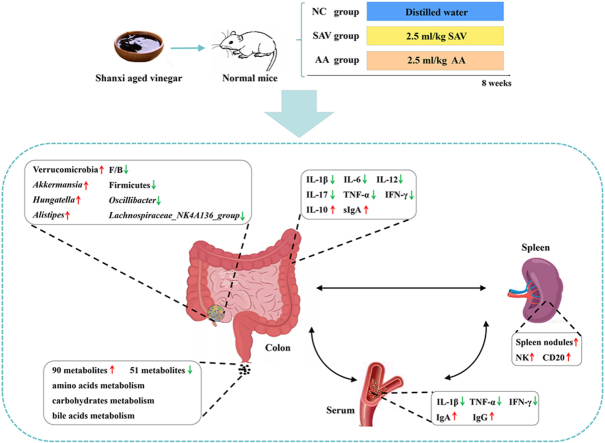
Abstract
Vinegar is used as fermented condiment and functional food worldwide. Vinegar contains many nutrients and bioactive components, which exhibits health benefits. In this study, the potential effects of Shanxi aged vinegar (SAV) on gut microbiome and metabolome were explored in normal mice. The levels of inflammatory factors were significantly decreased in SAV-treated mice. Immunoglobulin, NK cells and CD20 expression were significantly increased after SAV administration. In addition, SAV intake altered gut microbiota structure by up-regulating Verrucomicrobia, Akkermansia, Hungatella and Alistipes, and down-regulating Firmicutes, Lachnospiraceae_NK4A136_group and Oscillibacter. The differential metabolites were mainly included amino acids, carbohydrates and bile acids. Furthermore, after SAV intake, Verrucomicrobia, and Akkermansia closely impacted the related gut metabolites. These alterations of gut microbiota-related metabolism further modulated some immunoregulatory and inflammatory factors, and confer potential health benefits. Our results imply that vinegar consumption has beneficial effects on regulating gut microbiome and metabolome.
Keywords: Vinegar, Health benefits, Gut microbiome, Metabolome, Immune, Inflammation
Graphical abstract
Highlights
-
•
The levels of inflammatory factors were significantly decreased in Shanxi aged vinegar (SAV) -treated mice.
-
•
Shanxi aged vinegar (SAV) intake altered gut microbiota structure.
-
•
Research imply that vinegar consumption has beneficial effects by regulating gut microbiome and metabolome.
Abbreviations:
- AAPA
amino acids peptides and analogues
- AA
acetic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAAD
bile acids alcohols and derivatives
- CCC
carbohydrates and carbohydrate conjugates
- F/B
Firmicutes/Bacteroidetes
- GAE
gallic acid equivalents
- HDL-C
high-density lipoprotein-cholesterol
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IL-1β
interleukin-1β
- IL-2
interleukin-2
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-12
interleukin-12
- IL-17
interleukin-17
- INF-γ
interferon-gama
- LDL-C
low-density lipoprotein-cholesterol
- NK
natural killer
- OPLS-DA
orthogonal partial least squares-discriminant analysis
- OTUs
operational taxonomic units
- PCA
principal component analysis
- RE
rutin equivalents
- SAV
Shanxi aged vinegar
- sIgA
secretory immunoglobulin A
- TC
total cholesterol
- TG
triglyceride
- Th0
T helper 0
- TNF-α
tumor necrosis factor-alpha
- VIP
variable importance for projection
1. Introduction
Vinegar is a traditional product that has been fermented with history over 3000 years worldwide. Vinegar is not only a kind of acid seasoning, but also a functional food. Previous studies have found that vinegar can prevent diseases including obesity (Kondo et al., 2009), liver damage (Xia et al., 2020a), metabolic disorders (Ousaaid et al., 2020) and cancer (Baba et al., 2013). Vinegar contains many nutritional and bioactive compounds including amino acids, carbohydrates, polyphenols, and melanoidins, which play a beneficial role on body health (Xia et al., 2018a; Kandylis et al., 2021 & Ousaaid et al., 2022).
Gut microbiome play a critical role in human digestion, which can break down complex carbohydrates, proteins, and other components (Oliphant and Allen-Vercoe, 2019). Some dietary substances with high molecular weight are not absorbed in intestine. These substances interact with gut microbiota can alter intestinal microbiome composition and function, and then affect gut metabolism and health of the body (Scott et al., 2013; Cianciosi et al., 2020; Li et al., 2021; Zhang et al., 2021a). It has been reported that about 90–95% of dietary polyphenols with high molecular weight reach the colon in a primary form, which are degraded into low-molecular-weight metabolites by gut microbiota (Higbee et al., 2022; Catalkaya et al., 2020; Prasain and Barnes, 2020). Polyphenols from food including cocoa, tea, and fruits interplay with gut microbiota, which modulate gut microbiota composition and play an essential part in body physiology and metabolism (Etxeberria et al., 2013; Zhang et al., 2021b). Other studies found that melanoidins, as large molecules of human diet, are fermented by gut microbiota, and conversely promote the beneficial bacteria growth and subsequently result in the increase of short-chain fatty acids production (Pérez-Burillo et al., 2020). Moreover, dietary polyphenols and melanoidins can modify and determine gut microbiota composition and gene expression, and even alter the metabolic profile of gut microbiome (Ursell et al., 2014). In addition, it has been reported that non-digestible polysaccharides in dietary has the effects of combating obesity and diabetes by altering gut microbiota and its metabolites, regulating bile acid composition, inhibiting fat absorption and protecting of intestinal barrier (Zhang et al., 2021a; Lin et al., 2021).
Accumulating researches have proved that gut microbiome and metabolome play a considerable role in the bridge of dietary nutrients and body health (Yang et al., 2020; Wang et al., 2020). Recent studies demonstrate that some metabolites which are transformed by gut microbiota have effects on intestinal and immune homeostasis, energy-metabolism, and inflammation response (Ursell et al., 2014; Zhang et al., 2021c). The metabolites, as chemical messengers, regulate host immune response and physiological metabolism, play a bridge role between gut microbiota and body (Wang et al., 2019). There is a close and multiple relationship among gut microbiome, metabolome and body's health and homeostasis (Rooks and Garrett, 2016).
In vinegar, acetic acid is a major composition, which has the effects of anti-inflammation, anti-obesity, and blood lipid regulation (Beh et al., 2017). A study reported that vinegar and acetic acid prevented ulcerative colitis through suppressing T helper 1 and T helper 17 cell, and maintained gut immune homeostasis (Shen et al., 2016). In addition, vinegar contains some macromolecular bioactive substances such as combined polyphenols, melanoidins and polysaccharides (Xia et al., 2020b). Our previous study have reported that polyphenol-rich vinegar extract improves gut microbiota and immunity, and inhibits inflammation in alcohol-treated mice (Xia et al., 2020a). Li et al. found that melanoidins in vinegar powder exert anti-inflammatory by inhibiting the mRNA expression and the secretion of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in ethanol-induced macrophages (Li et al., 2021). Nevertheless, it remains unclear how bioactive compounds in vinegar interact with gut microbiome and metabolom and further affect the body health.
In this study, biochemical parameters, immune and inflammation factors were evaluated in normal mice after Shanxi aged vinegar (SAV) intake. The effects of SAV on gut microbiome and metabolome were investigated in vivo. Furthermore, the relationship among body indexes, gut microbiota, and the metabolites were explored in normal mice. These data may illustrate the mechanisms of SAV intake influencing normal host.
2. Materials and methods
2.1. Chemicals and reagents
ELISA kits were provided by eBioscience (San Diego, CA, USA). Detection kits of biochemical indexes were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Vinegar samples were chosen SAV products with 8 year-aging time (Shanxi, China). Synthetic vinegar samples were purchased from local supermarkets. The samples were diluted 4 times with sterile water before administration. The constituents of SAV with 8 year-aging time (SAV-8) have been determined in our previous study (Xia, et al., 2018a). Total phenols and flavonoids contents were 3.732 ± 0.329 mg gallic acid equivalents (GAE)/mL and 3.425 ± 0.510 mg rutin equivalents (RE)/mL, respectively. The content of amino acids in SAV-8 was 9144.77 ± 2553.17 μg/mL. The content of acetic acid in SAV-8 was 5.6 × 104 ± 5.6 × 103 μg/mL, which is the main component in vinegar and occupied 45.70 ± 1.35% to 64.00 ± 2.22% of total organic acids.
