Abstract
Objective
The present study aimed to evaluate the clinicopathological characteristics and value of HER2-low expression evolution in breast cancer receiving neoadjuvant chemotherapy (NAC).
Methods
Patients with HER2 negative breast cancer receiving NAC from January 2017 to December 2020 were enrolled in this study. The clinicopathological characteristics, response to NAC, evolution of HER2 and prognostic value were retrospectively analyzed.
Results
410 patients were included. The proportion of HR positive disease in HER2-low cases was higher than in HER2-zero population (75.8 % vs. 65.8 %, P = 0.040). No statistical significant difference in pCR rate was observed between HER2-low and HER2-zero patients (33.8 % vs. 39.3 %, P = 0.290) when pCR was defined as ypTis/0ypN0. Exploratory analysis revealed that the pCR rate of HER2-low cases was significantly lower than HER2-zero patients in the entire population (19.8 % vs. 33.3 %, P = 0.004) and HR positive population (12.6 % vs. 29.9 %, P = 0.001) when pCR was defined as ypT0ypN0. The evolution rate of HER2 expression after NAC was 31.0 % in HER2-zero patients and 24.7 % in HER2-low patients. Compared with patients with HR positive disease, patients with TNBC had higher evolution rate of HER2 expression after NAC (37.7 % vs. 23.6 %). Significant association was observed between HER2 evolution with histology type and Ki-67 index in HER2-zero patients and with lymph node involvement, HR status and Ki-67 index in HER2-low patients. Prognostic impact of HER2 evolution was not observed.
Conclusions
HR positive and HR negative HER2-low breast cancer exhibit different clinicopathological features, response to NAC and HER2 evolution after treatment.
Keywords: Breast cancer, HER2-Low expression, Neoadjuvant chemotherapy, Pathological complete response, Prognosis
Highlights
-
•
The proportion of HER2-low in HR positive patients was higher than in TNBC.
-
•
The pCR rate of HER2-low was significantly lower than HER2-zero in the entire and HR positive population with pCR as ypT0ypN0.
-
•
Compared with HR positive disease, TNBC had higher evolution rate of HER2 expression after NAC.
-
•
HR positive and HR negative HER2-low breast cancer exhibit different HER2 evolution after NAC.
1. Introduction
Human epidermal growth factor receptor (HER2) is abnormally amplified and/or overexpressed in about 15–20 % of breast cancer. HER2 mediates cellular signaling pathway involved in the proliferation and differentiation of breast cancer cells by encoding transmembrane glycoprotein, which act as an important driving gene for the tumorigenesis and progression of breast cancer [1]. Anti-HER2 targeted therapies, represented by trastuzumab, have significantly improved the prognosis of HER2-positive breast cancer patients [2]. The positive standard of HER2 was initially determined according to the therapeutic effect of trastuzumab. The H0648g clinical trial suggested that the benefit from trastuzumab was limited to the immunohistochemistry (IHC) 3+ subgroup [3]. Subsequent studies such as M77001, N9831, BCIRG006 and HERA confirmed that the benefit of trastuzumab was limited to the in situ hybridization (ISH) positive subset [[4], [5], [6], [7]].
However, from pathological perspective, the expression level of HER2 is continuous, rather than a binary classification of absolute positive and negative. Retrospective studies have shown that the proportion of breast cancer with HER2 low expression is more than 50 % [[8], [9], [10]]. For these breast cancer with low HER2 expression, many previous studies have shown that traditional anti-HER2 targeted drugs have no clinical benefits [11]. In the preliminary exploration of phase 1/2 studies such as DS8201-AJ101, trastuzumab deruxtecan (T-DXd) showed positive anti-tumor activity for HER2-low metastatic breast cancer [12,13]. The results of the Phase 3 DESTINY-Breast04 trial revealed that T-DXd significantly improved the progression-free survival (PFS) and overall survival (OS) of HER2-low metastatic breast cancer [14]. The advent of novel anti-HER2 antibody-drug conjugates (ADC) opened the new precision therapeutic pattern of breast cancer with low HER2 expression, and pushed the concept of HER2-low breast cancer to an unprecedented peak era.
As a highly heterogeneous disease, breast cancer exhibited significant temporal and spatial heterogeneity. Especially in metastatic breast cancer, it has been recognized that there is inconsistency in hormone receptor (HR) and HER2 status between primary and metastatic lesions [[15], [16], [17]]. The evolution of HER2 status (positive/negative) was also identified in neoadjuvant chemotherapy (NAC) setting from previous studies. Similarly, the state of low expression of HER2 may also change during the progression of disease. However, the evolution of HER2 low expression is rarely investigated so far in breast patients undergoing NAC. The present study was conducted to assess the clinical characteristics and value of HER2-low expression evolution in breast cancer patients receiving NAC.
2. Materials and methods
2.1. Patient
This was a retrospective study conducted in Henan Provincial People's Hospital, that includes patients with HER2 negative breast cancer who have received NAC from January 2017 to December 2020. The criteria for patients inclusion in analysis: 1) histopathological confirmed breast cancer; 2) HER-2 negative confirmed by IHC or fluorescence in situ hybridization (FISH); 3) have received NAC and followed by surgery after NAC. Patients who did not undergo surgery after NAC and those with missing follow-up data were excluded.
2.2. Clinical and pathological characteristics
Clinical stage was determined by the seventh edition of American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system. Estrogen receptor (ER) and progesterone receptor (PR) were detected by IHC, and the cut-off value was set to ≥1 %. HR positive was defined as ER or PR positive and HR negative was defined as ER and PR both negative. The HER2 status was determined by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for HER2 testing, including IHC 0, IHC 1+, or IHC 2+ and FISH negative. HER2-zero was defined as IHC 0, and HER2-low was defined as IHC 1+, or IHC 2+ and FISH negative.
