Abstract
The hippocampus (HC) and the orbitofrontal cortex (OFC) jointly encode a map-like representation of a task space to guide behavior. It remains unclear how the OFC and HC interact in encoding this map-like representation, though previous studies indicated that both regions have different functions. We acquired the functional magnetic resonance imaging data under a social navigation task in which participants interacted with characters in a two-dimensional “social space.” We calculate the social relationships between the participants and characters and used a drift-diffusion model to capture the inner process of social interaction. Then we used multivoxel pattern analysis to explore the brain-behavior relationship. We found that (i) both the HC and the OFC showed higher activations during the selective trial than the narrative trial; (ii) the neural pattern of the right HC was associated with evidence accumulation during social interaction, and the pattern of the right lateral OFC was associated with the social relationship; (iii) the neural pattern of the HC can decode the participants choices, while the neural pattern of the OFC can decode the task information about trials. The study provided evidence for distinct roles of the HC and the OFC in encoding different information when representing social space.
Keywords: hppocampus, orbitofrontal cortex, social cognition, decision-making, decoding
Introduction
Understanding how humans represent social relationships in social contexts is important for studying social behavior. Map-like representations, which integrate information obtained through observation for reasoning or guiding behavior (Epstein et al. 2017), may also be constructed and used in social interactions. Studies have found the existence of map-like representations in various social contexts. For instance, Tavares et al. (2015) found that the brain could track the location of participants in a two-dimensional social space when they were performing social interactions in a role-playing game Park et al. (2020) found that a map-like representation could be constructed by learning the social hierarchy in the task and could further guide the reasoning about the social information. Moreover, the map-like representations were also found in other types of context, such as defining sensory space based on olfactory (Bao et al. 2019), concept learning (Theves et al. 2019; Theves et al. 2020), and transitive inference (Morton et al. 2020). Although there were differences between the tasks used in the studies above, studies jointly illustrated the characteristics of a map-like representation, that is, consisting of the Euclidean metric (distance and angle) and being capable of facilitating non-spatial behaviors.
The hippocampus (HC) is considered a crucial brain region for map-like representation. The HC is capable of supporting episodic memories that consist of spatial or temporal information and integrating them into map-like representations (Eichenbaum and Cohen 2014). Another important function of the HC is to track the current location in either physical (Derdikman and Moser 2010) or non-physical spaces (i.e. the structure of a cognitive task) (Stachenfeld et al. 2017). In addition, the parahippocampus (PHC), which is anatomically and functionally related to the HC (Insausti and Amaral 2012), is involved in encoding map-like representations. The PHC was related to the reorientation using landmarks when navigating in a physical space with map-like representation (Sutton et al. 2012).
The orbitofrontal cortex (OFC) is also associated with map-like representations in task spaces. The main function of the OFC is to support the value encoding during value-based decision-making (Rushworth et al. 2011; Padoa-Schioppa and Conen 2017; Murray and Rudebeck 2018). In addition, the OFC is involved in behaviors without extrinsic value, for instance, social cognition and social behavior. When cooperating with other people, the OFC showed stronger activation compared with competing with other people (Decety et al. 2004). Patients with OFC lesions showed difficulty in following social norms (Beer et al. 2006). Thus, these studies indicated that the OFC may have a more general function of representing the task space using map-like representations (Wilson et al. 2014; Schuck et al. 2016; Wikenheiser and Schoenbaum 2016; Zhou et al. 2019) across value-based or other types of tasks.
Previous studies also reported the relationship between the HC and the OFC in representing a task space. The OFC was found to encode map-like representation of a task space together with the HC by receiving the inputs from the HC (Wikenheiser et al. 2017). In an associative learning task, the HC and the OFC jointly represented the task structure (Mızrak et al. 2021). However, the HC and the OFC, although contributing to map-like representations, show some levels of domain specificity in their information processing and more evidence is needed to clarify the exact roles of the HC and the OFC in representing map-like representation (Wikenheiser and Schoenbaum 2016). There was also evidence supporting that the HC and the OFC had specialized functions in representing the task space. During social decision-making, the OFC, rather than the HC, was found to be capable of representing the personality traits which was defined in a map-like form (Kobayashi et al. 2022). In concept learning, the HC was suggested to encode the distance in a map-like concept space which was important for acquiring novel concepts (Theves et al. 2019; Theves et al. 2020).
To provide a better understanding of the neural mechanism of the HC and the OFC in representing map-like representation and guiding behavior, we performed a task-fMRI study to analyze the dissociable contribution of the HC and the OFC in representing the task space. We used the datasets from Zhang et al. (2022), in which the participants performed a role-playing social navigation task adapted from Tavares et al. (2015) with functional magnetic resonance imaging (fMRI), to explore the specific function of the HC and the OFC at a more fine-grained level. The social navigation task simulates real-life social interactions as a dynamic process within a two-dimensional (affiliation and power) social space, which allowed us to better explore the role of the HC and the OFC. In the social navigation task, participants were required to interact with characters on both dimensions in certain trials by making choices, in which the characters’ locations in the social space might change depending on their choices. The dimension of affiliation (including warmth, intimacy, trustworthiness) and power (including competence, dominance) are the two key factors that define social relationships in social psychology, and their interactions is the fundamental element of social perception (Wiggins 1979; Fiske 2012). In such a space, participants needed to construct a map-like two-dimensional social space to represent the social relationship.
We applied several approaches to explore the role of the HC and the OFC in the task. For the behavioral data, we measured the geometric social relationship between the participants and characters by vectors, which reflected the results of final social interactions. Moreover, we used the drift-diffusion model (DDM; Wiecki et al. 2013) as the computational model to analyze the behavioral data in the study, allowing us to decompose the complex psychological processes behind social behavior. The DDM describes the psychological process of social interaction by modeling trial-by-trial reaction times using several free parameters, including the evidence accumulation and decision threshold (see Materials and method for details). For the fMRI data, we used the intersubject representational similarity analysis (IS-RSA) to explore the brain-behavior relationship. The IS-RSA constructed a “behavioral similarity” matrix and we used IS-RSA to explore whether participants who were more similar in their neural response in the HC and the OFC were also more similar in their behavior (i.e. the individual difference). To test the robustness of the brain-behavior relationship, we conducted a multivariate pattern analysis (MVPA) decoding based on the neural pattern of the HC and the OFC and the behavioral data.