2.2. Animal experiments
Experiments on mice were executed following animal ethics. Male ICR mice (4-6 week-old, Beijing Vital River Laboratory Animal Technology Co., Ltd, China) were raised in pathogen-free environment provided by the Animal Ethics Committee of Nankai University (SYXK 2019-0001). The mice were randomly divided into three groups (n = 6–10) including normal control (NC) group, acetic acid (AA) group and SAV group. NC group were orally administrated with distilled water, AA group were treated with synthetic vinegar (2.5 mL/kg b. w.), and SAV group was given with SAV (2.5 mL/kg b. w.) by gavage for 8 weeks (Xia et al., 2018b). They were allowed free access to standard diet and water. In the end, the mice were anesthetized with sodium pentobarbital (50 mg/kg, i. p.) and euthanized by cervical dislocation. Fecal samples were obtained in the penultimate day and placed in −80 °C. In the end, spleen and intestine samples were immediately dissected and placed in −80 °C.
2.3. Biochemical analysis
These serum biochemical indexes levels (alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C)) were detected following the instructions of detection kits.
2.4. Enzyme-linked immunosorbent assay (ELISA)
Colon and spleen samples were grinded into homogenate. The supernatant in each sample was obtained after centrifugation (3500 rpm, 10 min, 4 °C). BCA protein assay kit (Nanjing Jiancheng) was used to measure total protein contents. Levels of IL-1β, interleukin-2 (IL-2), IL-6, interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-17 (IL-17), TNF-α, interferon-gama (INF-γ), immunoglobulin A (IgA), secretory immunoglobulin A (sIgA), immunoglobulin G (IgG) and natural killer (NK) cells in colon, spleen or serum were detected following the instructions by ELISA kits and ELISA reader (Tecan, Salzburg, Austria).
2.5. Histopathological analysis
The spleen was removed by laparotomy and fixed in neutral formaldehyde solution. The samples were embedded in paraffin wax and cut into 5 μm. Then the sections were stained with hematoxylin-eosin (H&E) and observed under a light microscope (Nikon, Tokyo, Japan).
2.6. Immunohistochemistry analysis
Immunohistochemistry was performed on the spleen to investigate expression changes of CD3 and CD20. The samples were embedded in paraffin after incubating in paraformaldehyde (4%) for 24 h, and then sliced into 5-μm sections. Then samples were stained with CD3 and CD20 antibodies, respectively (overnight, 4 °C). Secondary antibody was added to the tissues for 2 h in the next day. The samples were observed by light microscope to analyze CD3 and CD20 expressions. Morphometric analysis of immunohistochemical results was performed by ImageJ software (version 6.0).
2.7. Gut microbiota analysis
The cecal contents samples of each group were collected in sterilization tubes, and frozen in liquid nitrogen immediately. The total bacterial DNA of cecal contents was measured by E. Z.N.A.® Soil DNA kit (Omega Bio-tek, Norcross, GA, U.S.). DNA integrity were evaluated by 1% agarose gel electrophoresis. The purity was evaluated by NanoDrop (2000) (Thermo Scientific, Wilmington, USA). 16 S rRNA V3–V4 hypervariable regions from gut microbiota were amplified by PCR. These PCR amplicons were quantified and then sequenced on Illumina MiSeq platform (Illumina, San Diego, CA, USA). Sequenced reads and operational taxonomic units (OTUs) were bases of all the data.
2.8. Feces metabolomics
50 mg sample was put into 1.5 mL centrifuge tube, and 400 μL extracting solution was added. Then the sample was extracted by low temperature ultrasound. After centrifugation at 13,000 rpm for 15 min at 4 °C, the supernatant was removed and dried with nitrogen, and dissolved with 120 μL mixture. Then the samples were extracted with low-temperature ultrasound, and centrifuged at 13,000 rpm for 5 min at 4 °C. The supernatant was removed into an injection vial with internal tube for machine analysis. Quality control samples (QCs) were prepared by mixing aliquots of all the samples to make a pooled sample. QCs were injected at every 10 samples and provided a set in which repeatability could be assessed. Chromatographic separation was conducted on BEH C18 (100 mm × 2.1 mm, 1.8 μm) with injection 10 μL. The mobile phase was composed of (A) water (containing 0.1% formic acid) and (B) acetonitrile/Isopropanol (1/1) (containing 0.1% formic acid). Gradient parameters were as follows: 95-80% A and 5–20% B in 0–3 min, 80-5% A and 20–95% B in 3–9 min, 5% A and 95% B in 9–13 min, 5–95% A and 95-5% B in 13.0–13.1 min, and holding at 95% A and 5% B in 13.1–16 min. Flow rate 0.40 mL/min; column temperature 40 °C; mass range: 50–1000 m/z. Mass spectrometer operation parameters: ion spray voltage 5000 V (+) and 4000 V (−); spray gas 50 psi, auxiliary heating gas 50 psi, air curtain gas 30 psi; ion source heating temperature 500 °C; 20–60 V cyclic collision energy.
2.9. Metabolomics data analysis
After selected and normalized, the data were matched with the public databases Human Metabolome Database (HMDB) and Metlin to obtain metabolite information. The data was imported into Majorbio (https://cloud.majorbio.com) to conduct principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA). The selection of significant difference metabolites was according to the value of variable importance for projection (VIP) generated by OPLS-DA model, and p value generated by student's t-test. Differential metabolites (VIP >1 and p < 0.05) were chose to have further analysis by using software Origin (version 2021b) and Cytoscape (version 3.6.1).
2.10. Correlation analysis of network and heatmap
To explore the mechanisms of SAV in normal mice, the data was visualized by multiscale network and heatmap. Pearson's rank correlation was applied to display parameters relations. Only correlations with Pearson's correlation coefficient < −0.6 or >0.6 and p < 0.05 (generated by Origin, version 2021b) were selected for network visualization. Network and heatmap were generated by using Origin (version 2021b) and Cytoscape (version 3.6.1).
2.11. Statistical analysis
Species classification, richness diversity and microbial community functions prediction were finished on Omicsmart platform (https://www.omicsmart.com/). Independent t-test was applied to the analysis of statistical significance. All data were expressed as mean ± standard deviation (SD) (n = 6–10) using GraphPad prism (version 8.0.2). All the parameters were repeated for 3 times. The levels of p < 0.05 represent statistically significant difference. *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC group, n. s. indicates no significant difference.
3. Results and discussion
3.1. Effects of SAV on host parameters in normal mice
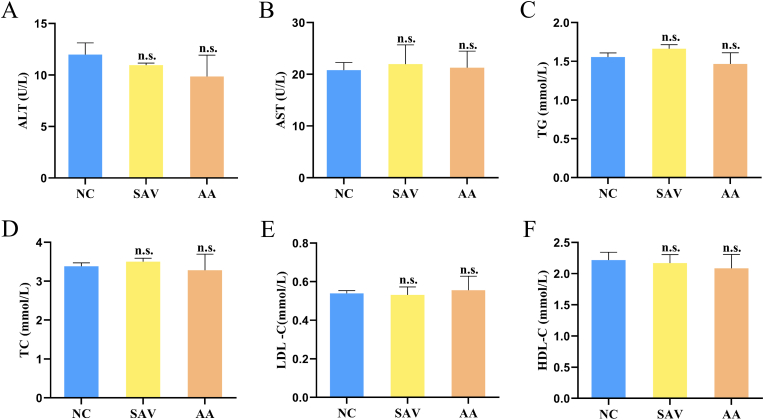
In this study, hepatic enzymes (ALT and AST) levels in serum had no obvious differences between SAV group and NC group, and between AA group and NC group (Fig. 1A and B). In addition, there was not significant alteration among blood lipids and lipoproteins (TG, TC, LDL-C and HDL-C) after AA and SAV intake (Fig. 1C–F). Beheshti et al. reported that vinegar consumption reduced TG, TC and LDL-C levels in hyperlipidemia patients, and had no significant influence on HDL-C level (Beheshti et al., 2013). Another study reported that schisandra vinegar significantly reduced serum TG, TC and LDL-C content, and increased HDL-C content in high-fat diet rats (Yuan et al., 2020). In present study, SAV has no effects on blood lipids in normal mice. AA was the main component of vinegar, and the content of AA in SAV aged 8 years old was about 5.6% (Xia et al., 2018a). Several studies has found that AA can reduce blood lipids levels in obese organism (Kondo et al., 2009; Yamashita, 2016). However, our results showed that AA had no effects on blood lipids in normal mice. Few studies reported that the effects of vinegar and AA on normal mice. The discrepant results between our study and other reports were mainly due to different animal models.