2.3. Neoadjuvant chemotherapy and efficacy evaluation
All the patients in this study received NAC. The programme for NAC include: anthracycline and taxane-based regimens, taxane combined with platinum regimens and others based on the recommendations of the guidelines. During treatment, imaging examinations were performed after every two cycles of in all patients to evaluate clinical efficacy including ultrasound and MRI. The vast majority of patients underwent surgery after completing all cycle of NAC. Pathological evaluation criteria for pCR after NAC was determined as ypTis/0ypN0, which was defined as absence of invasive cancer in the breast (regardless of ductal carcinoma in situ) and axillary lymph nodes. For patients who did not achieve pCR, the HER2 status of residual disease was determined. In addition, we also conducted exploratory analysis on another criteria ypT0ypN0, which was defined as absence of invasive or noninvasive cancer in the breast and axillary lymph nodes. The evolution rate means the overall rate of HER2 discordance from primary breast cancer to residual breast cancer. Decision making for adjuvant therapy after neoadjuvant chemotherapy: decide whether to undergo radiotherapy based on the initial clinical stage and surgical manner; HR positive patients who have achieved pCR and those not achieving pCR receive adjuvant endocrine therapy. Premenopausal patients undergoing ovarian function suppression (OFS) combined with aromatase inhibitor (AI), and postmenopausal patients receive AI treatment. Due to the fact that cyclin-dependent kinase (CDK) 4/6 inhibitors had not yet been recommended by guidelines at the time, and thus HR + patients not achieving pCR did not receive intensified treatment with CDK4/6 inhibitors. TNBC patients who did not reach pCR received intensified treatment with capecitabine.
2.4. Statistical analysis
The patients had regular inpatient or outpatient follow-up including laboratory indexes and imaging examination. The follow-up deadline is December 31, 2022. Disease free survival (DFS) was defined as from the date of surgery to the date of locoregional relapse, distant relapse, death or last follow-up. Overall survival (OS) was defined as from the date of surgery to patient death or last follow-up. Difference between groups were determined by Pearson's chi squared test or Fisher's exact test. Survival curves of patients were estimated by the Kaplan-Meier method and Log-rank test was performed for survival analysis. Multivariate Cox proportional hazards regression analyses were used to determine independent prognostic factors affecting DFS and OS in HR positive population. All the statistical descriptive analyses were performed with SPSS 22.0 software (SPSS Inc., IL, US) software. P < 0.05 was considered significant.
3. Results
3.1. Features of patient and treatment
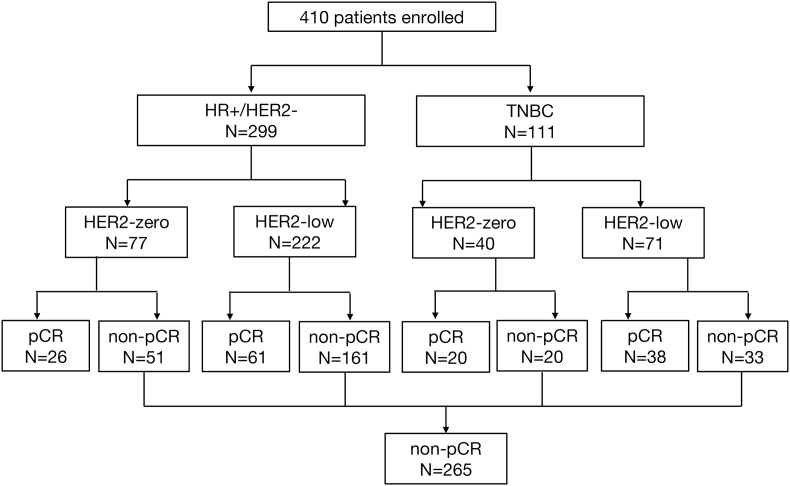
The present study enrolled a total of 410 breast cancer patients (Fig. 1). Features of patient and treatment are summarized in Table 1. In the entire population, HER2 status in 117 (28.5 %) patients was HER2-zero and in 293 (71.5 %) patients was HER2-low expression. Most patients analyzed in this study were locally advanced breast cancer, the proportion of T3-4 and N2-3 were 31.5 % and 42.7 %, respectively. The clinical stage in 185 (45.1 %) was stage II and in 225 (54.9 %) patients was stage III. In this study, 299 (72.9 %) patients were HR positive breast cancer, and the other 111 (27.1 %) patients were TNBC. The proportion of HR positive patients in HER2-low breast cancer was higher than in HER2-zero breast cancer patients (75.8 % vs. 65.8 %, P = 0.040). Anthracycline and taxane-based treatment was the most common regimen of NAC. The proportion of Ki-67 > 30 % in the entire population was 76.6 %. After NAC, 81.7 % patients underwent mastectomy surgery, only 20 patients (17.1 %) in HER2-zero and 55 patients (18.8 %) in HER2-low breast cancer performed breast conserving surgery or immediate breast reconstruction (IBR).
Fig. 1.
Flowchart of the patient queue.
Table 1.
Patient and treatment characteristics.
| Characteristic | Total (n = 410) |
TNBC(n = 111) |
HR positive (n = 299) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HER2-zero n = 117 | HER2-low n = 293 | P | HER2-zero n = 40 | HER2-low n = 71 | P | HER2-zero n = 77 | HER2-low n = 222 | P | |
| Age(years, median) | 47 | 47 | – | 51 | 50 | – | 45 | 46 | – |
| Histology type | 0.716 | 0.936 | 0.627 | ||||||
| IBC-NST | 98 (83.8) | 241 (82.3) | 33 (82.5) | 59 (83.1) | 65 (84.4) | 182 (82.0) | |||
| Other | 19 (16.2) | 52 (17.7) | 7 (17.5) | 12 (16.9) | 12 (15.6) | 40 (18.0) | |||
| cT | 0.324 | 0.269 | 0.047 | ||||||
| T1-2 | 76 (65.0) | 205 (70.0) | 31 (77.5) | 48 (67.6) | 45 (58.4) | 157 (70.7) | |||
| T3-4 | 41 (35.0) | 88 (30.0) | 9 (22.5) | 23 (32.4) | 32 (41.6) | 65 (29.3) | |||
| cN | 0.369 | 0.069 | 0.014 | ||||||
| N0-1 | 63 (53.8) | 172 (58.7) | 30 (75.0) | 41 (57.7) | 33 (42.9) | 131 (59.0) | |||
| N2-3 | 54 (46.2) | 121 (41.3) | 10 (25.0) | 30 (42.3) | 44 (57.1) | 91 (41.0) | |||
| Clinical stage | 0.292 | 0.012 | 0.003 | ||||||
| II | 48 (41.0) | 137 (46.8) | 24 (60.0) | 25 (35.2) | 24 (31.2) | 112 (50.5) | |||
| III | 69 (59.0) | 156 (53.2) | 16 (40.0) | 46 (64.8) | 53 (68.8) | 110 (49.5) | |||
| HR | 0.040 | – | – | ||||||
| Positive | 77 (65.8) | 222 (75.8) | 0 (0) | 0 (0) | 77 (100.0) | 222 (100.0) | |||
| Negative | 40 (34.2) | 71 (24.2) | 40 (100.0) | 71 (100.0) | 0 (0) | 0 (0) | |||
| Ki-67 | 0.536 | 0.363 | 0.484 | ||||||
| ≤ 30 % | 25 (21.4) | 71 (24.2) | 5 (12.5) | 4 (5.6) | 20 (26.0) | 67 (30.2) | |||
| >30 % | 92 (78.6) | 222 (75.8) | 35 (87.5) | 67 (94.4) | 57 (74.0) | 155 (69.8) | |||
| Chemotherapy regimen | 0.189 | 0.254 | 0.542 | ||||||
| Anthracycline + taxane | 110 (94.0) | 283 (96.6) | 35 (87.5) | 66 (93.0) | 75 (97.4) | 217 (97.7) | |||
| Platinum | 2 (1.7) | 6 (2.0) | 2 (5.0) | 4 (5.6) | 0 (0) | 2 (0.9) | |||
| Others | 5 (4.3) | 4 (1.4) | 3 (7.5) | 1 (1.4) | 2 (2.6) | 3 (1.4) | |||
| Surgery | 0.901 | 0.098 | 0.416 | ||||||
| Mastectomy | 97 (82.9) | 238 (81.2) | 38 (95.0) | 58 (81.7) | 59 (76.6) | 180 (81.1) | |||
| Breast conserving | 14 (12.0) | 40 (13.7) | 2 (5.0) | 7 (9.9) | 12 (15.6) | 33 (14.9) | |||
| IBR | 6 (5.1) | 15 (5.1) | 0 (0) | 6 (8.5) | 6 (7.8) | 9 (4.1) | |||
IBC-NST, invasive breast cancer, no special type; HR, estrogen receptor; IBR, immediate breast reconstruction.
