.26 An annual 3% discount rate was applied to each outcome in both settings.27,28
2. Input parameters
1) Transition probabilities and treatment proportions
Input parameters applied in the model and references are summarized in Tables 1, 2 and Supplementary Table 1. Transition probabilities including incidence, progression and mortality rates of HCC as well as proportions of patients receiving HCC stage-specific treatments were obtained from comprehensive literature review with preferable data sources from systematic review with meta-analysis and prospective studies. Mortality and recurrence rates of each treatment were stratified by pretreatment ALBI grade 1 and grade 2-3.
Table 1.
Costs of Surveillance Modalities and Treatments
| Parameter | Thailand value | U.S. value (range) | Reference |
|---|---|---|---|
| Surveillance costs, $ | |||
| USG with AFP | 40 | 161 (71–285) | HITAP/CGD, Ref29 |
| Contrast-enhanced MRI | 306 | 528 (264–1,056) | HITAP/CGD, Ref29 |
| Non-contrast aMRI | 102 | 233 (175–291) | HITAP/CGD, Ref30 |
| Treatment costs, $ | |||
| Liver transplantation | 26,160 | 49,906 | HITAP/CGD, Ref29 |
| Liver resection | 1,750 | 25,086 | HITAP/CGD, Ref23 |
| RFA | 1,308 | 18,386 | HITAP/CGD, Ref23 |
| TACE | 1,600 | 25,961 | HITAP/CGD, Ref23 |
| Radioembolization | 7,988 | 41,371 | HITAP/CGD, Ref31 |
| External radiation | 2,332 | 13,040 | HITAP/CGD, Ref31 |
| Sorafenib (/yr) | 25,567 | 34,644 | HITAP/CGD, Ref32 |
| Lenvatinib (/yr) | 22,572 | 61,942 | HITAP/CGD, Ref33 |
| Atezolizumab/bevacizumab (/cycle) | 3,114 | 13,065 | HITAP/CGD, Ref34 |
USG, ultrasonography; AFP, alpha-fetoprotein; MRI, magnetic resonance imaging; aMRI, abbreviated MRI; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; HITAP, Health Intervention and Technology Assessment Program; CGD, The Comptroller General’s Department.
Table 2.
Utilities of Health States, and Performance of Surveillance and Diagnostic Modalities
| Parameter | Value (range) | Reference |
|---|---|---|
| Annual utility | ||
| ALBI 1 cirrhosis | 0.80 (0.64–0.96) | Ref35,36 |
| ALBI 2-3 cirrhosis | 0.60 (0.48–0.72) | Ref35,36 |
| Early HCC in early cirrhosis | 0.72 (0.58–0.86) | Ref35 |
| Early HCC in late cirrhosis | 0.72 (0.46–0.68) | Ref35 |
| Late HCC in early cirrhosis | 0.40 (0.36–0.44) | Expert |
| Late HCC in late cirrhosis | 0.40 (0.36–0.44) | Expert |
| Post-transplantation | 0.85 (0.70–0.90) | Ref11,35,37 |
| Post-resection | 0.70 (0.40–0.90) | Ref11,37 |
| Post-RFA | 0.76 (0.57–0.95) | Ref37,38 |
| Post-TACE | 0.65 (0.52–0.77) | Ref37,39 |
| Post-radioembolization | 0.75 (0.56–0.94) | Expert, Ref31 |
| Post-external radiation | 0.68 (0.47–0.88) | Ref40 |
| Post-systemic | 0.76 (0.60–0.80) | Expert, Ref37,41 |
| Palliative treatment | 0.50 (0.45–0.55) | Expert |
| Sensitivity for the diagnosis of early HCC* | ||
| USG with AFP | 0.64 (0.45–0.79) | Supplementary Table 2 |
| aMRI | 0.81 (0.76–0.85) | Supplementary Table 3 |
| Full diagnostic MRI | 0.85 (0.67–0.95) | Ref42 |
| Sensitivity for the diagnosis of overall HCC | ||
| USG with AFP | 0.81 (0.54–0.94) | Supplementary Table 2 |
| aMRI | 0.84 (0.76–0.89) | Supplementary Table 3 |
| Full diagnostic MRI† | 0.90 (0.59–1.00) | Ref42 |
| Specificity for the diagnosis of overall HCC | ||
| USG with AFP | 0.84 (0.73–0.91) | Supplementary Table 2 |
| aMRI | 0.95 (0.89–0.98) | Supplementary Table 3 |
| Full diagnostic MRI | 0.97 (0.96–0.98) | Ref42 |
ALBI, albumin-bilirubin score; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; USG, ultrasonography; AFP, alpha-fetoprotein; MRI, magnetic resonance imaging; aMRI, abbreviated MRI.