Fig. 1.
Host parameters in normal mice treated by SAV and AA. Hepatic enzymes ALT (A) and AST (B) levels. Blood lipids indexes TG (C), TC (D), LDL-C (E), and HDL-C (F) levels.
It is well known that T helper 0 cells (Th0) can secrete IL-12, which induce Th1 cells to generate TNF-α and IFN-γ. Th0 can also promote Th17 cells to produce IL-17. These factors (TNF-α, IFN-γ and IL-17) further enhance the secretion of inflammatory factors (Curciarello et al., 2019). In our study, pro-inflammatory factors including IL-1β, TNF-α, and IFN-γ levels in serum, and IL-1β, IL-6, IL-12, IL-17, TNF-α, and IFN-γ levels in colon were obviously decreased by SAV administration. However, AA treatment only significantly reduced the levels of IL-17 and INF-γ, with no significant impact on other indexes in this study (Table 1). Shen et al. reported that vinegar inhibited inflammation through suppressing Th1 and Th17 responses in mice with ulcerative colitis. Inflammatory factors (INF-γ, IL-17, IL-1β and TNF-α) in colon were significantly inhibited after vinegar (5% v/v) and acetic acid (0.3% v/v) treatment, which showed the same protective effect (Shen et al., 2016). The inhibition of inflammation was also observed in our study, and the effect of AA on inflammatory response was less than that of SAV. Above data suggest that SAV can regulate immune response, and lower inflammatory levels in normal mice.
Table 1.
Immune factors and immuneglobulins in mice.
| NC | SAV | AA | ||
|---|---|---|---|---|
| Serum | ||||
| IL-2 (pg/mL) | 24.86 ± 2.74 | 23.49 ± 2.99 | 23.04 ± 2.08 | |
| IL-1β (pg/mL) | 38.47 ± 2.25 | 33.66 ± 1.27* | 34.29 ± 2.80 | |
| IL-6 (pg/mL) | 60.48 ± 0.86 | 59.03 ± 1.14 | 59.86 ± 4.98 | |
| TNF-α (pg/mL) | 204.9 ± 13.82 | 173.3 ± 10.69* | 197.75 ± 20.64 | |
| IFN-γ (pg/mL) | 43.02 ± 4.36 | 32.79 ± 4.46* | 39.88 ± 2.29 | |
| IL-10 (pg/mL) | 200.20 ± 10.15 | 218.70 ± 17.69 | 198.34 ± 11.98 | |
| IgA (ug/mL) | 270.00 ± 3.52 | 288.70 ± 3.23** | 280.51 ± 13.04 | |
| IgG (mg/mL) | 15.57 ± 0.37 | 16.35 ± 0.18* | 15.53 ± 0.21 | |
| Colon | ||||
| IL-1β (pg/mg prot) | 19.00 ± 0.69 | 17.60 ± 0.16* | 16.82 ± 1.16 | |
| IL-6 (pg/mg prot) | 32.79 ± 0.58 | 30.60 ± 0.53** | 33.60 ± 2.42 | |
| IL-12 (pg/mg prot) | 21.49 ± 0.62 | 19.42 ± 0.61* | 20.89 ± 1.14 | |
| IL-17 (pg/mg prot) | 12.03 ± 0.19 | 10.49 ± 0.44** | 11.07 ± 0.21* | |
| TNF-α (pg/mg prot) | 123.10 ± 4.15 | 112.60 ± 3.02* | 114.29 ± 6.26 | |
| IFN-γ (pg/mg prot) | 20.51 ± 0.82 | 17.20 ± 0.77** | 16.33 ± 2.27* | |
| IL-10 (pg/mL prot) | 68.30 ± 2.75 | 76.13 ± 1.83* | 72.75 ± 2.67 | |
| sIgA (ug/mg prot) | 82.50 ± 0.18 | 95.43 ± 1.27*** | 98.62 ± 8.11 | |
3.2. Effects of SAV on spleen immunity in normal mice
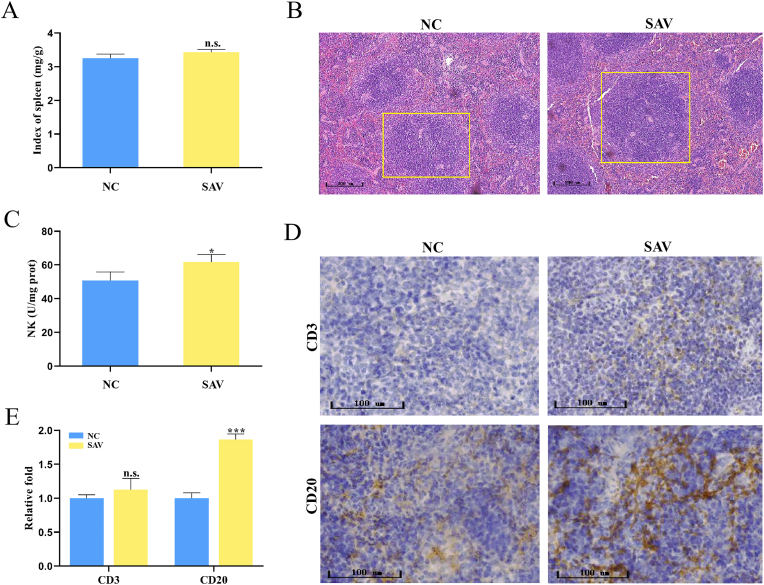
There is a central lymphoid tissue in the spleen. The increase of splenic nodule size is related to immune activation (Bo et al., 2016). As shown in Fig. 2A, there was no obvious difference among the spleen index between the two groups. However, the spleen nodules in the SAV group were obviously enlarged after SAV intake, indicating that SAV intake can activate lymphocytes and improve the immune responses in the spleen of normal mice (Fig. 2B). NK cells are essential for maintaining homeostasis and controlling immune response, which have a close correlation in promoting more effective Th1 cells immunity and regulating autoimmunity (Mikelez-Alonso et al., 2021). We found that the activity of NK cells was significantly increased after SAV administration (Fig. 2C), which contribute to maintain immune homeostasis in the spleen of normal mice. IL-2 is important for the proliferation of T cells and the generation of effector and memory cells (Mizui, 2019). We found that the levels of CD3 expression and IL-2 were not obvious changed in two groups (Table 1, Fig. 2D and E). Mohamad et al. reported that population of CD4+ and CD8+ T cells in the spleen of normal mice were increased after coconut juice vinegar consumption for 14 days, which was associated with higher levels of serum IL-2 and IFN-γ cytokines (Mohamad et al., 2018). In our study, there was no obvious alteration of T cells after SAV intake, this may be caused by the different raw materials, resulting in different active ingredients of the vinegars. The immunomodulatory activity of vinegar may be closely related to these bioactive compounds. The immunomodulatory activity of coconut juice vinegar may be partly due to its protein content (Mohamad et al., 2018), while grain vinegar was used in this study with low protein content. Meanwhile, the CD20 expression level were significantly added by SAV administration (Fig. 2D and E). CD20, as a B lymphocyte surface antigen, regulates the activation of B cells (Kuijpers et al., 2010). B lymphoid cells differentiate into plasmocytes, which secrete immunoglobulin and mediate immune response (LeBien and Tedder, 2008). This alteration was corresponded to the increase of IgA and IgG levels in serum (Table 1). Taken together, these results imply that SAV intake enhance immune function by improving the activities of NK cells and B lymphoid cells, and serum immunoglobulins levels.