In the entire population, expect for HR status, there were no statistically significant differences in histology type, clinical tumor size, lymph node status, clinical stage, Ki-67 index, NAC regimen and operation between HER2-zero and HER2-low breast cancer. However, in subgroup of TNBC and HR positive disease, HER2-zero and HER2-low patients exhibited different clinicopathological characteristics. In TNBC, although no statistical significance was found, the proportion of T3-4 and N2-3 lymph node involvement in HER2-low were higher than in HER2-zero breast cancer. And simultaneously, the proportion of clinical stage III HER2-low patients in was higher than in HER2-zero breast cancer (64.8 % vs. 40.0 %, P = 0.012). However, HER2-low breast cancer patients in HR positive breast cancer exhibit lower tumor burden, including lower proportion of T3-4 (29.3 % vs. 41.6 %, P = 0.047), less lymph node metastatic burden (N2-3: 41.0 % vs. 57.1 %, P = 0.014) and earlier clinical stage (clinical stage III: 49.5 % vs. 68.8 %, P = 0.003) than HER2-zero breast cancer patients. Other features of patient and treatment were similar between HER2-low and HER2-zero patients in both HR positive breast cancer patients and TNBC.
3.2. HER2 and efficacy of neoadjuvant chemotherapy
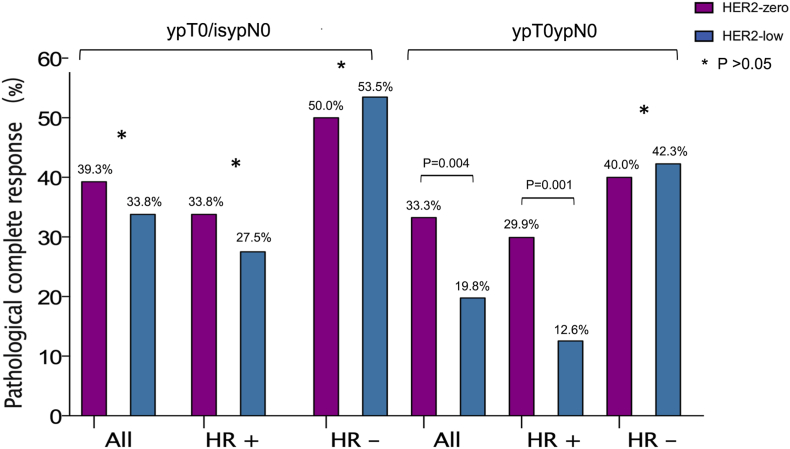
When pCR was defined as ypTis/0ypN0, no statistical significant differences in pCR rate were found between HER2-low and HER2-zero breast cancer among the entire population (33.8 % vs. 39.3 %, P = 0.290, Fig. 2), HR positive breast cancer patients (27.5 % vs. 33.8 %, P = 0.295) and TNBC (53.5 % vs. 50.0 %, P = 0.721).
Fig. 2.
The pCR rate in the entire cohort, HR-positive disease and TNBC with different evaluation criteria for pCR after NAC, including ypTis/0ypN0 and ypT0ypN0.
In addition, another criteria of pCR which was defined as ypT0ypN0 was also exploratively analyzed. In the entire population, 97 patients achieved pCR and the pCR rate was 23.7 % with the pCR defined as ypT0ypN0. The pCR rate of HER2-low patients was significantly lower than HER2-zero patients in the entire population (19.8 % vs. 33.3 %, P = 0.004) and HR positive breast cancer patients (12.6 % vs. 29.9 %, P = 0.001). However, no significant differences in pCR rate were found between HER2-low and HER2-zero breast cancer in TNBC (42.3 % vs. 40.0 %, P = 0.817).