According to the previous studies, it could be inferred that HC might be capable of encoding the map-like representation of the task space and track one’s location in the space, while the OFC might be capable of encoding the task-related context information which was associated with the map-like representation of the task space in social context. Specifically, we hypothesized that (i) the HC was involved in representing the task space and tracking the characters’ location in the space; (ii) the OFC was involved in representing the task space and encoding the social information in the task; and (iii) the neural activity of the HC and the OFC was capable of reflecting different aspects of individual difference in the task.
Materials and methods
Participants
This study recruited 41 healthy adult students from South China Normal University (SCNU). All of the participants had normal or corrected-to-normal vision. None of them had a history of neurological or psychiatric disorders. The study was approved by the Human Research Ethnics Committee of SCNU. Each participant provided written informed consent prior to the experiment and was compensated for their participation. To increase the statistical power and the robustness, we used a different head movement exclusion criterion (mean framewise displacement > 0.2 mm) by following (Zhang et al. 2022) to ensure a larger sample size. Two participants’ data were excluded from further analysis because of excessive head movement and one participant’s data were also excluded because of a technical glitch which resulted in the field-map images not being saved after the scanning. Thus, a total of 38 participants’ data (18 M/20F, mean age = 20.21, SD = 1.30, ranged 18–23 years old) were used in the following analysis.
Social navigation task
The social navigation task was adapted from Tavares et al. (2015). In this task, the participants were required to go through a storyline in the form of role-playing and to interact with six fictional characters to achieve two goals, finding a job and renting a house. The six fictional characters could be further divided into five main characters and one neutral character, that is, a bystander. The participants needed to interact with the five main characters on two dimensions, affiliation and power. In the task, a two-dimensional social space representing the social relationship between the participants and the main characters was constructed. Before any interactions, all the main characters had the same initial affiliation and power in the social space. For the neutral character, the interaction was on neither the affiliation dimension nor the power one but was merely neutral greetings. To avoid any possible gender-related stereotypes, we used two versions (I and II) of the social navigation task in which the male characters in version I were switched to female characters in version II and vice versa.
There were a total of 216 trials in the task, which could be categorized into two types: narrative trials (153 trials) and selective trials (63 trials). In the narrative trials, the information, including the background of the story, personal information about the characters, and the relationship between the characters, was presented to the participants. In each selective trial, the participants were asked to interact with a specific character. There were two options for the participants to choose, each representing a different way of interaction. The participants made a choice between two options by pressing a button (1 or 2) on a keypad. Participants needed to perform 12 trials with each of the five main characters and 3 trials (neutral trials) with the neutral character. According to the definition of social space, the 12 trials could be further divided into two trial types (i.e. the dimensions of trials), including 6 affiliation trials in which participants choose whether to accept engagement in intimate interactions with others or not and 6 power trials in which participants to choose whether to accept instructions/demands from others or not (see examples in Table S2). The task design is shown in Fig. 1A.
Fig. 1.
Task design. (A) the schematic of the social navigation task. Participants needed to find a job and to rent a house in the role-playing game. The social navigation task included two types of trials, narrative trials and selective trials (affiliation dimension). In the narrative trials, the participants needed to watch slides that provided information about the story or the words of a character in a dialog box. The narrative trials lasted 2–10 s, depending on the length of the text. In the selective trials, the participants were required to make a choice from two options (whether they interacted with the character or not) in 12 s. After the participant made their choice, a blank screen was presented for (12—Response time) seconds. (B) the two-dimensional social space defined by the social navigation task. In the social space, the x-axis represents the affiliation dimension, and the y-axis represents the power dimension. The coordinate (x, y) represents the location of the specific main character in the social space and would vary according to the participants’ response in the selective trials. The dot (0, 0) on the left side of the x-axis is defined as the initial point of the main character before having any interactions. The dot (6, 0) on the right side of the x-axis is defined as the point of view of the participants. The line between the character’s origin and the main character indicates the moving trajectory of the main character, which varied along with the choice of a participant in the selective trials. For instance, in the schematic, the main character went through three selective trials in the following order: (i) power increased by one, (ii) affiliation increased by one, and (iii) power increased by one. After finishing the task, the main character stopped at a specific location in the space and the vector from the point of view to the final location of the main character was determined in the 2D space. The vector length (L) indicates the social distance between the participants and the main character and the angle (θ) indicates the first-person orientation of the participant.
The trial sequence was fixed in the task and the duration of the task was also controlled to ensure that all the participants experienced identical storylines. Specifically, for each narrative trial, the duration was fixed and ranged from 2 to 10 s depending on the length of text. For each selective trial, the maximum response time was limited to 12 s. If the participants responded within 12 s, a blank screen was presented after their response to fill the remaining time of the 12 s (lasted 12 s—response time). If participants failed to respond within 12 s, the blank screen was skipped and the next trial started. The complete task lasted 25 min, and the response time of the participants, the trial type (narrative or selective), the trial contexts (only available in selective trials, including affiliation, power, and neutral), and the onset of the trials were recorded. The task was programmed using PsychoPy 3.0 (http://psychopy.org).
MRI data acquisition
All imaging data were collected on a 3 T Siemens Trio MRI scanner with a 32-channel phased-array head coil. The fMRI data were obtained using a single-shot simultaneous multi-slice or multi-band gradient-echo EPI sequence with the following parameters: repetition time (TR) = 1200 ms, echo time (TE) = 41.6 ms, flip angle = 52°, slice acceleration factor = 5, field of view (FOV) = 211 mm × 211 mm, data matrix = 88 × 88, slice thickness = 2.4 mm without inter-slice gap, voxel size = 2.4 × 2.4 × 2.4 mm3, anterior-to-posterior phase encoding direction (A≫P), and 65 interleaved axial slices covering the whole brain. To correct for susceptibility-induced geometric distortions and MRI signal loss in the acquired functional images, we also acquired a field map of the whole brain by using a double-echo FLASH sequence. The locations of the slices and the geometric properties for the field-map scan were the same as those in the fMRI scan. Specifically, the sequence parameters for the field-map scan were: TR = 735 ms, TE1/TE2 = 5.04 ms/7.50 ms, flip angle = 60°, FOV = 211 mm × 211 mm, voxel size = (2.4 mm)3, and 65 axial slices. In addition, high-resolution brain structural images were acquired using a T1-weighted 3D MP-RAGE sequence with the following parameters: TR = 1600 ms, TE = 2.98 ms, flip angle = 9°, slice thickness = 1 mm, FOV = 256 mm × 256 mm, data matrix = 256 × 256, voxel size = 1× 1 × 1 mm3, and 176 sagittal slices covering the whole brain.