Fig. 2.
The SAV effects on immune function in normal mice spleen. (A) The spleen indexes. (B) Spleen histopathological was observed by H&E staining with microscopy (100× magnification). (C) The activity of NK cells was measured by ELISA kit. (D) CD3 and CD20 levels were measured by immunohistochemical staining (200× magnification). (E) The relative folds of CD3 and CD20 expression by morphometric analysis.
3.3. Effects of SAV on gut microbiome composition and function in normal mice
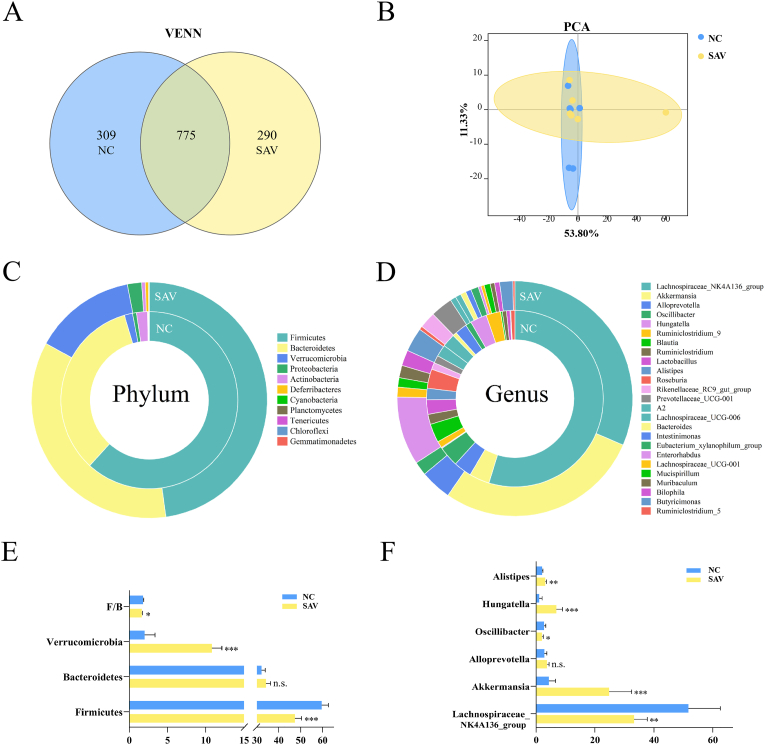
According to the sequence similarity of N97%, averages of 1163 and 1299 OTUs were identified in two groups (Table 2). A healthier individual has higher microbial richness. Good's coverage was applied to evaluate whether the sequencing method can be truly characterized gut microbiota composition (Rinninella et al., 2022). In present study, all the sequences in the two groups had nearly complete Good's coverage (Table 2). Eventhough the diversity had no clearly changes between the two groups, the richness of gut microbiota in SAV group was remarkably higher than that in NC group (Table 2), indicating the healthy status is well-being after SAV intake. In addition, Venn diagram showed that two groups had their own unique OTUs and shared OTUs (Fig. 3A), and PCA plot displayed that individuals in two groups were partly separated from their own group (Fig. 3B). These results suggest that gut microbiota profile is partly altered by SAV intake in healthy individuals.
Table 2.
Gut microbiota diversity in mice.
| Groups | NC | SAV | |
|---|---|---|---|
| OTUs | 1163 ± 77.90 | 1299 ± 72.99* | |
| Good's coverage | 0.9942 ± 0.0011 | 0.9934 ± 0.0025 | |
| Richness | Chao1 | 1548.00 ± 100.20 | 1776.00 ± 23.94*** |
| Ace | 1628.00 ± 112.00 | 1860.00 ± 57.96** | |
| Diversity | Shannon | 6.38 ± 0.41 | 6.56 ± 0.48 |
| Simpson | 0.96 ± 0.02 | 0.97 ± 0.02 | |
Fig. 3.
The SAV effects on gut microbiota composition and microbial community functions prediction. (A) Venn diagram. (B) The PCA plots. (C) Composition of microbial at phylum level. (D) Relative abundances of bacterial taxa at genus level. (E) Relative abundances of Firmicutes, Bacteroidetes, Verrucomicrobia, and the ratio of F/B. (F) Relative abundances of Lachnospiraceae_NK4A136_group, Akkermansia, Alloprevotella, Oscillibacter, Hungatella, Alistipes.
It is well known that Firmicutes, Bacteroidetes and Verrucomicrobia are major phyla in gut microbiota, and decrease in the abundance ratio of Firmicutes/Bacteroidetes (F/B) is associated with health benefits (Carmody et al., 2015). In our study, SAV intake significantly cut F/B ratio, which would be beneficial to health after SAV consumption (Fig. 3E, Table S1). In addition, the abundances of Verrucomicrobia Akkermansia, and Alistipes were remarkably higher after SAV intake (Fig. 3C–F, Tables S1 and S2). Akkermansia, a dominant genus of Verrucomicrobia, can increase the thickness of mucus and enhance the intestinal barrier function, which is negatively associated with inflammation and metabolic syndrome incidence (Dao et al., 2016). Alistipes have protective effects in liver fibrosis, colitis and cancer immunotherapy (Parker et al., 2020). Food and beverage products derived from fruit and vegetables are rich in polyphenols, which are closely related with health benefits. Kemperman et al. found that polyphenols extract from a red wine grape increased Akkermansia and Alistipes growth in vitro gut microbial ecosystem (Kemperman et al., 2013). In our study, SAV contains various polyphenols, and the increase of Verrucomicrobia and Akkermansia may promote gut barrier and decrease host inflammation. On the contrary, the abundances of Firmicutes, Lachnospiraceae_NK4A136_group and Oscillibacter displayed a significant decline after SAV intake (Fig. 3E and F). The genera of Lachnospiraceae_NK4A136_group and Oscillibacter belong to phylum of Firmicutes. Increased abundance of Lachnospiraceae_NK4A136_group and Oscillibacter were closely associated with ulcerative colitis, gastritis and obesity (Zhou et al., 2022). Oscillibacter promotes intestinal permeability, and generation of inflammatory factors (IL-6 and IL-1β) (Wu et al., 2019). Generally, our data demonstrate that dietary SAV can alter gut microbiota structure and composition, which may exhibit benefit for host health.