3.3. Evolution of HER2 after neoadjuvant chemotherapy
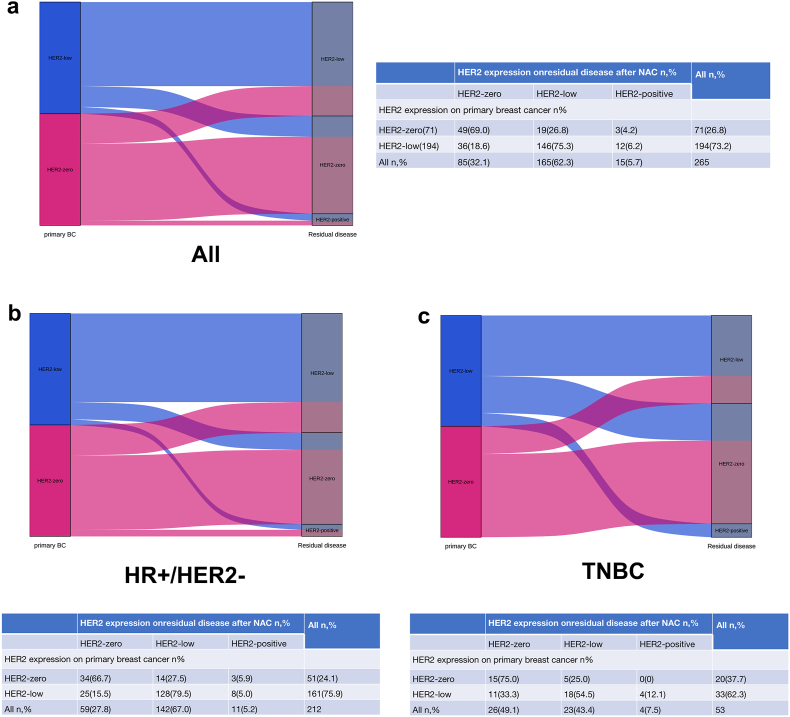
In the entire population, 265 patients did not obtain pCR and evolution of HER2 expression was analyzed before and after NAC in these patients. In the entire population, the overall evolution rate of HER2 expression after NAC was 26.4 % (Fig. 3). Among the 71 HER2-zero patients, 49 (69.0 %) cases remained HER2-zero, 19 (26.8 %) cases transitioned to HER2-low expression, and the remaining 3 (4.2 %) patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 31.0 %. Among the 194 HER2-low patients, 146 (75.3 %) cases remained HER2-low, 36 (18.5 %) cases transitioned to HER2-zero, and the remaining 12 (6.2 %) patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 24.7 %.
Fig. 3.
HER2 evolution from baseline biopsy to residual disease after NAC in the entire cohort (a), HR-positive disease (b) and TNBC (c).
In patients with HR positive disease, the overall evolution rate of HER2 expression after NAC was 23.6 %. Among the 51 HER2-zero patients, 34 (66.7 %) cases remained HER2-zero, 14 (27.5 %) cases transitioned to HER2-low expression, and the remaining 3 (5.9 %) patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 33.3 %. Among the 212 HER2-low patients, 128 (79.5 %) cases remained HER2-low, 25 (15.5 %) cases transitioned to HER2-zero, and the remaining 8 (5.0 %) patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 20.5 %.
In TNBC, the overall evolution rate of HER2 expression was 37.7 %. Among the 20 HER2-zero patients, 15 (75.0 %) cases remained HER2-zero, 5 (25.0 %) cases transitioned to HER2-low expression, with no patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 25.0 %. Among the 33 HER2-low patients, 18 (54.5 %) cases remained HER2-low, 11 (33.3 %) cases transitioned to HER2-zero, and the remaining 4 (12.1 %) patients transitioned to HER2-positive. The overall evolution rate of HER2 expression after NAC was 45.5 %.
3.4. Clinicopathological characteristics of HER2 evolution
The relationship between HER2 evolution and clinicopathological characteristics was further analyzed. Among HER2-zero population, significant association was observed between HER2 evolution with histology type and Ki-67 index (Table 2). Compared with HER2 stable cases, the proportion of pure invasive breast cancer (IBC-NST) was higher in HER2 gain (HER2-zero transitioned to HER2-low and HER2-positive) cases (100.0 % vs. 69.4 %, P = 0.009). Simultaneously, the proportion of Ki-67 ≤ 30 % was also higher in HER2 gain cases than HER2 stable cases (40.9 % vs. 16.3 %, P = 0.025). In patients with HR positive disease, patients with Ki-67 ≤ 30 % were more prone to the occurrence of HER2 evolution (52.9 % vs. 23.5 %, P = 0.036). In TNBC, patients with T3-4 were more prone to the occurrence of HER2 evolution (100.0 % vs. 11.8 %, P = 0.009).
Table 2.
Clinicopathological characteristics of HER2-zero Expression Evolution.
| Characteristic | Total (n = 71) |
TNBC(n = 20) |
HR positive (n = 51) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HER2-stable n = 49 | HER2-gain n = 22 | P | HER2-stable n = 15 | HER2-gain n = 5 | P | HER2-stable n = 34 | HER2-gain n = 17 | P | |
| Histology type | 0.009 | 0.260 | 0.051 | ||||||
| IBC-NST | 34 | 22 | 9 | 5 | 25 | 17 | |||
| Other | 15 | 0 | 6 | 0 | 9 | 0 | |||
| cT | 0.714 | 0.009 | 0.234 | ||||||
| T1-2 | 29 | 12 | 15 | 2 | 14 | 10 | |||
| T3-4 | 20 | 10 | 0 | 3 | 20 | 7 | |||
| cN | 0.406 | 0.266 | 0.135 | ||||||
| N0-1 | 23 | 8 | 10 | 5 | 13 | 3 | |||
| N2-3 | 26 | 14 | 5 | 0 | 21 | 14 | |||
| Clinical stage | 0.313 | 0.347 | 1.000 | ||||||
| II | 17 | 5 | 10 | 2 | 7 | 3 | |||
| III | 32 | 17 | 5 | 3 | 27 | 14 | |||
| HR | 0.495 | – | – | ||||||
| Positive | 34 | 17 | 0 | 0 | 34 | 17 | |||
| Negative | 15 | 5 | 15 | 5 | 0 | 0 | |||
| Ki-67 | 0.025 | – | 0.036 | ||||||
| ≤ 30 % | 8 | 9 | 0 | 0 | 8 | 9 | |||
| >30 % | 41 | 13 | 15 | 5 | 26 | 8 | |||
IBC-NST, invasive breast cancer, no special type; HR, estrogen receptor.
Among HER2-low expression population, significant association was observed between HER2 evolution with clinical lymph node involvement, HR status and Ki-67 index (Table 3). The evolution rate of HER2 expression [HER2-low transitioned to HER2-zero (HER2 loss) and HER2-positive (HER2 gain)] was much higher in cN2-3 (31.2 % vs. 18.8 %, P = 0.025), HR negative (45.5 % vs. 20.5 %, P = 0.010) and Ki-67 > 30 % (30.3 % vs. 9.6 %, P = 0.008) patients. In TNBC, patients, with other histology type (85.7 % vs. 34.6 %, P = 0.047) and cT3-4 (50.0 % vs. 40.0 %, P = 0.016) were more prone to the occurrence of HER2 evolution. In patients with HR positive disease, patients with N2-3 (25.0 % vs. 16.0 %, P = 0.014) and stage III (22.2 % vs. 18.3 %, P = 0.030) were more prone to the occurrence of HER2 evolution.