The MRI scan procedure was as follows: the scanning started with a short localizer scan, followed by a functional field-map scan, the resting-state fMRI (rs-fMRI) before task, a task-fMRI scan of the social navigation task, the rs-fMRI after task, a T1-weighted brain structural scan, the diffusion tensor imaging (DTI) scan, a DTI field-map scan, and a T2-weighted 3D brain structural scan. All the scans were completed in the same session and took approximately 70 min and all participants underwent the same MRI scan procedure.
Functional data preprocessing
Functional images were preprocessed using fMRIPrep (ver 21.0.2) (Esteban et al. 2019), which is based on Nipype 1.6.1 (Gorgolewski et al. 2011). The preprocessing included the following steps: (i) estimation of head-movement in 6-parameters and slice-timing correction, (ii) alignment between functional images to T1-weighted structural images, (iii) spatial normalization to the (2 mm)3 MNI standard space, (iv) signal distortion correction with the field-map, and (v) estimation of the confounds, including the mean global signals of brain white matter and cerebrospinal fluid (CSF) as well as framewise displacement (FD) which estimates the head movement over time for a participant. Spatial smoothing was applied with an isotropic, Gaussian kernel of 6-mm full-width half-maximum (FWHM). In addition, for each participant, the preprocessed fMRI data were denoised using independent component analysis (ICA) for Automatic Removal of Movement Artifacts (ICA-AROMA) and the movement components were removed in the MNI space. A high band pass-filter of 1/100 Hz was applied to the time-series.
Calculation of geometric social relationships
The analysis of behavioral data mainly focused on calculating the final location of the main characters in the social space. Referring to Tavares et al. (2015), we first defined and calculated the coordinates which represented the location of the certain main character in the two-dimensional (affiliation for the x-axis and power for the y-axis) social space. The character’s origin (0,0) was defined as the initial location of the main characters, assuming that the main characters were neutral for the participants on both affiliation and power before any interactions. The location of the main characters changed as the task proceeded. In the selective trials, the main characters moved one unit in a single direction (power or affiliation) depending on the trial context. For instance, for a specific selective trial in the affiliation dimension, if the interaction option was chosen by the participant (e.g. giving a hug to the main character), the coordinate of the main character would increase by 1 on the x-axis. If the non-interaction option in the same trial was chosen by the participant (e.g. refused to give a hug), the coordinate of the main character would decrease by 1 on the x-axis. The participant needed to interact with each main character 6 times on each dimension, so the possible coordinates ranged from (−6, −6) to (6,6).
Next, we calculated the vectors which represent the social relationship between participants and main characters in geometric form. For each main character, the final coordinate, that was, the final location of the specific main character in the social space after 12 trials, was calculated. The vectors were defined by the final coordinates and the point of view of the participants in the social space. The point of view, which represented the location of the participants themselves in the social space, was defined as (6, 0), with the highest intimacy (most intimate with themselves, maximum 6 in the social navigation task) and the neutral of power (neither dominating nor being dominated by themselves, 0 in the social navigation task). We calculated the lengths of the vectors (L) and the angles of the vectors (cos θ) for each main character using the following formulas:
 |
where x and y are the final coordinates for a main character, ranging from −6 to 6; L indicates the absolute social distance between a participant and a main character; cos θ represents the normalized function of the power modulated by affiliation. The illustration of the geometric social relationships is shown in Fig. 1B.
Effects of main characters on decision-making
We performed a single linear mixed model (LMM) to explore the effect of each character on the decision-making: LMM: 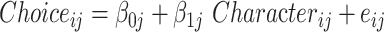 , where Character denotes the main characters of the social navigation task. Choice denotes whether the participants chose the option that increased the affiliation or power of the main characters or not (coded as 1 if they chose to increase, coded as 0 if not), i and j indicate the ith trials of the jth participant (i = 1 to 12 for each main characters in the current study), and
, where Character denotes the main characters of the social navigation task. Choice denotes whether the participants chose the option that increased the affiliation or power of the main characters or not (coded as 1 if they chose to increase, coded as 0 if not), i and j indicate the ith trials of the jth participant (i = 1 to 12 for each main characters in the current study), and 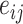 is the residuals. The intercept of the jth participant,
is the residuals. The intercept of the jth participant, 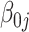 , varied across the participants with a random intercept. The LMM model parameters were estimated using the lmeTest package in R language (https://github.com/runehaubo/lmerTestR).
, varied across the participants with a random intercept. The LMM model parameters were estimated using the lmeTest package in R language (https://github.com/runehaubo/lmerTestR).
Fitting the drift-diffusion model
To capture the participants’ decision-making behavior in the social space, we used a model-based approach to estimate the parameters describing the participants’ decision-making process. Drift-diffusion models (DDMs), which are widely used to estimate binary-choice decision-making processes, have become a popular tool for evaluating dynamic decision processes, especially in social cognition and decision-making (Ratcliff et al. 2016). In the DDMs, four parameters are defined to describe an individual’s choice behavior. These include the drift rate (v), decision threshold (a), initial bias (z), and non-decision time (ndt). The drift rate (v) refers to the speed of evidence accumulation. The higher the drift rate the more rapid the evidence accumulation during the decision-making. The decision threshold (a) was used to define the distance between the two decision boundaries (i.e. binary options). Smaller thresholds indicate that it is easier to reach the decision boundaries, suggesting a faster but less accurate decision process. The initial bias (z) refers to the starting point in the decision-making process, for instance, showing a preference toward the upper boundary (z > 0.5) or the lower boundary (z < 0.5). The non-decision time (ndt) illustrates the response time phase, which is unrelated to the evidence accumulation process during decision-making, such as perceptual processing (reading the text) and motor execution (eye/head movement). The schematic of the DDM is illustrated in Fig. 2.
Fig. 2.

Schematic of the drift-diffusion model (DDM) for the two-option decision tasks. The upper and lower boundaries indicate the two options in the selective trials. The threshold (a) between the two boundaries reflects the difficulty of making a decision; specifically, a smaller threshold means that the participants more easily reached one of the two boundaries to make a choice. The starting point is theoretically located in the middle of the two boundaries but may shift toward one of the boundaries (i.e. having preference toward one of the options). The initial bias (z) measures the initial preference toward the options at the beginning of the decision-making process. The drift rate (v) indicates the speed of evidence accumulation in the decision-making process. Participants with larger drift rates (v) responded more quickly. The dashed line represents the response time unrelated to evidence accumulation (ndt). The curve between the two thresholds simulates the decision-making process based on evidence accumulation. The curves above and below the upper and lower boundaries represent the reaction time (RT) distribution of the trials in which the participants select the corresponding option.