*Value (95% CI); †Sensitivity for the diagnosis of late HCC.
Systematic reviews and meta-analyses were conducted as reference for the sensitivity and specificity of ultrasonography with AFP and aMRI for early and overall HCC detection. The selection criteria including studies and forest plots for each meta-analysis are shown in Supplementary Tables 2, 3 and Supplementary Figs 2, 3. The meta-analyses showed that ultrasonography with AFP and aMRI had a pooled sensitivity of 0.64 (95% confidence interval [CI], 0.45 to 0.79) and 0.81 (95% CI, 0.76 to 0.85) for early HCC detection as well as 0.81 (95% CI, 0.54 to 0.94) and 0.84 (95% CI, 0.76 to 0.89) for overall HCC detection, respectively. Pooled overall specificity of ultrasonography with AFP and aMRI were 0.84 (95% CI, 0.73 to 0.91) and 0.95 (95% CI, 0.89 to 0.98), respectively. Due to very limited studies reported the sensitivity of aMRI for the diagnosis of late HCC, pooled sensitivity for overall HCC detection was used as an alternative. Previous studies showed that the sensitivities full diagnostic MRI for early and late HCC detection were 0.85 (95% CI, 0.67 to 0.95) and 0.90 (95% CI, 0.59 to 1.00), respectively, The overall specificity of full diagnostic MRI were 0.97 (95% CI, 0.96 to 0.98).42 Treatment proportion for early and late HCCs in Thailand were obtained from a cohort in our center.43 Whereas, in the U.S. setting, the treatment proportions were derived from an average proportion from previous studies conducted in the U.S. population.44-47
2) Costs and utilities
Detailed costs and utilities with references are shown in Tables 1, 2 and Supplementary Table 1. Both direct medical costs (e.g., procedures, medication, hospitalization) and direct non-medical costs (e.g., travel and food costs for patients and caregivers) were included in the model. Costs were obtained from The Standard Cost List provided by Health Intervention and Technology Assessment Program48 and The Comptroller General’s Department49 in the Thailand setting, and Medicare50 as well as previous studies in the U.S. setting. Costs were adjusted for inflation by Consumer Price Index. Utility data were referenced from recommendations for cost-effectiveness analysis and previous economics studies.35 For utilities not available in previous literature, i.e., late HCC after stratification by ALBI grade and the utility of palliative treatment, experts in clinical hepatology were consulted on whether utility for similar states could be inferred.
3. Analysis
Base-case analysis was conducted using deterministic analysis with a cohort of 100,000 cirrhotic patients, aged 40 years. ICERs were calculated by dividing the difference in costs by the difference in QALYs between surveillance modalities. Patient-level microsimulations were performed with automatically updated variables as trackers to capture each patient’s disease history such as the number of transplantations, resections and TACEs, that the patient would undergo. To represent realistic situations, patients were limited to only one transplantation as well as no more than two resections or TACEs before proceeding other treatment lines.
One-way sensitivity analyses were conducted to determine the robustness of the models with the following input parameters: age of surveillance initiation, gender, annual HCC incidence, annual HCC incidence by different etiologies of cirrhosis, sensitivity of aMRI (upper and lower limits of range of the studies included in meta-analyses), costs of aMRI, HCC treatment options and proportions, and treatment-related mortality rates for resection and transplantation. Two-way sensitivity analysis for the annual HCC incidence rate and cost ratio of aMRI and ultrasonography with AFP was conducted to assess the relationship between these parameters to the cost-effectiveness results. The cost ratio was calculated by the cost of aMRI divided by the cost of ultrasonography with AFP.
To examine the effect of uncertainty of model inputs, probabilistic sensitivity analysis was conducted using second-order Monte Carlo simulation which repeatedly sampling input parameters from relevant distributions at random for 1,000 iterations. We used beta distribution for transition probabilities such as the performance of surveillance protocols and gamma distribution for costs. The results from the simulations were used to generate a cost-effectiveness acceptability curve.