Table 3.
Clinicopathological characteristics of HER2-low Expression Evolution.
| Characteristic | Total (n = 194) |
TNBC(n = 33) |
HR positive (n = 161) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2-stable n = 146 | HER2-loss n = 36 | HER2-gain n = 12 | P | HER2-stable n = 18 | HER2-loss n = 11 | HER2-gain n = 4 | P | HER2-stable n = 128 | HER2-loss n = 25 | HER2-gain n = 8 | P | |
| Histology type | 0.104 | 0.047 | 0.149 | |||||||||
| IBC-NST | 121 | 30 | 7 | 17 | 7 | 2 | 104 | 23 | 5 | |||
| Other | 25 | 6 | 5 | 1 | 4 | 2 | 24 | 2 | 3 | |||
| cT | 0.278 | 0.016 | 0.734 | |||||||||
| T1-2 | 97 | 21 | 10 | 9 | 2 | 4 | 88 | 19 | 6 | |||
| T3-4 | 49 | 15 | 2 | 9 | 9 | 0 | 40 | 6 | 2 | |||
| cN | 0.025 | 0.077 | 0.014 | |||||||||
| N0-1 | 82 | 17 | 2 | 14 | 4 | 2 | 68 | 13 | 0 | |||
| N2-3 | 64 | 19 | 10 | 4 | 7 | 2 | 60 | 12 | 8 | |||
| Clinical stage | 0.201 | 0.472 | 0.030 | |||||||||
| II | 63 | 15 | 2 | 5 | 2 | 2 | 58 | 13 | 0 | |||
| III | 83 | 21 | 10 | 13 | 9 | 2 | 70 | 12 | 8 | |||
| HR | 0.010 | – | – | |||||||||
| Positive | 128 | 25 | 8 | 0 | 0 | 0 | 128 | 25 | 8 | |||
| Negative | 18 | 11 | 4 | 18 | 11 | 4 | 0 | 0 | 0 | |||
| Ki-67 | 0.008 | 0.150 | 0.066 | |||||||||
| ≤ 30 % | 47 | 5 | 0 | 4 | 0 | 0 | 43 | 5 | 0 | |||
| >30 % | 99 | 31 | 12 | 14 | 11 | 4 | 85 | 20 | 8 | |||
IBC-NST, invasive breast cancer, no special type; HR, estrogen receptor.
3.5. Survival analysis of baseline HER2 expression and HER2 evolution
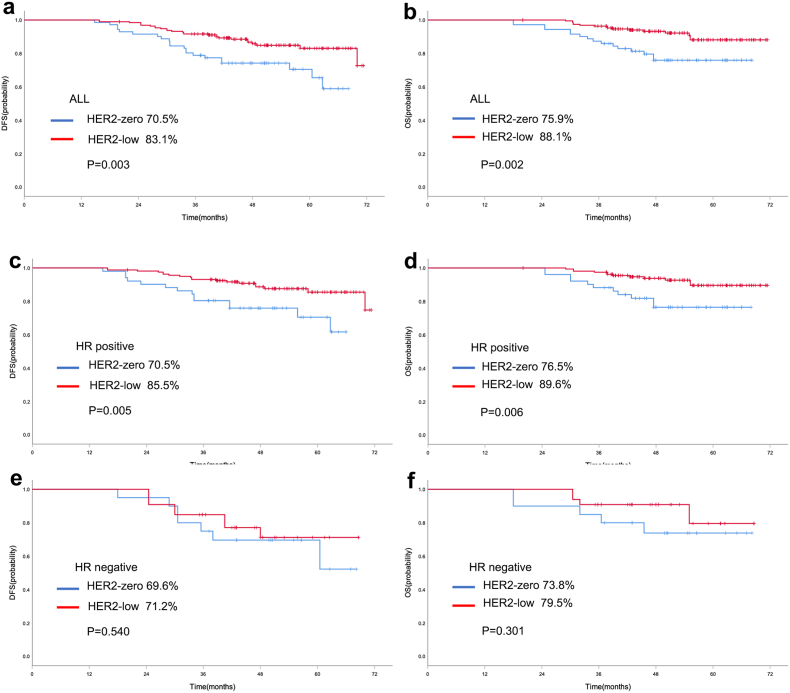
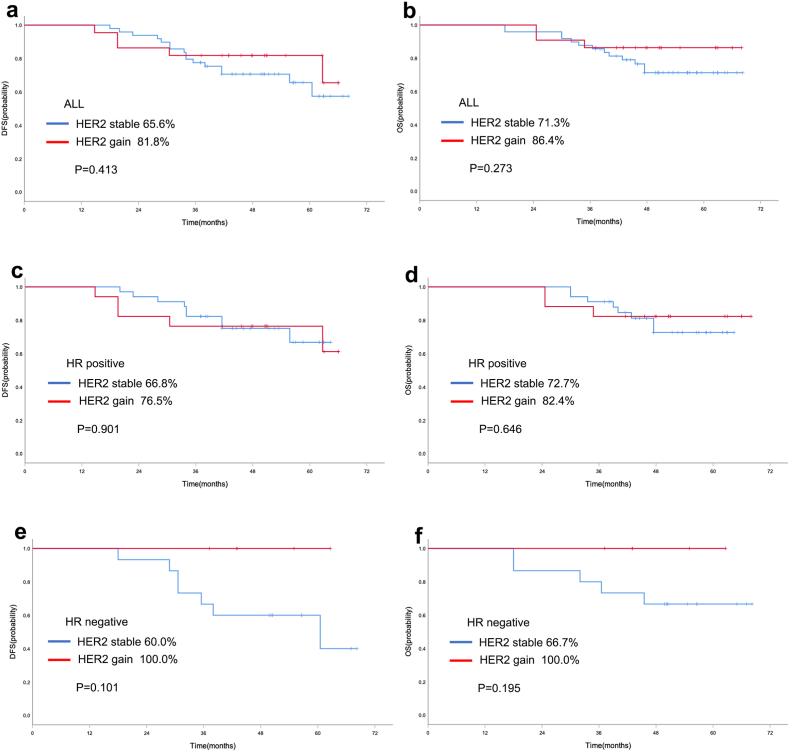
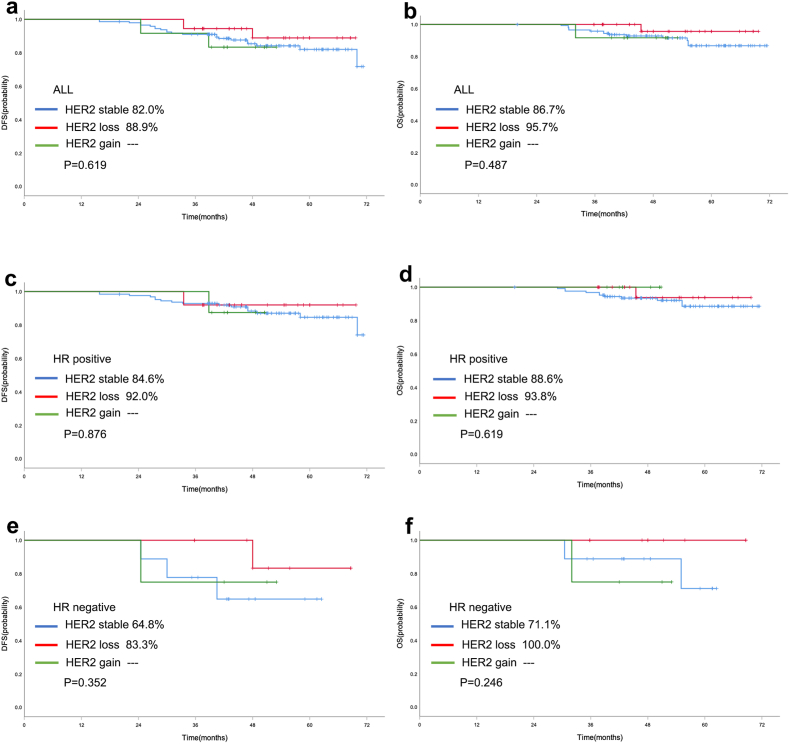
Survival analysis was performed in the 265 patients who did not obtain pCR. For baseline HER2 expression, in the entire population, the 5-year rate of DFS in HER2-low cases was significantly better than HER2-zero breast cancer patients (83.1 % vs. 70.5 %, P = 0.003, Fig. 4a). And the 5-year rate of OS in HER2-low cases was also significantly better than HER2-zero breast cancer patients (88.1 % vs. 75.9 %, P = 0.002, Fig. 4b). Similarly, HER2-low cases also showed better survival data of DFS (85.5 % vs. 70.5 %, P = 0.005, Fig. 4c) and OS (89.6 % vs. 76.5 %, P = 0.006, Fig. 4d) than HER2-zero patients in patients with HR positive disease. Multivariate Cox proportional hazards regression analyses also showed that HER2-low was independent prognostic factors affecting DFS and OS in HR positive population (Supplementary Table 1). However, there were no statistically significant survival differences in DFS (P = 0.540, Fig. 4e) and OS (P = 0.301, Fig. 4f) between HER2-low and HER2-zero breast cancer in TNBC cohort. For the evolution of HER2, in the HER2-zero population, no statistically significant differences in DFS (65.6 % vs. 81.8 %, P = 0.413, Fig. 5a) and OS (71.3 % vs. 86.4 %, P = 0.273, Fig. 5b) were found between HER2 stable and HER2 gain cases. And similarly, no statistically significant differences were observed between HER2 stable and HER2 gain cases with respect to HR positive disease (Fig. 5c/d) and TNBC (Fig. 5e/f) subgroup. In the HER2-low population, no differences were found in DFS and OS according to the evolution of HER2, including HER2 stable, HER2 loss and HER2 gain (Fig. 6).