Because the social navigation task had fewer selective trials, a hierarchical Bayesian framework was used to enhance the statistical power and to diminish the impact of outliers (Wiecki et al. 2013). By applying the hierarchical Bayesian framework, we first extracted the parameters of the DDM for each participant from the group-level, and then estimated both the group-level and the individual-level parameters by using Bayesian Markov chain Monte Carlo (MCMC) sampling. The response time (RT) and the participants’ choices (i.e. to increase the affiliation or power of the main character or not) in the selective trials in the social navigation task were used to fit the DDM. Five MCMC chains with 10,000 samples including the first 1,000 samples, which served as burn-in and were discarded, were estimated. The Gelman–Rubin approach was used to assess the convergence of the MCMC chains. If all variables in the model had  <1.01, the model was convergent. The model fitting and parameter estimation were conducted using the python-based toolbox HDDM 0.9.6 (Wiecki et al. 2013). Since we had no prior hypothesis about whether participants would prefer one option vs. the other in the task, the fitting of DDM took an initial bias (z) as a constant (z = 0.5).
<1.01, the model was convergent. The model fitting and parameter estimation were conducted using the python-based toolbox HDDM 0.9.6 (Wiecki et al. 2013). Since we had no prior hypothesis about whether participants would prefer one option vs. the other in the task, the fitting of DDM took an initial bias (z) as a constant (z = 0.5).
Region of interest (ROI) definition
For the fMRI data analysis, we selected the HC and the PHC (together as HC_PHC ROI), and the OFC as the ROIs in accordance with our hypotheses. In addition, to ensure the specificity, we included the motor region (the postcentral gyrus), which is not considered to be related to the representation of social space, as the control region. These ROIs were defined bilaterally using the Brainnetome atlas (Fan et al. 2016), a fine-grained parcellation based on functional and structural connectivity. The ROIs are shown in Fig. 4A.
Fig. 4.
The ROIs and results of the fMRI analysis (GLM1 and MVPA). (A) Selection of ROIs. These ROIs were defined according to the Brainnetome atlas. The OFC covers the orbital gyrus, including BAs 11, 12, 13, and 47. The HC_PHC covers both the HC and the PHC. The ROI of the motor region, which serves as a control ROI, covers the postcentral gyrus, mainly BAs 1, 2, and 3. (B) Regions showing significant higher activation in the selective trials than in the narrative trials. The results were obtained from the voxel-based GLM analysis for the whole-brain. The color bar indicates the range of t-values. Abbreviations: OFC, orbitofrontal cortex. (C) Clusters with a high decoding accuracy (> 0.6) in the choices of the participants in the social navigation task in the ROI-based analysis of the HC. (D) Clusters with a high decoding accuracy (> 0.6) in the dimension (affiliation or power) of the current trial in the social navigation task in the ROI-based analysis of the OFC. For better visualization, only clusters with t > 3.1 and cluster sizes > 50 voxels are shown in the figure. Abbreviations: L (R), left (right) hemisphere.
Univariate analysis of the fMRI data for trial types
To identify which brain regions were involved in processing the selective trials than narrative trials, we used a general linear model (GLM1) to analyze the preprocessed fMRI data. In the participant-level analysis of the GLM1, we included eight regressors, including: (i) the effect of the narrative trials and (ii) the effect of the selective trials, and six motion parameters. The contrast between the selective trials and the narrative trials was defined. After the β values of the parameters were estimated at the participant level, we performed a group-level analysis to determine the brain regions that were significantly related to the contrast between the selective trials and the narrative trials. A Gaussian random field correction was used to control for multiple comparisons. The significant threshold at the voxel level was set to P < 0.05 (one-sample t-test) and the results were corrected by the cluster-defining threshold Z > 3.1 (cluster-level P < 0.001). The univariate analyses were performed using FSL/Feat (ver 6.0.4; https://fsl.fmrib.ox.ac.uk).
Inter-subject representational similarity analysis
To identify the brain regions that reflected individual difference in the behavioral data, for instance, the vector in the social navigation task and the parameters in the DDM, we conducted inter-subject representational similarity analyses (IS-RSA) (Finn et al. 2020) based on both the behavioral data and the preprocessed fMRI data without spatial smoothing and ICA-AROMA. The IS-RSA can explore the brain-behavior relationships by calculating the similarity between a behavioral similarity matrix based on a certain behavioral index and a neural pattern similarity matrix across all the participants.
Four behavioral similarity matrices were constructed in the study. We first calculated four types of behavioral data, including: i) the average lengths of the vectors (mean L) in the task space; (ii) the average angles of the vectors (mean cos θ) in the task space; (iii) the estimated individual-level drift rate (v) of the DDM; and (iv) the estimated individual-level threshold (a) of the DDM. Next, for each type of behavioral data, we ranked all the participants’ data using rankdata from the python package SciPy and calculated the pairwise Euclidean distance between each pair of participants based on the nearest neighbors (NN) model (i.e. participants with closer specific behavioral data should be more similar to one another, regardless of where they fall on the behavioral data). Then, the Euclidean distance metrics were converted to similarity matrices using Adjacency from the python package nltools.
To construct the neural pattern similarity matrices, we extracted the timeseries of the fMRI data using a parcellation method and calculated the similarities. For each participant, the timeseries were extracted from each region in the Brainnetome atlas (246 regions in total) by averaging the signal across all voxels for the given region. Then, similar to the calculation of the behavioral similarity matrices, we calculated the similarity of the timeseries based on the pairwise correlation distance based on the NN model and transformed the correlation distance into similarities (1—correlation distance) in the ROIs defined above, including the HC_PHC, the OFC, and the motor region.
After calculating the behavioral similarity matrices and neural pattern similarity matrices, we tested the brain-behavior similarities. For each ROI, the non-parametric Mantel test was used to test the significance of the brain-behavior similarity. Specifically, the participants were randomly permuted for one of the two similarity matrices (5000 permutations), and the correlation between the two matrices was re-calculated to form a null distribution of surrogate correlation values. Then, the correlation coefficient was compared to the null distribution to obtain the P-values for each region for each participant. The P-values were further corrected using false discovery rate (FDR, with q = 0.05) for multiple comparison correction with the permutation sign test (5000 permutations). The IS-RSA was conducted using the python package nltools and the schematic is shown in Fig. 3.
Fig. 3.