RESULTS
1. Base-case analysis
In the Thailand setting, surveillance with ultrasonography and AFP every 6 months and aMRI gained 8.23 and 8.42 QALYs/person, respectively. The total costs of surveillance with ultrasonography plus AFP and aMRI per patient were $8,300 and $9,001, respectively. Using ultrasonography with AFP as reference, surveillance protocol using aMRI was cost-effective, providing an ICER of $3,667/QALY which is within the cost-effectiveness threshold of $4,665 in Thailand (Table 3). In the U.S. setting, HCC surveillance by combined ultrasonography with AFP and aMRI gained 8.12 and 8.29 QALYs/person, respectively. The costs were $83,565 and $89,799 for the combination of ultrasonography with AFP and aMRI. Compared to ultrasonography and AFP, aMRI provided an ICER of $37,062 which was within the cost-effectiveness threshold of $50,000/QALY (Table 3).
Table 3.
Deterministic Base-Case Cost-Utility Analysis and Patient-Level Microsimulations
| Strategy | USG+AFP (95% CI) | aMRI (95% CI) |
|---|---|---|
| Deterministic analysis | ||
| Thailand setting | ||
| QALY/person | 8.23 | 8.42 |
| Cost/person, $ | 8,300 | 9,001 |
| ICERs (USG with AFP as baseline) | 3,667 | |
| U.S. setting | ||
| QALY/person | 8.12 | 8.29 |
| Cost/person, $ | 83,565 | 89,799 |
| ICERs (USG with AFP as baseline) | 37,062 | |
| Patient-level microsimulations | ||
| Thailand setting | ||
| QALY/person | 8.14 (8.05–8.22) | 8.32 (8.23–8.41) |
| Cost/person, $ | 8,272 (7,979–8,565) | 8,874 (8,673–9,075) |
| ICER (USG with AFP as baseline) | 3,277 | |
| U.S. setting | ||
| QALY/person | 8.04 (7.95–8.12) | 8.20 (8.11–8.29) |
| Cost/person, $ | 82,610 (81,031–84,189) | 88,569 (86,865–90,274) |
| ICER (USG with AFP as baseline) | 36,742 | |
USG, ultrasonography; AFP, alpha-fetoprotein; CI, confidence interval; aMRI, abbreviated magnetic resonance imaging; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio.
2. Patient-level microsimulation
In the Thailand setting, HCC surveillance by ultrasonography with AFP and aMRI gained 8.14 (95% CI, 8.05 to 8.22) and 8.32 (95% CI, 8.23 to 8.41) QALY/person, respectively, with total costs of $8,272 (95% CI, 7,979 to 8,565) and $8,874 (95% CI, 8,673 to 9,075), respectively. aMRI was cost-effective, providing an ICER of $3,277/QALY (Table 3). In the U.S. setting, ultrasonography with AFP and aMRI provided total QALYs of 8.04 (95% CI, 7.95 to 8.12) and 8.20 (95% CI, 8.11 to 8.29), respectively, with total costs of $82,610 (95% CI, 81,041 to 84,189) and $88,569 (95% CI, 86,865 to 90,274), respectively. The ICER of aMRI was $36,742 which was within the cost-effectiveness threshold of $50,000/QALY (Table 3).
3. One-way sensitivity analyses
The results for one-way sensitivity analyses are shown in Table 4. Adjusting the age of surveillance initiation from 40 to 50 years or adjusting the compliance rates to surveillance program from 100% to 30% and 50% did not affect the cost-effectiveness of aMRI. By adjusting the annual HCC incidence, implementing surveillance in population with higher incidences resulted in lower ICERs. For example, in Thailand, aMRI surveillance in the female population with a lower HCC incidence of 2% provided an ICER of 5,085, which was slightly over the WTP threshold. Whereas aMRI surveillance in the male population resulted in a lower ICER of 3,398 due to a higher HCC incidence of 3.3%. In the U.S. population, aMRI was cost-effective for both male and female populations. Adding the treatment-related mortality rates for resection and transplantation of 2% to 3%51 did not affect the cost-effectiveness results in both Thailand and the U.S. settings (Supplementary Fig. 4).
Table 4.