3.4. Effects of SAV on metabolome in the feces of normal mice
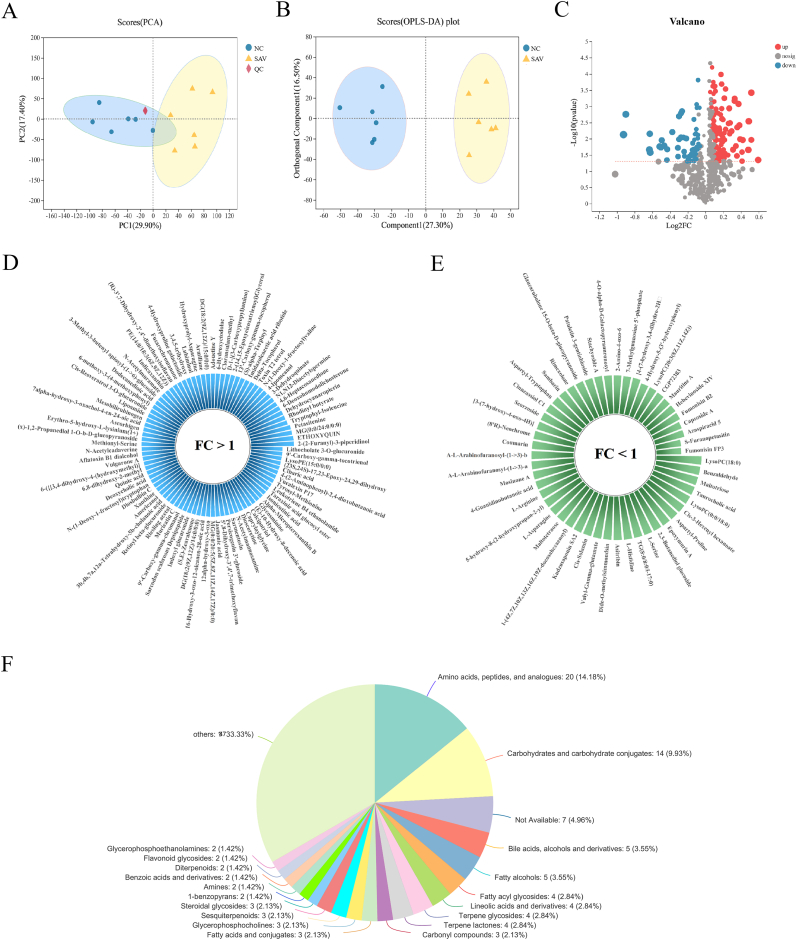
To explore the effects of SAV on metabolic profile, LC-MS was applied to detect fecal metabolome. The results showed that PCA plots displayed a clear separation of two groups in positive mode (Fig. 4A) and negative mode (Fig. S1A). In addition, OPLS-DA analysis was used to estimate metabolic fingerprints, which presented an excellent separation of two groups in positive and negative modes (Fig. 4B and Fig. S1B), indicating that the established method had good stability and repeatability. Then, VIP value and p value were applied to be the screening criteria for identifying differential metabolites. 141 differential metabolites between NC and SAV group were shown in volcano plot in positive and negative modes (Fig. 4C). 90 of these 141 differential metabolites were up-regulated, and 51 of them were down-regulated in the SAV-treated group compared with NC group (Fig. 4D and E). These results suggest SAV consumption also alter gut metabolome in normal mice. The classification of these metabolites was analyzed based on HMDB database (Table S3). In the classification pie-chart, the differential metabolites between the two groups mainly included 20 amino acids, peptides, and analogues (AAPA) (14.18%), 14 carbohydrates and carbohydrate conjugates (CCC) (9.93%) and 5 bile acids, alcohols and derivatives (BAAD) (3.55%) (Fig. 4F). Gut metabolites generated by gut microbiota is important in maintaining health and disease development (Wang et al., 2019). Johnston et al. found that daily vinegar ingestion for 4 weeks significantly increased 3 amino acid metabolites, including sarcosine, piperic acid and acetylcysteine, 3 fatty acid metabolites (3-adipic acid, lauric acid and caproic acid), and 2 carbohydrate metabolites in healthy adults (Johnston et al., 2021). The alteration of amino acid metabolites was associated with mood improvement. We also found amino acids metabolites were the main metabolite alterations, which might predict the mood benefit after SAV intake.
Fig. 4.
The SAV effects on fecal metabolism in normal mice. (A) PCA plot in positive mode. (B) The OPLS-DA scores plot in positive mode. (C) Volcano plot of variables between NC group and SAV group. (D) Metabolites of significant differences were up-regulated in SAV group. Fold change (FC) > 1 means up-regulation of metabolites. (E) Metabolites of significant differences were down-regulated in SAV group. FC < 1 means down-regulation of metabolites. (F) Metabolites of significant differences between two groups were classified according to HMDB classification.
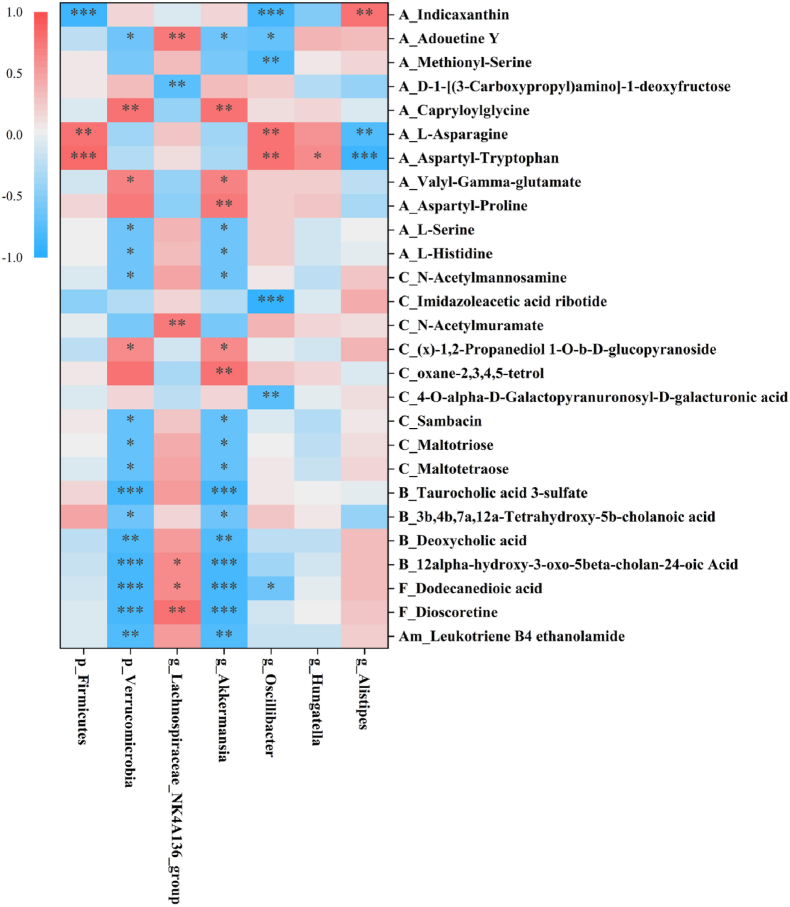
3.5. Correlation between gut microbiome and metabolome after dietary vinegar
To further illuminate the relationship between gut microbiome and differential metabolome after dietary vinegar, the correlation analysis was conducted by Pearson correlation analysis. Differential metabolites associated with gut microbiota were selected and displayed in heatmap, including 11 AAPA, 8 CCC, 3 BAAD, and others (Fig. 5). It is reported that Firmicutes can be significantly increased by using amino acids from dietary food, and promotes the occurrence of intestinal inflammatory reaction (Zeng et al., 2022; Lin et al., 2023). Tanes reported that Firmicutes play an important role in amino acid metabolites, which that may modify host health (Tanes et al., 2021).
Fig. 5.
Correlation analysis between differential gut microbiota and metabolites. Classification of metabolites were described at the abbreviation in metabolite name (A: AAPA, B: BAAD, C: CCC, F: fatty acids and conjugates, Am: amines).
In our study, Firmicutes and Oscillibacter were mainly correlated with AAPA (indicaxanthin, L-asparagine, and aspartyl-tryptophan). The genus of Oscillibacter belongs to phylum of Firmicutes, these bacteria were decreased after SAV consumption, and influenced amino acids metabolites and inhibited inflammatory reaction. We also found that Lachnospiraceae_NK4A136_group were closely associated with AAPA and fatty acids and conjugates. Akkermansia is the representative of Verrucomicrobia phylum, which consumes amino acids for protein synthesis (Sharon et al., 2019). Kordy et al. found that Akkermania abundance was decreased, which induced the increase of gene expression of amino acid metabolism in the liver disease (Kordy et al., 2021). In our study, Verrucomicrobia and Akkermansia presented obviously association with the fecal metabolites including 6 AAPA, 6 CCC and 4 BAAD and 2 fatty acids and conjugates. Tu et al. found that a black raspberry-rich diet selectively enhanced Akkermania population in normal C57BL/6 J mice, which impact aromatic amino acid metabolism and carbohydrate metabolism (Tu et al., 2018). Another study reported that Akkermansia administration showed correlation with bile acids metabolism, which alleviated obesity and non-alcoholic fatty liver disease in mice (Juárez-Fernández, et al., 2021). Generally, our data indicated that SAV intake may improve host health via gut microbiome-related metabolism.