Fig. 4.
Kaplan-Meier curve of DFS (a) and OS (b) in the entire cohort with different baseline HER2 status (HER2-low vs. HER2-zero). Kaplan-Meier curve of DFS (c) and OS (d) in HR positive patients with different baseline HER2 status (HER2-low vs. HER2-zero). Kaplan-Meier curve of DFS (e) and OS (f) in TNBC with different baseline HER2 status (HER2-low vs. HER2-zero).
Fig. 5.
Kaplan-Meier curve of DFS (a) and OS (b) in the HER2-zero entire cohort with different HER2 evolution. Kaplan-Meier curve of DFS (c) and OS (d) in HR positive HER2-zero patients with different HER2 evolution. Kaplan-Meier curve of DFS (e) and OS (f) in TNBC HER2-zero patients with different HER2 evolution.
Fig. 6.
Kaplan-Meier curve of DFS (a) and OS (b) in the HER2-low entire cohort with different HER2 evolution. Kaplan-Meier curve of DFS (c) and OS (d) in HR positive HER2-low patients with different HER2 evolution. Kaplan-Meier curve of DFS (e) and OS (f) in TNBC HER2-low patients with different HER2 evolution.
4. Discussion
HER2 is the most important therapeutic target for solid tumors, including breast cancer. In the past, breast cancer was divided into HER2-positive and HER2-negative. HER2-positive breast cancer was initially defined as breast cancer with HER2 overexpression caused by ERBB2 gene amplification. The precise classification of HER2 positive breast cancer accounts for about 15–20 % of all breast cancer, and has evolved into a synonym for predicting the effectiveness of HER2 targeted therapy such as trastuzumab. Anti-HER2 targeted therapies have experienced the era of single target, dual target, tyrosine kinase inhibitor (TKI) and ADC. Trastuzumab deruxtecan (T-DXd), a new generation ADC drug, has a unique bystander effect and shows good efficacy in breast cancer population with low expression of HER2. Approximately 50 %–60 % of breast cancers are scored as having low HER2 expression by IHC, which had unique and differential biological behavior [[18], [19], [20], [21]]. However, one study shows that HER2-low vs HER2-0 do not differ from the prognostic perspective in the metastatic BC setting [22]. Another study emphasized the large biological heterogeneity of HER2-low BC, and the need to implement reproducible and sensitive assays to measure low HER2 expression [23]. But, some studies also showed that the genomic landscape of HER2-low and HER2-0 tumors does not differ significantly, apart from a higher ERBB2 copy count among HER2-low tumors, and a higher rate of ERBB2 hemideletions in HER2-0 tumors [24]. The consensus of clinical diagnosis and treatment of HER2-low breast cancer state that although HER2-low breast cancer is expected to become a new treatment subtype, but should not be considered a new pathological molecular subtype [25,26]. However, this does not hinder HER2-low breast cancer as a new direction of basic research and clinical treatment of breast cancer.