One-Way Sensitivity Analysis
| Strategy | ICER for aMRI ($/QALY) | |
|---|---|---|
| Thailand | U.S. | |
| Age of initiating surveillance (annual HCC incidence) | ||
| 40 yr (base-case) | 3,667 | 37,062 |
| 50 yr (3%) | 4,182 | 36,765 |
| Sex (annual HCC incidence) | ||
| Male (3.3% in Thailand, 3.1% in the U.S.)* | 3,398 | 37,018 |
| Female (2.0% in Thailand, 1.9% in the U.S.)* | 5,085 | 37,803 |
| Annual HCC incidence | ||
| 1% | 9,159 | 39,464 |
| 2% | 5,085 | 37,706 |
| 2.5% | 4,245 | 37,328 |
| 3% (base-case) | 3,667 | 37,062 |
| 4% | 2,911 | 36,707 |
| 5% | 2,426 | 36,475 |
| Compliance to surveillance program | ||
| 30% | 3,332 | 34,672 |
| 50% | 3,502 | 36,192 |
| Etiology of cirrhosis (annual HCC incidence) | ||
| Hepatitis B cirrhosis (3.6%)19 | 3,169 | 36,829 |
| HCV cirrhosis (2.4%)19 | 4,386 | 37,392 |
| HCV-infected cirrhosis with SVR after DAA treatment (1.82%)53 | 5,494 | 37,888 |
| Alcoholic cirrhosis (2.9%)22 | 3,768 | 37,109 |
| NAFLD cirrhosis (3.78%)21 | 3,047 | 36,772 |
| Sensitivity of aMRI | ||
| Lower limit (early HCC 0.72, late HCC 0.77)7 | 4,020 | 39,497 |
| Upper limit (early HCC 0.91, late HCC 0.95)54 | 3,560 | 36,300 |
| Cost of aMRI, $ | ||
| Highest costs of aMRI which remain cost-effective ($112 in Thailand, $350 in the U.S.) | 4,627 | 49,816 |
| Treatment options and proportions for early HCC | ||
| 1% Transplantation, 15% resection, 22% RFA, 51% TACE, 11% BSC44 | 863 | 30,810 |
| 5% Transplantation, 14% resection, 38% RFA, 43% TACE23 | 1,813 | 31,702 |
| 3% Transplantation, 42% resection, 35% RFA, 19% TACE, 1% BSC55 | 2,740 | 26,714 |
| 12% Transplantation, 16% resection, 72% RFA24 | 4,129 | 30,328 |
| Alternative treatment options: transplantation, resection, RFA, TACE, radioembolization, radiation, sorafenib, lenvatinib, atezolizumab/bevacizumab and BSC† | 3,919 | 36,113 |
ICER, incremental cost-effectiveness ratio; aMRI, abbreviated magnetic resonance imaging; QALY, quality-adjusted life-year; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virological response; DAA, direct-acting antivirals; NAFLD, nonalcoholic fatty liver disease; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; BSC, best supportive care.
*HCC risk was calculated using the ADRESS-HCC risk model for patients aged 55 years, viral group etiology and Child-Pugh score of 5 in Thailand setting and patients aged 64 years, 27.3% viral group etiology and Child-Pugh score of 5 in the U.S. setting56; †Treatment proportions are shown in Supplementary Table 1.
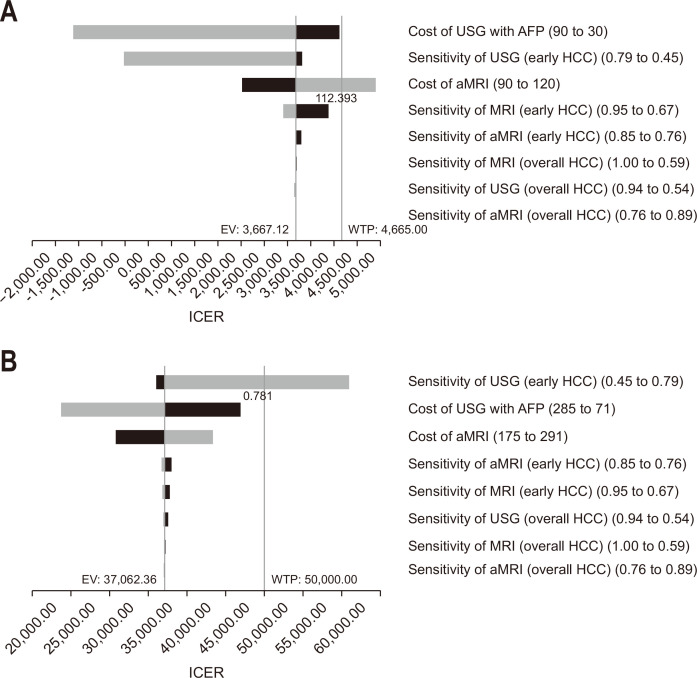
In Thailand, aMRI would not be cost-effective if the cost of aMRI was more than $112 (Fig. 2A). In the United States, if the sensitivity of ultrasonography with AFP for the detection of early HCC was above 0.781, the ICER would be $50,523/QALY which was above the $50,000/QALY threshold (Fig. 2B). Using the upper sensitivity margin of 0.79 for the detection of early HCC by ultrasonography with AFP resulted in an ICER of $60,931/QALY which was under the less conservative WTP threshold of $100,000/QALY, proposed in the United States.52 Adjusting other sensitivities and costs of surveillance protocols did not affect the cost-effectiveness of aMRI in both settings. Furthermore, aMRI surveillance protocol would remain cost-effective, until the costs of aMRI were over $112 and $350 which were higher than the referenced costs in Thailand and the United States, respectively. Sensitivity analysis according to alternative HCC treatment options, including radioembolization, external radiation and lenvatinib did not affect the cost-effectiveness of aMRI (Table 4).