Schematic of the inter-subject representational similarity analysis (IS-RSA). For a given behavioral similarity matrix, ranks were first assigned to the behavioral data, dealing with ties appropriately. Next, the matrix was constructed based on the pairwise Euclidean distance between each pair of participants’ rank using a nearest neighbors (NN) model. For the neural similarity matrix, a parcellation mask was used to segment the whole brain into many regions to extract the time-series. Then, for each region, a matrix was constructed based on the pairwise correlation distance between each pair of participants’ timeseries using the NN model. Finally, a permutation-based mantel test was used to compare these matrices.
Multivoxel pattern analysis decoding
A trial-based analysis was performed to obtain the input data of the subsequent MVPA approach. We conducted a trial-based general linear model (GLM2) to estimate the BOLD coefficient (beta-values) for each trial and integrated these beta-values into beta-maps based on the preprocessed fMRI data used in IS-RSA. Nine regressors were defined in the trial-based GLM2, including the onset of selective trials and other eight regressors as nuisance (six motion parameters and the signal of white matter and cerebrospinal fluid). These regressors were then convolved with the hemodynamic response functions. The least squares-separate method was applied to the GLM2 to generate beta values. The estimation of the beta-maps was conducted with the python package Nibetaseries (https://github.com/HBClab/NiBetaSeries).
To explore the functional differentiation of the HC_PHC and OFC, we used a ROI-based searchlight MVPA approach to test whether the neural pattern of the two brain regions could decode different behavioral information of the social navigation task. According to our hypothesis, the neural pattern in the HC should be capable of decoding the choice of participants in the selective trials (i.e. whether they chose to interact with main characters on the dimension of affiliation or power, which was related to the increase/decrease of the value on that dimension and the change of the location in the space). For the neural pattern of the OFC, it should be capable of decoding the social relationships between the participants and main characters, for instance, the vector length or the vector angle in the social navigation task, which was associated with social cognition. A support vector machine (SVM) was used as the classifier in the MVPA decoding.
The ROI-based searchlight MVPA decoding was conducted as follows: (i) the beta-maps obtained from the trial-based GLM2 were taken as the input data. (ii) A k-fold approach was applied to the input data to define the training set and testing set. Specifically, the input data was divided into k (k = 4 in the current study) groups of samples, and the prediction was trained using k-1 folds (training set) and tested in the left-out fold (testing set). (iii) A searchlight sphere with a 5 mm radius moved progressively over the whole brain and iterated on the volume. (iv) The neural activity of each voxel was selected as the feature to train the two SVM classifiers in the training set, one for the decoding of the decision-making behavior (the choice of participants in the social navigation task) and the other for the decoding of task information (the dimension of the current trial). (v) After the SVM classifiers were trained, they were applied to decode the choice or the task information of each trial in the testing sets. We used a 4-fold cross validation so that the above steps were repeated four times with different training and testing sets. The individual-level MVPA decoding was performed using the python toolbox Nilearn.
After conducting the MVPA of decoding on the individual-level, we obtained MVPA maps for each participant. These MVPA maps contained the voxels that were capable of distinguishing the choice of participants or the task information together with the scoring (i.e. accuracy in the cross validation) of the voxels. We set a threshold of scoring > 0.6 on the MVPA maps to select the voxels that performed better in the decoding and smoothed the MVPA maps with a 4 mm FWHM kernel. Subsequently, the MVPA maps were assembled for the group-level inference using a one-sample permutation test (5000 permutations) and a family-wise error (FWE) correction was applied to the group-level result for a multiple comparison correction. The group-level inference and the voxel-wise FWE correction were conducted using FSL/Randomize (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise).
Results
Geometric social relationships and decision-making preferences
By conducting the LMM, we found a significant main effect of the characters on participants’ choices (F(1,4) = 17.89, P < 0.001). Considering that participants’ decision-making in the social navigation task was related to the geometric social relationships, such as the vector length, we also calculated the average vector length (L) of each main character and compared the difference in the average vector length between characters, as shown in Fig. S1A (Supplementary Materials). We found that only the difference in L between main character 1 (L1) and main character 4 (L4) was significant (L1 = 5.63 ± 2.28, L4 = 7.69 ± 2.13, t (37) = −4.34, pBonferroni < 0.001, Cohen’s d = 0.93) after correction for multiple comparisons using a Bonferroni approach.
From fitting the DDM, we found that all chains had an autocorrelation close to 0 and that all variables had 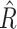 < 1.01, indicating a successful convergence of all five chains to our target posterior distributions. The estimated group-level parameters were as follows: drift rate (v) = 0.14, threshold (a) = 3.11, and non-decision time (ndt) =1.68. The individual-level parameters distribution is presented in Fig. S1B (Supplementary Materials).
< 1.01, indicating a successful convergence of all five chains to our target posterior distributions. The estimated group-level parameters were as follows: drift rate (v) = 0.14, threshold (a) = 3.11, and non-decision time (ndt) =1.68. The individual-level parameters distribution is presented in Fig. S1B (Supplementary Materials).
To explore the relationship between decision-making preference and social interactions, we also calculated Pearson’s correlation between the drift rate (v), threshold (a), and the average vector length. We found that the drift rate (v) was significantly negatively correlated with the length of the vectors (r = −0.41, P < 0.01). No significant correlation between the average vector length and the average response time (r = −0.20, P = 0.22).
Brain activation in the selective trials relative to the narrative trials
Figure 4B shows the significant activation brain clusters for the contrast between the selective trials and the narrative trials, which were obtained from the GLM1 in the whole-brain analysis. Specifically, we found significantly higher activation in the left HC (t = 4.35, peak MNI: −18, −6, −8, cluster size = 59 voxels) and the right PHC (t = 4.48, peak MNI: 30, −42, −4, cluster size = 64 voxels) for the selective trials than the narrative trials. Moreover, we also found higher activation in the OFC in the selective trials than narrative trials, including Brodmann’s area (BA) 10 (t = 4.98, peak MNI: 20, 46, 6, cluster size = 316 voxels) and BA 11 (t = 4.78, peak MNI: −14, 26, −10, cluster size = 227 voxels). In addition, there were other regions showed significantly higher activation in selective trials than narrative trials, including the bilateral temporopolar area, right putamen, motor region (BA 1, BA 5, and BA 6), ventral posterior cingulate cortex (vPCC), dorsal anterior cingulate cortex (dACC), and the superior temporal gyrus. The detailed information about the significant clusters obtained from the whole-brain analysis is provided in Table S1 (Supplementary Materials).