3.6. Multi-dimensional network and heatmap analysis of SAV
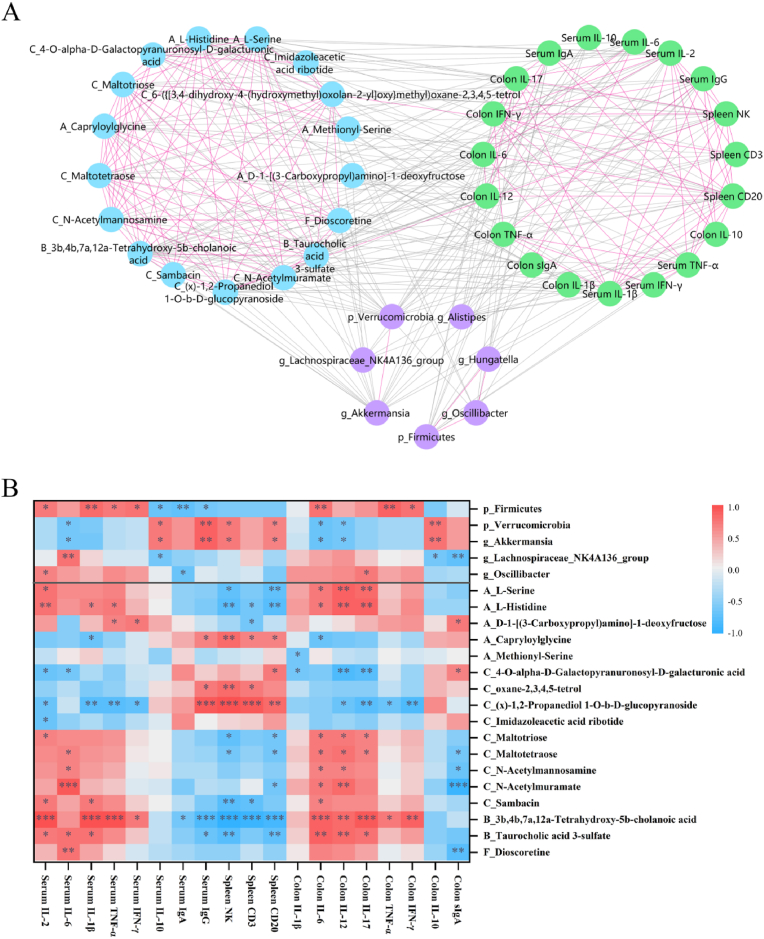
In present study, the metabolites were chosen from AAPA, CCC, BAAD, and fatty acids and conjugates with false discovery rate <0.05. As shown in Fig. 6A, 19 host indexes, 17 metabolites, and 7 taxa in gut microbiota were integrated into the network. The nodes in host indexes (green circle), gut microbiota layer (purple circle), and fecal metabolism layer (blue circle) were closely connected with each other. Then, the relationship between gut microbiota, metabolites and host indexes was presented in heatmaps (Fig. 6B). 3 differential gut microbiota (Firmicutes, Verrucomicrobia, and Akkermansia), 6 differential metabolites (Maltotetraose, L-Histidine, 4-O-alpha-D-Galactopyranuronosyl-D-galacturonic acid, (x)-1,2-Propanediol 1-O-b-D-glucopyranoside, Taurocholic acid 3-sulfate, 3 b,4 b,7a, 12a-Tetrahydroxy-5b-cholanoic acid) were more correlated with host indexes.
Fig. 6.
Correlations among gut microbiota, metabolites and indexes by Pearson correlation analysis. (A) Multi-dimensional network analysis among gut microbiota, fecal metabolites and immune factors. The edges are showed when Pearson's correlation coefficient < −0.6 or >0.6 and p < 0.05. Edges in the same classification layer are pink color, and the edges in different classification layers are black color. (B) Heatmap of gut microbiota and metabolites, and indexes. Classification of metabolites were described at the abbreviation in metabolite name (A: AAPA, B: BAAD, C: CCC, F: fatty acids and conjugates). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Emerging data proved that gut metabolome such as amino acid metabolites, short-chain fatty acids and bile acids played a bridge role between gut microbiome and host physiological function (Ursell et al., 2014). L-histidine is an amino acid derived from dairy food, which can be obtained through protein hydrolysis. Stražar et al. found that Akkermansia and Bifidobacterium mediated histidine metabolism, and affected cytokines (IL-6, TNF-α, IL-1β, and IFN-γ) as immunomodulatory pathways (Stražar et al., 2021). In our study, L-histidine was closely correlated with Verrucomicrobia, and Akkermansia (Fig. 5), and essentially influenced serum IL-2, IL-1β, and IFN-γ. These factors as potential pathways to further modulate inflammation and immune.
Gut microbiota plays a key part in regulating host energy-balance and immune response by participating in carbohydrate metabolism (Wang et al., 2019). It has been reported Akkermania participated in amino acid metabolism and carbohydrate metabolism, which conferred health benefits (Tu et al., 2018). After SAV intake, we discovered carbohydrate metabolites were closely related with Verrucomicrobia, and Akkermansia (Fig. 5), and affected influenced serum IL-2, IL-6, spleen NK cells and CD20, colon IL-12 and IL-17. These data indicated that gut bacterial especially Verrucomicrobia, and Akkermansia altered carbohydrate metabolism, and then involved in immune homeostasis by modulating immune cells and factors. Juárez-Fernández et al. reported that Akkermansia was linked to bile acids metabolism, and caused with the amelioration of liver steatosis and inflammation in mice with obesity and non-alcoholic fatty liver disease (Juárez-Fernández et al., 2021). 3 b,4 b,7a, 12a-Tetrahydroxy-5b-cholanoic acid is known as a kind of polyhydroxylated bile acids, can reduced inflammatory and fibrotic genes, and protected from cholestatic liver in mice (Ling et al., 2012). We found that bile acid metabolism was mainly affected by Verrucomicrobia, and Akkermansia (Fig. 5), and exhibited significantly associated with inflammatory and immune response. Collectively, the results revealed that SAV consumption caused remarkable upregulation of Verrucomicrobia, and Akkermansia and had an impact on the metabolisms of amino acids, carbohydrates, and bile acids, and further affected the host anti-inflammation and immunoregulation process, which exert beneficial health effects.
4. Conclusion
This study illustrated the effects of SAV intake on host indexes, gut microbiome, and fecal metabolome of normal mice. SAV administration increased immunoglobulin and the splenic immunity, and inhibit serum and intestine inflammation. In addition, SAV intake made the structure of gut microbiota tend to a healthy state. SAV intake can regulated intestinal metabolites including AAPA, CCC, and BAAD. SAV was able to maintain body health involved in the interaction among physiological parameters, gut microbiota, and metabolites. Furthermore, the gut metabolism changes of amino acids, carbohydrates and bile acids significantly affected by Verrucomicrobia, and Akkermansia, which were association with some immunoregulation factors and inflammatory indexes. These findings provide crucial evidence for beneficial effects of SAV, and encourage SAV further applications as a functional food for human health.
Funding
This work was supported by the Foundation of Shanxi Provincial Key Laboratory for Vinegar Fermentation Science and Engineering, P.R. China (No. 20220401931002); the Innovation fund of Haihe Laboratory of Synthetic Biology, P.R. China; the Key Research and Development Program of Ningxia (No. 2022BBF02010), P.R. China.
Ethics statement
We ensured that all the animal procedures were performed in compliance with relevant laws and the guidelines of the institutional animal ethics committee. Animal experiments were approved by the animal ethics committees of Nankai University (SYXK, 2019–0001, permission date: Jan 11, 2019).