Fig. 2.
Tornado diagram for Thailand setting (A) and the U.S. setting (B). USG, ultrasonography; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; aMRI, abbreviated MRI; EV, expected value; WTP, willingness-to-pay; ICER, incremental cost-effectiveness ratio.
4. Two-way sensitivity analysis
Two-way sensitivity analysis showed that an acceptable cost ratio of aMRI/ultrasonography with AFP, in which aMRI remained cost-effective, depended on the incidence of HCC (Supplementary Fig. 5). With a higher HCC incidence, aMRI would remain cost-effective at a higher aMRI/ultrasonography with AFP cost ratio. For example, with 3% annual HCC incidence, aMRI/ultrasonography with AFP cost ratio of 2.80 and 2.18 in Thailand and the U.S. setting would be the highest cost ratio in which aMRI remained cost-effective.
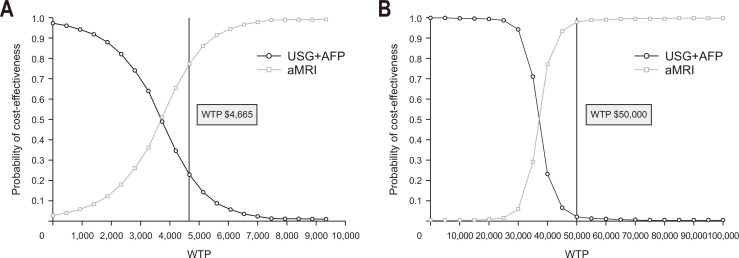
5. Probabilistic sensitivity analysis
The cost-effectiveness acceptability curve is shown in Fig. 3. aMRI had a probability of 0.77 for being the optimal surveillance strategy (superior or cost-effective, compared to ultrasonography with AFP) in Thailand and 0.98 in the United States. Scatter plots representing the repeated iterations of cost-effective analyses are shown in Supplementary Fig. 6.
Fig. 3.
Cost-effectiveness acceptability curve in Thailand setting (A) and in U.S. setting (B). USG, ultrasonography; AFP, alpha-fetoprotein; aMRI, abbreviated magnetic resonance imaging; WTP, willingness-to-pay.
DISCUSSION
This study demonstrated the cost-effectiveness of using non-contrast aMRI as a surveillance tool for early HCC detection in cirrhotic patients. When using the standard surveillance protocol of ultrasonography with AFP as a reference, all analyses, including base-case analysis, patient-level simulations and probabilistic sensitivity analysis consistently showed that aMRI was cost-effective in both low and high medical cost settings. These findings were likely due to the high performance of aMRI for the detection of early HCC.
Although the cost-effectiveness results in Thailand were in the same direction with the U.S. setting, some differences were observed. One-way sensitivity analysis showed that if the cost of aMRI was above $112, the ICER would exceed the WTP threshold in Thailand. However, adjusting the cost of aMRI in the U.S. setting provided ICER within the WTP threshold. This was because the costs of aMRI surveillance is closer to the WTP threshold in Thailand than in the United States. Moreover, in Thailand setting, aMRI surveillance in female population resulted in an ICER slightly above the WTP, which was due to the lower HCC incidence in female than male population. While aMRI remained cost-effective in both male and female populations in the U.S. setting. Probabilistic sensitivity analysis showed that aMRI had a higher cost-effectiveness probability of 0.98 in the U.S. setting, compared to 0.77 in Thailand. These differences between the two settings are most likely due to the larger gap between the WTP threshold and surveillance costs in the United States, compared to Thailand. As a result, adjusting the incidence of HCC or the sensitivity of surveillance protocols in the United States had a lesser impact on cost-effectiveness results than in Thailand. Therefore, cost-utility analysis using costs and WTP in each setting would help determine the most appropriate surveillance protocol before implementation.