Our present study enrolled 410 HER2 negative breast cancer patients receiving NAC, and HER2-low breast cancer accounted for a considerable proportion, reaching 71.5 %. The proportion of HR positive patients in HER2-low breast cancer was higher than in HER2-zero breast cancer patients, which was consistent with previous studies. The ratio of HER2-low was proportional to the expression level of estrogen receptor. Some studies have shown that most of the clinicopathological differences between HER2-low and HER2-zero breast cancer actually depend on the HR expression level, and there was no significant difference between HER2-low and HER2-zero breast cancer after correcting for HR status [27]. However, our study did not support this view. In subgroup of TNBC and HR positive disease, HER2-zero and HER2-low patients exhibited different clinicopathological characteristics. In TNBC, HER2-low patients had a greater tumor burden. However, HER2-low patients exhibited smaller tumor size, less node involvement and earlier clinical stage in HR positive disease. And thus, our present study supported that HER2-low breast cancer had unique clinicopathological features, and it was proposed for the first time that the biological behavior of HER2-low breast cancer was related to HR status in NAC setting.
Currently, whether HER2-low expression will affect the efficacy of NAC for breast cancer is inconsistent [[28], [29], [30]]. A study summarized the data of 1098 HER2-low and 1212 HER2-zero breast cancer patients who received NAC in four prospective neoadjuvant clinical trials (Geparsepto; Geparocto; GeparX; Gain-2), which showed that the pCR rate of HER2-low tumors was significantly lower than that of HER2-zero patients (29.2 % vs 39.0 %, p = 0.0002) [31]. However, another study showed that there was no significant difference in pCR rate between HER2-low and HER2-zero breast cancer patients receiving NAC [32]. In our present study, with the pCR defined as ypTis/0ypN0, no statistical significant differences in pCR rate were found between HER2-low and HER2-zero breast cancer among the entire population, HR positive breast cancer patients and TNBC. However, the pCR rate of HER2-zero patients was significantly lower than HER2-zero patients in the entire population and HR positive disease with the pCR defined as ypT0ypN0. Therefore, we proposed for the first time that different criteria of pCR need to be considered when evaluating whether HER2-low will affect the response to NAC in breast cancer. This was attributed to that HER-2 low breast cancer had a higher proportion of ductal carcinoma in situ (DCIS) in the residual disease after NAC, which also reflected the different pathological response pattern of HER-2 low breast cancer to NAC compared with HER2-zero breast cancer.
The pCR rate in HR-positive patients was 29.1 % (ypTis/0ypN0) and 17.1 % (ypT0ypN0), which seems to be higher than previous clinical studies. For example, KEYNOTE-756 and CheckMate 7FL clinical studies explored the clinical efficacy of immune checkpoint inhibitors (ICIs) in HR positive breast cancer patients. The pCR rates of HR positive patients receiving chemotherapy alone were 15.6 % and 13.8 %, respectively. We speculate that there are two reasons. First, a considerable proportion of patients exhibit residual intraductal carcinoma in the primary tumor lesion in this study. We will continue to monitor the tumor response patterns of HR positive patients in future research. Second, the results of a Swedish breast cancer research group (241MO) released at the 2023 ESMO conference showed that there was no significant difference in OS, distant disease-free survival, and pCR rate between ER negative and ER low positive (1%–10 %) breast cancer patients. In the cohort of HR positive patients of this study, 35 patients had ER low positive expression, which may affect the overall pCR of the population.
The state of HER2 can change as the disease progresses. A study conducted on 512 patients with advanced TNBC by performing core needle biopsy in different stages of the disease showed that the proportion of HER2-low disease increased with consecutive biopsies, which ranged from 59 % (one biopsy), 73 % (two biopsies), 83 % (three biopsies), 83 % (four biopsies), and 100 % (≥ five biopsies). About one-third of HER2-zero patients transformed to HER2-low. Another study demonstrated that 31.7 % of metastatic breast cancer patients transitioned from HER2-zero to HER2-low during anti-HER2 targeted therapies, and also observed heterogeneity in HER2 status within metastatic lesions [17]. However, the evolution of HER2 status is rarely investigated so far in breast patients undergoing NAC. A previous study have suggested that the overall rate of HER2 evolution from baseline biopsy to residual disease after NAC was 26.4 %, mostly represented by cases switching from HER2-zero to HER2-low (8.9 %) and from HER2-low to HER2-0 (14.7 %) [33].
The current study was performed in 265 patients failing to achieve pCR to evaluate the evolution of HER2 expression from baseline biopsy to residual disease after NAC.The overall evolution rate of HER2 expression after NAC was 26.4 %, including HER2-zero cases transitioned to HER2-low and HER2-positive (31.0 %) and HER2-low cases transitioned to HER2-zero and HER2-positive (24.7 %). Compared with patients with HR positive disease, patients with TNBC had higher evolution rate of HER2 expression after NAC (37.7 % vs. 23.6 %). Our study demonstrated the high instability of HER2 expression from baseline to residual disease, suggesting the importance of re-pathological assessment of HER2 after NAC. Exploratory analysis of clinicopathological characteristics and HER2 evolution revealed that patients with high tumor burden were more prone to the occurrence of HER2 evolution, which may be related to the higher tumor heterogeneity in these patients. In addition to metastatic breast cancer, novel ADC drugs such as T-DXd have been moving forward to adjuvant and neoadjuvant therapies. Our study on the evolution of HER2 after NAC provides valuable insights for the design of clinical trials related to the application of ADC drugs in non-pCR patients.
Previous studies suggested that HER2-low patients seem to have a better prognosis, although this difference was relatively small. A retrospective study from the cancer database showed that in stage II − IV TNBC and stage III − IV HR positive breast cancer, patients with low HER2 expression had better OS [24]. Similarly, previous studies also revealed that in patients receiving NAC, after adjusting for factors such as tumor stage and HR status, low expression of HER2 was significantly associated with longer OS [9]. We also performed survival analysis to assess survival differences according to baseline HER2 status and HER2 evolution from primary to residual disease. Significantly improved in both DFS and OS was observed in baseline HER2-low patients compared with HER2-zero cases. It was worth noting that this survival difference was related to HR status, the survival improvement observed only in HR positive patients, rather than TNBC. However, evolution of HER2 expression from baseline biopsy to residual disease after NAC had no prognostic influence, regardless of HER2 loss or HER2 gain. Our research supported that intensive treatment was necessary for HER2 negative patients who did not obtain pCR after NAC.