Inter-subject correlation effect of HC and OFC
By conducting the IS-RSA, we found that the neural pattern of the HC and the OFC were associated with the task-related indexes. Specifically, the similarity of the neural pattern in the right HC (MNI: 29, −27, −10, r = 0.11, pFDR < 0.05) was significantly positively correlated with the similarity of the drift rates (v) of the participants. That is, the participants who were more similar in the speed of the evidence accumulation process were also more similar in their neural pattern in the right HC. Moreover, the similarity in the neural pattern of the lateral OFC (lOFC, MNI: 42, 31, −9, r = 0.09, PFDR < 0.05) was significantly positively correlated with the similarity in the average length of the vectors of the participants. The participants who moved a more similar distance in the two-dimensional social space were also more similar in their neural pattern of the right lOFC. In addition, no brain region was found to be significantly correlated with either the decision threshold (a) or the average vector angle (cos θ).
Functional differentiation of the HC and OFC in social navigation
Figure 4C and D shows the clusters that were capable of decoding the task-related information obtained from the MVPA. For the decoding of the participants’ choices, the clusters located in the bilateral PHC (left: peak MNI: −32, −26, −20, cluster size = 73 voxels; right: peak MNI: 22, −22, −20, cluster size = 69 voxels) and the right HC (peak MNI: 30, −10, −22, cluster size = 54 voxels) were found to be significant at PFWE < 0.05 level, as shown in Fig. 4C. No significant cluster was found in either the OFC or the motor region when decoding the participants’ choices.
For the decoding of the task information, we found that clusters in the OFC decoded the task information (power or affiliation) of a specific selective trial. These clusters were mainly located in the bilateral BA 11 (left: peak MNI: 22, 26, −16, cluster size = 59 voxels; right: peak MNI: −6, 44, −18, cluster size = 55 voxels, both pFWE < 0.05), as shown in Fig. 4D. No cluster was found in either the HC or the motor region when decoding the task information of the current trial.
Discussion
The current study explored the different functions of the HC and the OFC in a role-playing social navigation task using fMRI. From the behavioral analysis, we found that the participants who accumulate evidence faster were more likely to stay closer to the character’s origin location in the social space. From the fMRI data analysis, we found that the left HC, the right PHC, and the OFC showed significantly higher activations in the selective trials than in the narrative trials. Also, we found significant higher activation in the vPCC, the dACC, the temporopolar area, and the motor region in selective trials than narrative trials. The neural activity of the HC was correlated with the evidence accumulation of participants, whereas the neural activity of the lateral OFC was correlated with the average vector length between the participants and the main characters in the two-dimensional social space. The neural pattern of the HC was capable of decoding a participant’s choice, and the neural pattern of the OFC was capable of decoding the dimension of the current trial.
Involvement of the HC and OFC in social interaction
We found that the left HC, the right PHC and the OFC showed significantly higher activations in the selective trials than in the narrative trials (Fig. 4B, Table S1). In the social navigation task, we found that the location of the main characters in the social space varied depending on the participants’ choice in the selective trials; while in the narrative trials, we found the location of the main characters remained unchanged. Referring to previous studies showing that the HC could track one’s location in space (Derdikman and Moser 2010; Stachenfeld et al. 2017), we inferred that HC was recruited in the selective trials to track the location of main characters during social interaction. The connection between the HC and the PHC was associated with the memory formation, navigation, and temporal dynamics (Van Strien et al. 2009). The activation of the HC and the PHC indicated that a similar process of navigating in the social space might happen when navigating in the social space during a task. The stronger activation of the OFC was also observed in the selective trials than the narrative trials in the task. The OFC is recognized as one of the regions involving in social interaction (Decety et al. 2004; Beer et al. 2006). However, compared with other social cognition related brain regions, for instance, the temporopolar area and temporo-parietal junction that are responsive to more general social cognition, the OFC is more likely to be activated only in specific types of social interaction (Rilling et al. 2002; Shi et al. 2023). Previous studies also found that the OFC is capable of encoding specific variables in social interaction (Jiang et al. 2021). The exact role of the OFC in selective trials still needed to be validated by other results.
We also found several other regions, including the vPCC, dACC, temporopolar area, and motor region, were associated with the selective trials (Fig. 4B and Table S1). In particular, we found significant higher activation of the vPCC in the selective trials than narrative trials. The retrosplenial cortex (RSC), a part of the vPCC, was found to be implicated in human spatial orientation (Epstein et al. 2007; Epstein 2008). The function of spatial orientation even extended from the RSC to the vPCC (Burles et al. 2018). In the selective trials, the dimensions indicated the orientation in the social space, for instance, affiliation represented the direction along the y-axis. Thus, the activation of the vPCC also supported the spatial orientation function of the vPCC. The dACC was found to be capable of tracking a person’s position in a social hierarchy (Kumaran et al. 2016). This indicated that the dACC might share a similar function with the HC in a task. The temporopolar area was commonly reported in social cognition studies as the temporo-parietal junction and the medial prefrontal cortex (Rilling et al. 2004; Amodio and Frith 2006). This could explain the role of the temporopolar area in selective trials for supporting social interaction. We also observed a significant activation of the motor region in the selective trials; this could be related to the neural response of the participants when making a choice by pressing buttons on a keypad.
Inter-subject correlation of the HC and the OFC
By conducting the IS-RSA, we observed that the neural pattern of the right HC reflected individual differences in the speed of evidence accumulation. In addition, the neural pattern of the lateral OFC reflected the individual differences on the preference of social interactions.
A DDM can describe decision-making as drift-process accumulating evidence over time until reaching one of the two boundaries and initiating the corresponding response. Studies have found that the HC might be the brain region supporting the evidence accumulation process (Umbach et al. 2020; Nieh et al. 2021). For instance, Nieh et al. (2021) found that the neurons in the dorsal HC could jointly encode the accumulated evidence about the spatial position in mice when performing a decision-making task. In the social navigation task of the current study, we found that the evidence accumulation processes also existed before the participants made a decision as to whether to interact with the main characters or not, and participants’ choices were closely related to the location of the main characters in the social space. These evidence accumulation processes required the involvement of the HC. In addition, we also found that the neural pattern of the HC also reflected individual differences in evidence accumulation.