Sensitivity analyses revealed that varying HCC annual incidence had the greatest impact on the cost-effectiveness of HCC surveillance. aMRI may not be a cost-effective option in populations with a lower HCC incidence such as viral hepatitis B carriers or non-cirrhotic chronic viral hepatitis B-infected patients who have well-controlled disease after long-term antiviral treatments.57 With a lower incidence, the number of patients with early-stage HCC who will eventually be diagnosed and undergo curative treatment was significantly decreased, thus, providing less benefit of early diagnosis and less QALYs gained.
One of the strengths of this study is the incorporation of ALBI score instead of Child-Pugh score into the Markov model to predict the patients’ prognoses based on pretreatment liver reserve. Recently, ALBI score has been shown to be more accurate than the Child-Pugh classification to predict the prognosis of HCC patients.58 Furthermore, the model was adjusted to account for each patient’s history using treatment trackers to limit the number of liver transplantations, resections and TACEs per patient. Altogether, the present work provides a realistic model that closely simulates cost-effective results similar to real-world practice.
There are some limitations. First, survival benefit of HCC surveillance with aMRI needs to be further investigated in prospective randomized controlled studies. Nonetheless, a previous randomized controlled trial has shown that HCC surveillance with biannual ultrasonography and AFP significantly reduced the mortality of HCC patients.59 Due to the higher sensitivity of aMRI for early HCC detection, it is likely that aMRI would increase the chance of receiving curative treatments, thus, improve patients’ survival. Notably, aMRI may not be able to replace ultrasonography with AFP entirely. The implementation of aMRI surveillance would require more expensive and less readily available equipment than ultrasonography. Instead, the study supports the use of aMRI, a high-yielded test, for HCC surveillance in high-risk population such as cirrhotic patients with high HCC incidence. In low-risk patients, the use of ultrasonography with AFP may not result in a significant decrease in HCC surveillance effectiveness and is still a reasonable option. To stratify individual patient’s HCC risk, a number of prediction models have been studied. For instance, PAGE-B score60 and a risk model which predicting high-risk population with annual HCC risk of >5%.61 Nevertheless, further studies on the cost-effectiveness of HCC surveillance with aMRI in high-risk cirrhotic patients are needed. Second, the reported performance of each surveillance protocol varied among previous studies, given that patient population and studied imaging protocols were different. To address this, we performed new meta-analyses using studies with specific characteristics such as population and imaging protocols to be used as references for the model. To determine whether various performance inputs of each surveillance protocols affected the cost-effectiveness results, we conducted sensitivity analyses using upper and lower limits of range of sensitivity of surveillance modalities. There were no significant changes in cost-effectiveness results in most scenarios, except for after adjusting the sensitivity of ultrasonography with AFP for the detection of early HCC in the U.S. setting. Albeit this adjustment still provided an ICER within the higher acceptable margin of WTP threshold of $100,000. Furthermore, age of surveillance initiation, treatment options and proportions, and treatment-related mortality rates may vary across different settings. However, sensitivity analyses showed that adjusting starting of 40 and 50 years, adding alternative treatment options, or adding treatment-related mortality rates for resection and transplantation did not affect the cost-effectiveness results. This reflected the robustness of the results. Additionally, TreeAge model with spreadsheet front-end will be available upon request. Input parameters of the model will be changed according to the data inputted in the spreadsheet file. Lastly, our model did not account individual patient’s comorbidity such as underlying diseases which might over-estimate overall utilities. However, our model already accounted for the risk of liver decompensation which is the major causes of morbidity and mortality in cirrhotic patients.