CRediT authorship contribution statement
Ting Xia: conducted research and drafted the manuscript. Chaoyan Kang: was responsible for elaboration of main data and figures. Xiao Qiang: designed the proposal and edited the paper. Xiaodong Zhang: designed the proposal and edited the paper. Shaopeng Li: did some experiments and data analysis work. Kai Liang: did some data analysis work and submitted amendments to the manuscript. Yiming Wang: did some experiments and data analysis work. Jianxin Wang: submitted amendments to the manuscript. Hui Cao: provided some suggestions and approved the final version of manuscript. Min Wang: provided some suggestions and approved the final version of manuscript, All authors read and approved the final version of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Genedenovo Biotechnology Co. Ltd for excellent technical assistance of gut microbiota analysis, and Shanghai Majorbio Bio-pharm Technology Co. Ltd for excellent technical assistance of metabolites analysis.
Handling Editor: Dr. Quancai Sun
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100566.
Contributor Information
Ting Xia, Email: xiating@tust.edu.cn.
Chaoyan Kang, Email: chaoyan_kang@163.com.
Xiao Qiang, Email: qiangxiao0907@163.com.
Xiaodong Zhang, Email: Zhangxd915@outlook.com.
Shaopeng Li, Email: li17627820568@163.com.
Kai Liang, Email: jszx.lk@zlcy.com.
Yiming Wang, Email: frszzfy@163.com.
Jianxin Wang, Email: tkxiaoxin@gmail.com.
Hui Cao, Email: hui_cao0830@yahoo.com.
Min Wang, Email: minw@tust.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- Baba N., Higashi Y., Kanekura T. Japanese black vinegar “Izumi” inhibits the proliferation of human squamous cell carcinoma cells via necroptosis. Nutr. Cancer. 2013;65:1093–1097. doi: 10.1080/01635581.2013.815234. [DOI] [PubMed] [Google Scholar]
- Beh B.K., Mohamad N.E., Yeap S.K., Ky H., Boo S.Y., Chua J., Tan S.W., Ho W.Y., Sharifuddin S.A., Long K., Alitheen N.B. Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Sci. Rep. 2017;7(1):6664. doi: 10.1038/s41598-017-06235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheshti Z., Chan Y.H., Nia H.S., Hajihosseini F., Nazari R., Shaabani M., Taghi M., Omran S., Huak Y. Influence of apple cider vinegar on blood lipids. Life Sci. J. 2013;9:2431–2440. http://www.lifesciencesite.com [Google Scholar]
- Bo R., Zheng S., Xing J., Luo L., Niu Y., Huang Y., Liu Z., Hu Y., Liu J., Wu Y., Wang D. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016;85:294–301. doi: 10.1016/j.ijbiomac.2015.12.089. [DOI] [PubMed] [Google Scholar]
- Carmody R.N., Gerber G.K., Luevano J.M., Jr Gatti D.M., Somes L., Svenson K.L., Turnbaugh P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalkaya G., Venema K., Lucini L., Rocchetti G., Delmas D., Daglia M., De Filippis A., Xiao H., Quiles J.L., Xiao J., Capanoglu E. Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers. 2020;1:109–133. doi: 10.1002/fft2.25. [DOI] [Google Scholar]
- Cianciosi D., Forbes-Hernández T.Y., Giampieri F., Zhang J., Ansary J., Pacetti M., Quiles J.L., Simal-Gandara J., Battino M. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds and antioxidant activity of manuka honey. eFood. 2020;1(1):85–93. doi: 10.2991/efood.k.191011.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curciarello R., Canziani K.E., Docena G.H., Muglia C.I. Contribution of non-immune cells to activation and modulation of the intestinal inflammation. Front. Immunol. 2019;10:647. doi: 10.3389/fimmu.2019.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M.C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E.O., Kayser B.D., Levenez F., Chilloux J., Hoyles L., Micro-Obes Consortium Dumas M.E., Rizkalla S.W., Doré J., Cani P.D., Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- Etxeberria U., Fernández-Quintela A., Milagro F.I., Aguirre L., Martínez J.A., Portillo M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013;61(40):9517–9533. doi: 10.1021/jf402506c. [DOI] [PubMed] [Google Scholar]
- Higbee J., Solverson P., Zhu M., Carbonero F. The emerging role of dark berry polyphenols in human health and nutrition. Food Frontiers. 2022;3:327. doi: 10.1002/fft2.128. [DOI] [Google Scholar]
- Johnston C.S., Jasbi P., Jin Y., Bauer S., Williams S., Fessler S.N., Gu H. Daily vinegar ingestion improves depression scores and alters the metabolome in healthy adults: a randomized controlled trial. Nutrients. 2021;13(11):4020. doi: 10.3390/nu13114020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Fernández M., Porras D., Petrov P., Román-Sagüillo S., García-Mediavilla M.V., Soluyanova P., Martínez-Flórez S., González-Gallego J., Nistal E., Jover R., Sánchez-Campos S. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants. 2021;10(12):2001. doi: 10.3390/antiox10122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandylis P., Bekatorou A., Dimitrellou D., Plioni I., Giannopoulou K. Health promoting properties of cereal vinegars. Foods. 2021;10(2):344. doi: 10.3390/foods10020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemperman R.A., Gross G., Mondot S., Possemiers S., Marzorati M., Wiele T.V.D., Doré J., Vaughan E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013;53(2):659–669. doi: 10.1016/j.foodres.2013.01.034. [DOI] [Google Scholar]
- Kondo T., Kishi M., Fushimi T., Ugajin S., Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci. Biotechnol. Biochem. 2009;73:1837–1843. doi: 10.1271/bbb.90231. [DOI] [PubMed] [Google Scholar]
- Kordy K., Li F., Lee D.J., Kinchen J.M., Jew M.H., La Rocque M.E., Zabih S., Saavedra M., Woodward C., Cunningham N.J., Tobin N.H., Aldrovandi G.M. Metabolomic predictors of non-alcoholic steatohepatitis and advanced fibrosis in children. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.713234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T.W., Bende R.J., Baars P.A., Grummels A., Derks I.A., Dolman K.M., Beaumont T., Tedder T.F., van Noesel C.J., Eldering E., van Lier R.A. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J. Clin. Investig. 2010;120(1):214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Zhang J., Li S., Zheng B., Hu J. Polysaccharides isolated from Laminaria japonica attenuates gestational diabetes mellitus by regulating the gut microbiota in mice. Food Frontiers. 2021;2:208–217. doi: 10.1002/fft2.79. [DOI] [Google Scholar]
- Lin L., Lai Z., Yang H., Zhang J., Qi W., Xie F., Mao S. Genome-centric investigation of bile acid metabolizing microbiota of dairy cows and associated diet-induced functional implications. ISME J. 2023;17(1):172–184. doi: 10.1038/s41396-022-01333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, V., Wang, R., Sheps, J.A., 2012. Polyhydroxylated Bile Acids for Treatment of Biliary Disorders. US Pat., 20120277198.
