Abstract
Mounting evidence suggests considerable diversity in brain aging trajectories, primarily arising from the complex interplay between age, genetic, and environmental risk factors, leading to distinct patterns of micro- and macro-cerebral aging. The underlying mechanisms of such effects still remain unclear. We conducted a comprehensive association analysis between cerebral structural measures and prevalent risk factors, using data from 36,969 UK Biobank subjects aged 44–81. Participants were assessed for brain volume, white matter diffusivity, Apolipoprotein E (APOE) genotypes, polygenic risk scores, lifestyles, and socioeconomic status. We examined genetic and environmental effects and their interactions with age and sex, and identified 726 signals, with education, alcohol, and smoking affecting most brain regions. Our analysis revealed negative age-APOE-ε4 and positive age-APOE-ε2 interaction effects, respectively, especially in females on the volume of amygdala, positive age-sex-APOE-ε4 interaction on the cerebellar volume, positive age-excessive-alcohol interaction effect on the mean diffusivity of the splenium of the corpus callosum, positive age-healthy-diet interaction effect on the paracentral volume, and negative APOE-ε4-moderate-alcohol interaction effects on the axial diffusivity of the superior fronto-occipital fasciculus. These findings highlight the need of considering age, sex, genetic, and environmental joint effects in elucidating normal or abnormal brain aging.
Keywords: Alzheimer’s disease, APOE, brain aging, DTI & ROI volumes, lifestyle
Introduction
Aging is a complex biological process involving numerous molecular, metabolic, and cellular pathway alterations (Johnson et al. 1999; Ferrucci et al. 2020; Finch and Cohen 1997). Brain aging, characterized by age-related changes in brain structure such as regional morphometrics and white matter (WM) tract wiring patterns, plays a crucial role in reflecting and mediating aging risk factors (Gomez-Pinilla et al. 2008; Jolly et al. 2016; Logtenberg et al. 2021; Ye et al. 2021). A wealth of literature exists on human brain aging, with common findings including diminishing brain volumes, expanding ventricles, and cognitive decline (Fletcher et al. 2018), loss of WM microstructural integrity (Vernooij et al. 2009), and brain regions particularly susceptible to aging-related changes such as frontal and temporal cortex, putamen, thalamus, and accumbens (Fjell and Walhovd 2010). However, the heterogeneity in aging trajectories is complex in terms of brain structure, brain function, and presence or absence of specific cognitive and behavioral symptoms (Hueluer and Dodge 2018; Tang 2014; Naftali Raz et al. 2010). These heterogeneities primarily stem from a wide range of demographic, genetic, and environmental exposures, including non-modifiable factors such as age (Bethlehem et al. 2022), sex (Cowell et al. 1994; Murphy et al. 1996; Ingalhalikar et al. 2014), and genetics (Elliott et al. 2018; Zhao et al. 2019; Thompson et al. 2001), and modifiable factors like lifestyle choices (Gallinat et al. 2006; Hamer et al. 2018; Jensen et al. 2021; Daviet et al. 2022) and socioeconomic status (SES) (Nyberg et al. 2021; Kweon et al. 2022). These risk factors collectively and cumulatively affect an individual’s brain structure. Current studies primarily focus on individual risk factors, lacking a comprehensive understanding of brain structural regulation within this multifactorial biological system. Thus, it is essential to investigate how the interplay of these factors influences brain health and aging disparities.
Age, sex, and Apolipoprotein E (APOE) genotypes are three major non-modifiable risk factors that have been extensively studied for their impact on human brain aging. Age serves as a fundamental reference point for examining changes that occur over time. Research has shown that APOE-related effects display sex-dependent (Cowell et al. 1994; Coffey et al. 1998; Altmann et al. 2014) and age-dependent associations. One longitudinal investigation (Régy et al. 2022) discovered that APOE-ε4 carriers exhibited increased annual atrophy in brain temporal regions, highlighting a significant age-APOE-ε4 interaction. In contrast, research (Operto et al. 2019) has reported no age-APOE-ε4 interactions on WM diffusivity. While other investigations (Kuo et al. 2020) have examined the association of APOE-ε2 with aging-related clinical outcomes, the existing literature does not report associations on interactions of APOE-ε2 with age. In response to these findings, one large-scale brain-wide association study is being conducted to explore the synergistic effects between age, sex, and APOE-ε2/-ε4 genotypes.