In conclusion, aMRI is a cost-effective HCC surveillance option in cirrhotic patients. aMRI would be useful for HCC surveillance, especially in cirrhotic patients with high HCC risks. Multicenter randomized controlled trials in different countries to validate the clinical benefits of aMRI as a surveillance tool are warranted.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl230089
ACKNOWLEDGEMENTS
This study is supported mainly by The Royal College of Physicians of Thailand (grant number: 03/2565). The data that support the findings of this study are available upon request from the corresponding author. We would like to express our gratitude to the Faculty of Medicine Chulalongkorn University Library for providing TreeAge Software License.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: R.C. Data acquisition: P.D., T.P. Data analysis and interpretation: P.D., W.P. Drafting of the manuscript: P.D. Critical revision of the manuscript for important intellectual content: R.C., R.R. Statistical analysis: P.D., W.P. Obtained funding: R.C. Administrative, technical, or material support: R.C., W.P., N.T. Study supervision: R.C., S.T., P.T. Approval of final manuscript: all authors.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut. 2020;69:168–176. doi: 10.1136/gutjnl-2018-318193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 4.Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154:1706–1718. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY) 2016;41:71–90. doi: 10.1007/s00261-015-0592-8. [DOI] [PubMed] [Google Scholar]
- 7.Park HJ, Jang HY, Kim SY, et al. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J Hepatol. 2020;72:718–724. doi: 10.1016/j.jhep.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol. 2021;75:108–119. doi: 10.1016/j.jhep.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan MV, Huo YR, Trieu N, et al. Noncontrast MRI for hepatocellular carcinoma detection: a systematic review and meta-analysis: a potential surveillance tool? Clin Gastroenterol Hepatol. 2022;20:44–56. doi: 10.1016/j.cgh.2021.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98:679–690. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 12.Goossens N, Singal AG, King LY, et al. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol. 2017;8:e101. doi: 10.1038/ctg.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25:3–9. doi: 10.1016/j.jval.2021.11.1351. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver, author. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute (NCI), author Surveillance, Epidemiology, and End Results (SEER) program [Internet] NCI; Bethesda: c2022. [cited 2023 March 5]. Available from: http://seer . [Google Scholar]
- 16.Yang JD, Altekruse SF, Nguyen MH, Gores GJ, Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017;123:81–89. doi: 10.1002/cncr.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Toh MR, Wong EY, Wong SH, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164:766–782. doi: 10.1053/j.gastro.2023.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20:283–292. doi: 10.1016/j.cgh.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Ganne-Carrié N, Chaffaut C, Bourcier V, et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274–1283. doi: 10.1016/j.jhep.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Kim HL, An J, Park JA, Park SH, Lim YS, Lee EK. Magnetic resonance imaging is cost-effective for hepatocellular carcinoma surveillance in high-risk patients with cirrhosis. Hepatology. 2019;69:1599–1613. doi: 10.1002/hep.30330. [DOI] [PubMed] [Google Scholar]
- 24.Cadier B, Bulsei J, Nahon P, et al. Early detection and curative treatment of hepatocellular carcinoma: a cost-effectiveness analysis in France and in the United States. Hepatology. 2017;65:1237–1248. doi: 10.1002/hep.28961. [DOI] [PubMed] [Google Scholar]
- 25.Teerawattananon Y, Tritasavit N, Suchonwanich N, Kingkaew P. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwes. 2014;108:397–404. doi: 10.1016/j.zefq.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 27.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [DOI] [Google Scholar]
- 28.Chaikledkaew U, Kittrongsiri K. Guidelines for health technology assessment in Thailand (second edition): the development process. J Med Assoc Thai. 2014;97 Suppl 5:S4–S9. [PubMed] [Google Scholar]
- 29.Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-effectiveness of hepatocellular carcinoma surveillance: an assessment of benefits and harms. Am J Gastroenterol. 2020;115:1642–1649. doi: 10.14309/ajg.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2017;42:179–190. doi: 10.1007/s00261-016-0841-5. [DOI] [PubMed] [Google Scholar]
- 31.Marqueen KE, Kim E, Ang C, Mazumdar M, Buckstein M, Ferket BS. Cost-effectiveness analysis of selective internal radiotherapy with yttrium-90 versus sorafenib in locally advanced hepatocellular carcinoma. JCO Oncol Pract. 2021;17:e266–e277. doi: 10.1200/OP.20.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiss KA, Yu S, Mamtani R, et al. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective, multi-institutional study. J Clin Oncol. 2017;35:3575–3581. doi: 10.1200/JCO.2017.73.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medicare.gov, author. Health & drug plans [Internet] U.S. Centers for Medicare and Medicaid Services; Baltimore: c2023. [cited 2023 Apr 20]. Available from: https://www.medicare.gov/ [Google Scholar]