- Li Y., Jia X., Tang N., Tao H., Xia R., Cheng Y. Melanoidins, extracted from Chinese traditional vinegar powder, inhibit alcohol-induced inflammation and oxidative stress in macrophages via activation of SIRT1 and SIRT3. Food Funct. 2021;12(17):8120–8129. doi: 10.1039/d1fo00978h. [DOI] [PubMed] [Google Scholar]
- Mikelez-Alonso I., Magadán S., González-Fernández Á., Borrego F. Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: a look into how nanoparticles enhance NK cell activity. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113860. [DOI] [PubMed] [Google Scholar]
- Mizui M. Natural and modified IL-2 for the treatment of cancer and autoimmune diseases. Clinical Immunology (Orlando, Fla. 2019;206:63–70. doi: 10.1016/j.clim.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Mohamad N.E., Keong Yeap S., Beh B.K., Romli M.F., Yusof H.M., Kristeen-Teo Y.W., Sharifuddin S.A., Long K., Alitheen N.B. Comparison of in vivo toxicity, antioxidant and immunomodulatory activities of coconut, nipah and pineapple juice vinegars. J. Sci. Food Agric. 2018;98(2):534–540. doi: 10.1002/jsfa.8491. [DOI] [PubMed] [Google Scholar]
- Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousaaid D., Laaroussi H., Bakour M., ElGhouizi A., Aboulghazi A., Lyoussi B., ElArabi I. Beneficial effects of apple vinegar on hyperglycemia and hyperlipidemia in hypercaloric-fed rats. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/9284987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousaaid D., Mechchate H., Laaroussi H., Hano C., Bakour M., El Ghouizi A., Conte R., Lyoussi B., El Arabi I. Fruits vinegar: quality characteristics, phytochemistry, and functionality. Molecules. 2022;27(1):222. doi: 10.3390/molecules27010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B.J., Wearsch P.A., Veloo A., Rodriguez-Palacios A. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Burillo S., Rajakaruna S., Pastoriza S., Paliy O., Ángel Rufián-Henares J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020;316 doi: 10.1016/j.foodchem.2020.126309. [DOI] [PubMed] [Google Scholar]
- Prasain J.K., Barnes S. Cranberry polyphenols-gut microbiota interactions and potential health benefits: an updated review. Food Frontiers. 2020;1:459–464. doi: 10.1002/fft2.56. [DOI] [Google Scholar]
- Rinninella E., Cintoni M., Raoul P., Ianiro G., Laterza L., Ponziani F.R., Pulcini G., Gasbarrini A., Mele M.C. Diet-induced alterations in gut microbiota composition and function. Reference Module in Food Science. 2022:354–373. doi: 10.1016/B978-0-12-819265-8.00035-8. [DOI] [Google Scholar]
- Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Sharon G., Cruz N.J., Kang D.W., Gandal M.J., Wang B., Kim Y.M., Zink E.M., Casey C.P., Taylor B.C., Lane C.J., Bramer L.M., Isern N.G., Hoyt D.W., Noecker C., Sweredoski M.J., Moradian A., Borenstein E., Jansson J.K., Knight R., Metz T.O., Lois C., Geschwind D.H., Krajmalnik-Brown R., Mazmanian S.K. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. doi: 10.1016/j.cell.2019.05.004. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stražar M., Temba G.S., Vlamakis H., Kullaya V.I., Lyamuya F., Mmbaga B.T., Joosten L.A.B., van der Ven A.J.A.M., Netea M.G., de Mast Q., Xavier R.J. Gut microbiome-mediated metabolism effects on immunity in rural and urban African populations. Nat. Commun. 2021;12:4845. doi: 10.1038/s41467-021-25213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Feng J., Wang X., Qi Z., Shi X., An Y., Zhang Q., Wang C., Liu M., Liu B., Yu L. Vinegar treatment prevents the development of murine experimental colitis via inhibition of inflammation and apoptosis. J. Agric. Food Chem. 2016;64(5):1111–1121. doi: 10.1021/acs.jafc.5b05415. [DOI] [PubMed] [Google Scholar]
- Tanes C., Bittinger K., Gao Y., Friedman E.S., Nessel L., Paladhi U.R., Chau L., Panfen E., Fischbach M.A., Braun J., Xavier R.J., Clish C.B., Li H., Bushman F.D., Lewis J.D., Wu G.D. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29(3):394–407. doi: 10.1016/j.chom.2020.12.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P., Bian X., Chi L., Gao B., Ru H., Knobloch T.J., Weghorst C.M., Lu K. Characterization of the functional changes in mouse gut microbiome associated with increased Akkermansia muciniphila population modulated by dietary black raspberries. ACS Omega. 2018;3(9):10927–10937. doi: 10.1021/acsomega.8b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell L.K., Haiser H.J., Van Treuren W., Garg N., Reddivari L., Vanamala J., Dorrestein P.C., Turnbaugh P.J., Knight R. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology. 2014;146(6):1470–1476. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Huang S., Wang Y., Cai S., Yu H., Liu H., Zeng X., Zhang G., Qiao S. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci.: CM. 2019;76(20):3917–3937. doi: 10.1007/s00018-019-03190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.S., Mo Y.Y., Huang Y.W., Echeveste C.E., Wang H.T., Chen J., Oshima K., Yearsley M., Simal-Gandaraf J., Battino M., Xiao J., Chen J., Sun C., Yu J., Bai W. Effects of dietary interventions on gut microbiota in humans and the possible impacts of foods on patients' responses to cancer immunotherapy. eFood. 2020;1(4):279–287. doi: 10.2991/efood.k.200824.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Li P., An Y., Ren J., Yan D., Cui J., Li D., Li M., Wang M., Zhong G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019;150 doi: 10.1016/j.phrs.2019.104489. [DOI] [PubMed] [Google Scholar]
- Xia T., Zhang B., Li S., Fang B., Duan W., Zhang J., Song J., Wang M. Vinegar extract ameliorates alcohol-induced liver damage associated with the modulation of gut microbiota in mice. Food Funct. 2020;11(4):2898–2909. doi: 10.1039/c9fo03015h. [DOI] [PubMed] [Google Scholar]
- Xia T., Yao J., Zhang J., Duan W., Zhang B., Xie X., Xia M., Song J., Zheng Y., Wang M. Evaluation of nutritional compositions, bioactive compounds, and antioxidant activities of Shanxi aged vinegars during the aging process. J. Food Sci. 2018;83(10):2638–2644. doi: 10.1111/1750-3841.14356. [DOI] [PubMed] [Google Scholar]
- Xia T., Zhang B., Duan W., Zhang J., Wang M. Nutrients and bioactive components from vinegar: a fermented and functional food. J. Funct.Foods. 2020;64 doi: 10.1016/j.jff.2019.103681. [DOI] [Google Scholar]
- Xia T., Zhang J., Yao J., Zhang B., Duan W., Xia M., Song J., Zheng Y., Wang M. Shanxi aged vinegar prevents alcoholic liver injury by inhibiting CYP2E1 and NADPH oxidase activities. J. Funct.Foods. 2018;47:575–584. doi: 10.1016/j.jffff.2018.06.018. [DOI] [Google Scholar]
- Yamashita H. Biological function of acetic acid-improvement in obesity and glucose tolerance by acetic acid in type 2 diabetic rats. Crit. Rev. Food Sci. Nutr. 2016;56(1):S171–S175. doi: 10.1080/10408398.2015.1045966. [DOI] [PubMed] [Google Scholar]
- Yang M., Yan T., Yu M., Kang J., Gao R., Wang P., Zhang Y., Zhang H., Shi L. Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Frontiers. 2020;1:398–419. doi: 10.1002/fft2.49. [DOI] [Google Scholar]
- Yuan R., Sun G., Gao J., Yu Z., Yu C., Wang C., Sun J., Li H., Chen J. Schisandra fruit vinegar lowers lipid profile in high-fat diet rats. Evid. base Compl. Alternative Med. 2020;2020 doi: 10.1155/2020/7083415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.J., Xie Q.T., You L.J., Cheung P.C.K., Zhao Z.G. Behavior of non-digestible polysaccharides in gastrointestinal tract: a mechanistic review of its anti-obesity effect. eFood. 2021;2(2):59–72. doi: 10.2991/efood.k.210310.001. [DOI] [Google Scholar]
- Zhang H.L., Caprioli G., Hussain H., Khoi Le N.P., Farag M.A., Xiao J.B. A multifaceted review on dihydromyricetin resources, extraction, bioavailability, biotransformation, bioactivities, and food applications with future perspectives to maximize its value. eFood. 2021;2(4):164–184. doi: 10.53365/efood.k/143518. [DOI] [Google Scholar]
- Zhang J., Zhu S., Ma N., Johnston L.J., Wu C., Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021;41(2):1061–1088. doi: 10.1002/med.21752. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Qureshi N., Xue B., Xie Z., Li P., Gu Q. Preventive and therapeutic effect of Lactobacillus paracasei ZFM54 on Helicobacter pylori-induced gastritis by ameliorating inflammation and restoring gastric microbiota in mice model. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.972569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Xing X., Gupta M., Keber F.C., Lopez J.G., Lee Y.J., Roichman A., Wang L., Neinast M.D., Donia M.S., Wühr M., Jang C., Rabinowitz J.D. Gut bacterial nutrient preferences quantified in vivo. Cell. 2022;185(18):3441–3456. doi: 10.1016/j.cell.2022.07.020. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.