Our present study has some limitations. First, a retrospective study from a single center was the main limitation of our present work. Second, the patients enrolled in the study were small. Third, the pathology was retrospectively analyzed, which was neither revised nor centralized. Fourth, all the patients enrolled were Asian ancestry and have not been included in other ethnicities. Future prospective and confirmatory studies are needed to evaluate the clinicopathological characteristics and value of HER2-low expression evolution in breast cancer receiving NAC.
5. Conclusion
In conclusion, we reported that HR positive and HR negative HER2-low breast cancer exhibit different clinicopathological features, response to NAC and HER2 evolution after treatment.
Ethics approval and consent to participate
This study was carried out in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Henan Provincial People's Hospital. Written informed consent was obtained from all patients for the use of the medical records for research purposes.
Funding
This work was supported by Medical Science and Technique Foundation of Henan Province (No. LHGJ20210055) and Beijing Medical Award Foundation Project (No.YXJL-2020-0941-0748).
Availability of data and materials
The raw data of this article will be made available by contacting the corresponding author.
CRediT authorship contribution statement
Yingbo Shao: Writing - review & editing, Writing - original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Huijuan Guan: Methodology, Investigation, Formal analysis. Zhifen Luo: Methodology, Investigation, Formal analysis. Yang Yu: Software, Resources. Yaning He: Methodology, Investigation. Qi Chen: Investigation, Formal analysis. Chaojun Liu: Methodology, Investigation. Fangyuan Zhu: Writing - original draft, Software. Hui Liu: Writing - review & editing, Validation, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that no potential conflict of interest exist.
Acknowledgements
We gratefully acknowledge the follow-up team for their contribution to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.103666.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 3.Mass R.D., Press M.F., Anderson S., et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6(3):240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 4.Perez E.A., Reinholz M.M., Hillman D.W., et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28(28):4307–4315. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez E.A., Press M.F., Dueck A.C., et al. Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005) Breast Cancer Res Treat. 2013;138(1):99–108. doi: 10.1007/s10549-013-2444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon D., Eiermann W., Robert N., et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron D., Piccart-Gebhart M.J., Gelber R.D., et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarantino P., Hamilton E., Tolaney S.M., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 9.Horisawa N., Adachi Y., Takatsuka D., et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022;29(2):234–241. doi: 10.1007/s12282-021-01303-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Katerji H., Turner B.M., Audeh W., Hicks D.G. HER2-low breast cancers: incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod Pathol. 2022;35(8):1075–1082. doi: 10.1038/s41379-022-01019-5. [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbacher L., Cecchini R.S., Geyer C.E., Jr., et al. NSABP B-47/NRG Oncology phase III Randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38(5):444–453. doi: 10.1200/JCO.19.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi T., Shitara K., Naito Y., et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 13.Modi S., Park H., Murthy R.K., et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieci M.V., Barbieri E., Piacentini F., et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24(1):101–108. doi: 10.1093/annonc/mds248. [DOI] [PubMed] [Google Scholar]
- 16.Guarneri V., Giovannelli S., Ficarra G., et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncol. 2008;13(8):838–844. doi: 10.1634/theoncologist.2008-0048. [DOI] [PubMed] [Google Scholar]
- 17.Miglietta F., Griguolo G., Bottosso M., et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021;7(1):137. doi: 10.1038/s41523-021-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schettini F., Chic N., Braso-Maristany F., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G., Ren C., Li C., et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20(1):142. doi: 10.1186/s12916-022-02346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L., Zhang Y., Wang Z., et al. Comparisons of clinical characteristics, prognosis, epidemiological factors, and genetic susceptibility between HER2-low and HER2-zero breast cancer among Chinese females. Cancer Med. 2023;12(14):14937–14948. doi: 10.1002/cam4.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Liu Y., Han D., et al. Clinical genetic features and neoadjuvant chemotherapy response in HER2-low breast cancers: a retrospective, multicenter cohort study. Ann Surg Oncol. 2023;30(9):5653–5662. doi: 10.1245/s10434-023-13311-y. [DOI] [PubMed] [Google Scholar]
- 22.Viale G., Basik M., Niikura N., et al. Retrospective study to estimate the prevalence and describe the clinicopathological characteristics, treatments received, and outcomes of HER2-low breast cancer. ESMO Open. 2023;8(4) doi: 10.1016/j.esmoop.2023.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schettini F., Chic N., Brasó-Maristany F., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantino P., Gupta H., Hughes M.E., et al. Comprehensive genomic characterization of HER2-low and HER2-0 breast cancer. Nat Commun. 2023;14(1):7496. doi: 10.1038/s41467-023-43324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarantino P., Viale G., Press M.F., et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol. 2023;34(8):645–659. doi: 10.1016/j.annonc.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Wolff A.C., Somerfield M.R., Dowsett M., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology-college of American Pathologists guideline update. Arch Pathol Lab Med. 2023;147(9):991–992. doi: 10.5858/arpa.2023-0187-ED. [DOI] [PubMed] [Google Scholar]
- 27.Peiffer D.S., Zhao F., Chen N., et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the national cancer database. JAMA Oncol. 2023;9(4):500–510. doi: 10.1001/jamaoncol.2022.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Moura Leite L., Cesca M.G., Tavares M.C., et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–163. doi: 10.1007/s10549-021-06365-7. [DOI] [PubMed] [Google Scholar]
- 29.Domergue C., Martin E., Lemarie C., et al. Impact of HER2 status on pathological response after neoadjuvant chemotherapy in early triple-negative breast cancer. Cancers. 2022;14(10) doi: 10.3390/cancers14102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilie S.M., Briot N., Constantin G., et al. Pathologic complete response and survival in HER2-low and HER2-zero early breast cancer treated with neoadjuvant chemotherapy. Breast Cancer. 2023 doi: 10.1007/s12282-023-01490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denkert C., Seither F., Schneeweiss A., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S., Liu T., Kuang X., et al. Comparison of clinicopathological characteristics and response to neoadjuvant chemotherapy between HER2-low and HER2-zero breast cancer. Breast. 2023;67:1–7. doi: 10.1016/j.breast.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miglietta F., Griguolo G., Bottosso M., et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 2022;8(1):66. doi: 10.1038/s41523-022-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of this article will be made available by contacting the corresponding author.