- 34.Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4:e210037. doi: 10.1001/jamanetworkopen.2021.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.1996.03540160061034. [DOI] [PubMed] [Google Scholar]
- 36.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28:582–592. doi: 10.1177/0272989X08315240. [DOI] [PubMed] [Google Scholar]
- 37.Lima PH, Fan B, Bérubé J, et al. Cost-utility analysis of imaging for surveillance and diagnosis of hepatocellular carcinoma. AJR Am J Roentgenol. 2019;213:17–25. doi: 10.2214/AJR.18.20341. [DOI] [PubMed] [Google Scholar]
- 38.McKay A, Kutnikoff T, Taylor M. A cost-utility analysis of treatments for malignant liver tumours: a pilot project. HPB (Oxford) 2007;9:42–51. doi: 10.1080/13651820600994541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cucchetti A, Trevisani F, Cescon M, et al. Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012;56:1089–1096. doi: 10.1016/j.jhep.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Leung HW, Liu CF, Chan AL. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol. 2016;11:69. doi: 10.1186/s13014-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cammà C, Cabibbo G, Petta S, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054. doi: 10.1002/hep.26221. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, An J, Lim YS, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3:456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaiteerakij R, Chattieng P, Choi J, Pinchareon N, Thanapirom K, Geratikornsupuk N. Surveillance for hepatocellular carcinoma reduces mortality: an inverse probability of treatment weighted analysis. Ann Hepatol. 2017;16:421–429. doi: 10.5604/01.3001.0009.8597. [DOI] [PubMed] [Google Scholar]
- 44.Cabrera R, Singal AG, Colombo M, et al. A real-world observational cohort of patients with hepatocellular carcinoma: design and rationale for TARGET-HCC. Hepatol Commun. 2020;5:538–547. doi: 10.1002/hep4.1652.9b84ea20c906414cb172f617c7c11105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanwal F, Befeler A, Chari RS, et al. Potentially curative treatment in patients with hepatocellular cancer: results from the liver cancer research network. Aliment Pharmacol Ther. 2012;36:257–265. doi: 10.1111/j.1365-2036.2012.05174.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuo KL, Stenehjem D, Albright F, Ray S, Brixner D. Treatment patterns and outcomes in patients with hepatocellular carcinoma stratified by stage-guided treatment categories. J Natl Compr Canc Netw. 2015;13:987–994. doi: 10.6004/jnccn.2015.0119. [DOI] [PubMed] [Google Scholar]
- 47.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Health Intervention and Technology Assessment Program (HITAP), author The Standard Cost List for Health Technology Assessment [Internet] HITAP; Mueang: c2023. [cited 2023 Apr 20]. Available from: https://costingmenu.hitap.net . [Google Scholar]
- 49.Comptroller General's Department (CGD), author Medical Expense Database [Internet] CGD; Thailand: c2023. [cited 2023 Apr 20]. Available from: https://mbdb.cgd.go.th . [Google Scholar]
- 50.CMS.gov, author. Physician Fee Schedule [Internet] U.S. Centers for Medicare and Medicaid Services; Baltimore: c2023. [cited 2023 Apr 20]. Available from: https://www.cms.gov/medi . [Google Scholar]
- 51.European Association for the Study of the Liver, author; European Organisation for Research and Treatment of Cancer, author. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan RM, Bush JW. Health-related quality of life measurement for evaluation research and policy analysis. Health Psychol. 1982;1:61–80. doi: 10.1037/0278-6133.1.1.61. [DOI] [Google Scholar]
- 53.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Vandecaveye V, De Keyzer F, Verslype C, et al. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. Eur Radiol. 2009;19:2456–2466. doi: 10.1007/s00330-009-1431-5. [DOI] [PubMed] [Google Scholar]
- 55.Yen YH, Cheng YF, Wang JH, Lin CC, Chen CH, Wang CC. Adherence to the modified Barcelona Clinic Liver Cancer guidelines: results from a high-volume liver surgery center in East Asias. PLoS One. 2021;16:e0249194. doi: 10.1371/journal.pone.0249194.d944d40db9a247c0b100346b3d0c2cd3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen MH, Roberts LR, Engel-Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr Med Res Opin. 2022;38:2163–2173. doi: 10.1080/03007995.2022.2124070. [DOI] [PubMed] [Google Scholar]
- 57.Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 58.Demirtas CO, D'Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3:100347. doi: 10.1016/j.jhepr.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 60.Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Velázquez RF, Rodríguez M, Navascués CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.