The individual difference in average vector length, which reflected the results of social interaction, was correlated with the lateral OFC. The main function of the OFC is to evaluate associations between stimuli and outcomes, especially in value-based decision-making (Rolls 2000; Rushworth et al. 2011; Rudebeck and Murray 2014). Specifically, the medial OFC encodes reward value and pleasantness, and the lateral OFC encodes punishment value, unpleasantness, and non-reward (Rolls et al. 2020). The lateral OFC was also found to be associated with social cognition. For instance, the gray matter volume in the lateral OFC could predict social influence (Campbell-Meiklejohn et al. 2012). Xia et al. (2015) found that the lateral OFC supported the mechanism of applying social information in political decision-making. Consistent with Xia et al. (2015), we found that the lateral OFC was capable of encoding social information and applying the information to decision-making in a task space.
HC decoded choice and the OFC decoded dimension
By performing the searchlight MVPA, we found that the clusters in the HC were capable of decoding the choices of the participants (Fig. 4C) and that the clusters in the OFC could decode the dimensions (affiliation or power) of the current trial (Fig. 4D). In the social navigation task, if the participants chose to interact with a main character, the coordinates of the main character in the social space would increase by 1 on the specific dimension. If the opposite option was chosen, the coordinate of the main character in the social space would decrease by 1. The ability to decode the choice in the HC was in line with previous studies that showed that the HC could track the location of an individual in space (Eichenbaum and Cohen 2014; Tavares et al. 2015; Stachenfeld et al. 2017). However, the HC could not decode the dimension of the current trial. Combining these results, the HC can only track the moving direction (i.e. to increase the affiliation or power of the main character or not) in a single dimension in the task. We further inferred that participants might consider the single dimension in the current trial when making decisions, although the space in the task was two-dimensional. This phenomenon was also found by Park et al. (2020), their study found that the participants learned a two-dimensional social hierarchy but only used the single task-relevant dimension when making an inference.
The result of the MVPA also showed that the OFC was capable of decoding the dimension of the current trial (Fig. 4D). This result was consistent with previous studies that indicated that the OFC could represent task structure (Wikenheiser et al. 2017; Park et al. 2020). Yet the OFC could not decode the choice of participants in the current trial, which was somewhat different from the function of the interaction of HC and OFC in value-based decision-making. For instance, in a value-based decision-making task, the HC was capable of encoding a task space based on value and the OFC was capable of guiding individual’s decision-making behavior based on the information of the space (Knudsen and Wallis 2020). The reason might be that there was no extrinsic value or reward in the options in the social navigation task compared with typical economic decision-making tasks. In addition, the choices represented the social interaction behavior, which was not directly related to the reward. Thus, the OFC might not straight-forwardly guide participants’ choice during the task. The inconsistent result of the HC and OFC in decoding revealed that it was worthy to explore and compare the HC-OFC interaction across different contexts and different types of spaces in future studies.
Limitations and conclusions
There were several limitations in the current study. First, lateralization of the HC and the parahippocampus was observed in our study, but we did not investigate this further. In the whole-brain univariate analysis, we found the lateralized activation of the left HC and the right parahippocampus, and also in the IS-RSA, we also found a correlation between the neural pattern of the right HC and the drift rate (v). The lateralization of the HC is commonly seen in the map-like representation related behavior, for instance, spatial navigation. A meta-analysis conducted by our group found the existence of lateralization among 47 fMRI studies in spatial navigation (Li et al. 2021). To explore the lateralization is a little bit beyond the current study. Future studies are encouraged to take the lateralization of the HC and the OFC into account in order to provide a better understanding of the mechanism of representing the task space. Second, only the positive activations were considered in the univariate analysis in the current study. The negative activation, although still having controversy in fMRI studies, is also worthy of further exploration. Third, the number of the selective trials in the social navigation task was relatively small, preventing us from exploring the effect of each main character on each dimension in the storyline. Participants only interacted with a specific main character on one dimension a total of 6 times, which was insufficient to conduct statistical tests. In addition, the sample size in the current study was limited; preventing us from using other machine learning methods, for instance, support vector regression, to increase the robustness of the results of the IS-RSA for exploring and validating the individual differences of the brain-behavioral relationship.
In conclusion, we studied the function of the HC and the OFC in a social navigation task which constructed a two-dimensional social space by social interactions. We found that the HC was associated with the decision-making process which related to the spatial location in the task space, while the OFC was associated with the context-related task information which constituted the task space. Our study provided evidence for the distinct roles that the HC and the OFC played in representing task structure in social context, which might also provide better understanding of social behavior.
Supplementary Material
Acknowledgments
We thank the participants who contributed their time and energy to this work.
Contributor Information
Jiajun Liao, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Jinhui Li, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Yidan Qiu, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Xiaoyan Wu, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Bingyi Liu, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Lu Zhang, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Yuting Zhang, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Xiaoqi Peng, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
Ruiwang Huang, School of Psychology, Center for Studies of Psychological Application, Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, Guangdong, China.
CRediT statement
TaxonomyJiajun Liao (Conceptualization, Formal analysis, Methodology, Visualization, Writing—original draft, Writing—review & editing), Jinhui Li (Conceptualization, Formal analysis, Methodology, Writing—review & editing), Xiaoyan Wu (Investigation, Methodology, Visualization, Writing—review & editing), Bingyi Liu (Resources, Validation, Writing—review & editing), Lu Zhang (Conceptualization, Data curation, Resources, Software), Yuting Zhang (Formal analysis, Writing—review & editing), Xiaoqi Peng (Visualization, Writing—review & editing), Ruiwang Huang (Conceptualization, Funding acquisition, Project administration, Supervision, Writing—review & editing)
Funding
National Natural Science Foundation of China (Grant numbers: 32371101 and 82171914), National Key Research and Development Program of China (2018YFC1705000), and Natural Science Foundation of Guangdong Province (2022A1515011022).
Conflict of interest statement: The authors declare no competing financial interests.
Data and code availability
Behavioral data and codes (for the linear mixed model) used in the present fMRI study are available on Github (https://github.com/JJliao13/Social_Navigation).
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006:7(4):268–277. 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bao X, Gjorgieva E, Shanahan LK, Howard JD, Kahnt T, Gottfried JA. Grid-like neural representations support olfactory navigation of a two-dimensional odor space. Neuron. 2019:102(5):1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006:18(6):871–879. [DOI] [PubMed] [Google Scholar]
- Burles F, Umiltá A, McFarlane LH, Potocki K, Iaria G. Ventral—dorsal functional contribution of the posterior cingulate cortex in human spatial orientation: a meta-analysis. Front Hum Neurosci. 2018:12:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Kanai R, Bahrami B, Bach DR, Dolan RJ, Roepstorff A, Frith CD. Structure of orbitofrontal cortex predicts social influence. Curr Biol. 2012:22(4):R123–R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. NeuroImage. 2004:23(2):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Moser M-B. A dual role for hippocampal replay. Neuron. 2010:65(5):582–584. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014:83(4):764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008:12(10):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 2007:27(23):6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nat Neurosci. 2017:20(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder Met al. 2019. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016:26(8):3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Glerean E, Khojandi AY, Nielson D, Molfese PJ, Handwerker DA, Bandettini PA. Idiosynchrony: from shared responses to individual differences during naturalistic neuroimaging. NeuroImage. 2020:215:116828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske ST. Warmth and competence: stereotype content issues for clinicians and researchers. Can Psychol Can. 2012:53(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinformatics. 2011:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Hippocampal formation. In: Mai JK, Paxinos G, editors The human nervous system, 3rd rev. San Diego (MA): Elsevier; 2012. pp. 871–914. [Google Scholar]
- Jiang M, Wang M, Shi Q, Wei L, Lin Y, Wu D, Liu B, Nie X, Qiao H, Xu L, et al. Evolution and neural representation of mammalian cooperative behavior. Cell Rep. 2021:37(7):110029. [DOI] [PubMed] [Google Scholar]
- Knudsen EB, Wallis JD. Closed-loop theta stimulation in the orbitofrontal cortex prevents reward-based learning. Neuron. 2020:106(3):537–547.e4. https://linkinghub.elsevier.com/retrieve/pii/S0896627320301021 [accessed 2023 Jan 17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kable JW, Hsu M, Jenkins AC. Neural representations of others’ traits predict social decisions. Proc Natl Acad Sci. 2022:119(22):e2116944119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Banino A, Blundell C, Hassabis D, Dayan P. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron. 2016:92(5):1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang R, Liu S, Liang Q, Zheng S, He X, Huang R. Human spatial navigation: neural representations of spatial scales and reference frames obtained from an ALE meta-analysis. NeuroImage. 2021:238:118264. 10.1016/j.neuroimage.2021.118264. [DOI] [PubMed] [Google Scholar]
- Mızrak E, Bouffard NR, Libby LA, Boorman ED, Ranganath C. The hippocampus and orbitofrontal cortex jointly represent task structure during memory-guided decision making. Cell Rep. 2021:37(9):110065. https://linkinghub.elsevier.com/retrieve/pii/S2211124721015515 [accessed 2023 Jan 17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NW, Schlichting ML, Preston AR. Representations of common event structure in medial temporal lobe and frontoparietal cortex support efficient inference. Proc Natl Acad Sci. 2020:117(47):29338–29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Rudebeck PH. Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci. 2018:19(7):404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Schottdorf M, Freeman NW, Low RJ, Lewallen S, Koay SA, Pinto L, Gauthier JL, Brody CD, Tank DW. Geometry of abstract learned knowledge in the hippocampus. Nature. 2021:595(7865):80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Conen KE. Orbitofrontal cortex: a neural circuit for economic decisions. Neuron. 2017:96(4):736–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SA, Miller DS, Nili H, Ranganath C, Boorman ED. Map making: constructing, combining, and inferring on abstract cognitive maps. Neuron. 2020:107(6):1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, McKoon G. Diffusion decision model: current issues and history. Trends Cogn Sci. 2016:20(4):260–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002:35(2):395–405. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind within interpersonal interactions. NeuroImage. 2004:22(4):1694–1703. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000:10(3):284–294. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020:2(2):fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014:84(6):1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011:70(6):1054–1069. [DOI] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, Niv Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron. 2016:91(6):1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Meisner OC, Blackmore S, Jadi MP, Nandy AS, Chang SW. The orbitofrontal cortex: a goal-directed cognitive map framework for social and non-social behaviors. Neurobiol Learn Mem. 2023:203:107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld KL, Botvinick MM, Gershman SJ. The hippocampus as a predictive map. Nat Neurosci. 2017:20(11):1643–1653. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Twyman AD, Joanisse MF, Newcombe NS. Geometry three ways: an fMRI investigation of geometric information processing during reorientation. J Exp Psychol Learn Mem Cogn. 2012:38(6):1530–1541. [DOI] [PubMed] [Google Scholar]
- Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D. A map for social navigation in the human brain. Neuron. 2015:87(1):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theves S, Fernandez G, Doeller CF. The hippocampus encodes distances in multidimensional feature space. Curr Biol. 2019:29(7):1226–1231. [DOI] [PubMed] [Google Scholar]
- Theves S, Fernández G, Doeller CF. The hippocampus maps concept space, not feature space. J Neurosci. 2020:40(38):7318–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach G, Kantak P, Jacobs J, Kahana M, Pfeiffer BE, Sperling M, Lega B. Time cells in the human hippocampus and entorhinal cortex support episodic memory. Proc Natl Acad Sci. 2020:117(45):28463–28474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien N, Cappaert N, Witter M. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci. 2009:10(4):272–282. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Sofer I, Frank MJ. HDDM: hierarchical Bayesian estimation of the drift-diffusion model in python. Front Neuroinform. 2013:7. 10.3389/fninf.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JS. A psychological taxonomy of trait-descriptive terms: the interpersonal domain. J Pers Soc Psychol. 1979:37(3):395–412. [Google Scholar]
- Wikenheiser AM, Schoenbaum G. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci. 2016:17(8):513–523. 10.1038/nrn.2016.56[accessed 2023 Jan 17]. http://www.nature.com/articles/nrn.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron. 2017:95(5):1197–1207.e3. 10.1016/j.neuron.2017.08.003[accessed 2023 Jan 17]. https://linkinghub.elsevier.com/retrieve/pii/S0896627317306943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014:81(2):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Stolle D, Gidengil E, Fellows LK. Lateral orbitofrontal cortex links social impressions to political choices. J Neurosci. 2015:35(22):8507–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen P, Schafer M, Zheng S, Chen L, Wang S, Liang Q, Qi Q, Zhang Y, Huang R. A specific brain network for a social map in the human brain. Sci Rep. 2022:12(1):1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Montesinos-Cartagena M, Wikenheiser AM, Gardner MPH, Niv Y, Schoenbaum G. Complementary task structure representations in hippocampus and orbitofrontal cortex during an odor sequence task. Curr Biol. 2019:29(20):3402–3409.e3. https://linkinghub.elsevier.com/retrieve/pii/S0960982219310905[accessed 2023 Jan 17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral data and codes (for the linear mixed model) used in the present fMRI study are available on Github (https://github.com/JJliao13/Social_Navigation).