Beyond the APOE gene, numerous other genetic factors may impact brain aging. One approach to account for these factors is through the polygenic risk score (PRS), a cumulative index that synthesizes the effects of numerous common genetic variants on a particular trait or disorder. PRSs have been used to investigate the associations of brain structure and various brain disorders, such as schizophrenia (SCH) (Ranlund et al. 2018; van der Merwe et al. 2019; Grama et al. 2020; Stauffer et al. 2021) or Alzheimer’s disease (AD) (Chandler et al. 2020). In this study, to differentiate the potential impacts of PRS and APOE on brain aging, we extend our analyses beyond the APOE gene by including PRSs from a wide array of brain diseases and behavioral factors. These factors encompass AD (Braskie et al. 2011), attention deficit hyper-activity disorder (ADHD) (Krain and Castellanos 2006), anxiety disorder (Wang et al. 2016), Autism spectrum disorder (ASD) (Shukla et al. 2011), bipolar disorder (Strakowski et al. 1999), major depression disorder (MDD) (Vasic et al. 2015), obsessive-compulsive disorder (OCD) (Pujol et al. 2004), Panic disorder (PD) (Han et al. 2008), SCH (Davis et al. 2003), and Tourette syndrome (TS) (Fredericksen et al. 2002).
In addition to the non-modifiable factors discussed above, numerous modifiable factors, such as lifestyle factors and SES, contribute to brain diseases and structural changes. The 2020 report of the Lancet Commission on dementia prevention, intervention, and care estimated that 40% of dementia cases could potentially be prevented or delayed through modifications of 12 major risk factors. Among these lifestyle factors, smoking, alcohol consumption, healthy diet, and physical activity have been extensively studied, as they are closely related to the risk of cardiovascular and neurodegenerative diseases.
Recent studies highlight a significant association between the structural and genetic attributes of both the heart and brain (Zhao et al. 2023). The brain depends on the heart to supply it with oxygen and essential nutrients. In turn, the heart receives modulatory signals from the brain that can influence its rhythm and contractile force. The disruption in cardiovascular function, due to risk factors, can impede blood flow to the brain. This intrinsic connection between the heart and brain emphasizes the necessity of exploring how cardiovascular risk factors impact brain health.
Smoking has been identified as a well-established cardiovascular risk factor (Ambrose and Barua 2004; Doll et al. 2004; Cox et al. 2019) and is associated with risks of brain disorders (Zhong et al. 2015; van Ewijk et al. 2015) and accelerated brain aging (Habes et al. 2016; Smith et al. 2019; Cole 2020; Ning et al. 2020). Similarly, heavy alcohol intake has been extensively researched and linked to cardiovascular disease (Ronksley et al. 2011; Piano 2017) as well as negative impact on brain health, such as accelerated brain volume loss (Pfefferbaum et al. 1992) and the loss of WM integrity (Daviet et al. 2022). However, the impact of moderate alcohol consumption on brain aging is more complex and remains a subject of debate.
Moderate alcohol consumption, defined as up to one drink per day for women or up to two drinks per day for men, has been associated with a lower risk of cardiovascular disease due to its potential effects on high-density lipoprotein cholesterol levels (Rimm et al. 1999; O’Keefe et al. 2007) and possible anti-inflammatory and antithrombotic properties (Brien et al. 2011). Since cardiovascular health is closely related to brain health, it is important to consider the potential impact of moderate alcohol consumption on brain structure. The findings in this area have been controversial, potentially due to differences in study design, sample size, or population characteristics. Some studies have reported moderate alcohol intake as being positively associated with whole brain volume (WBV) in elderly subjects (Gu et al. 2014), while others found no effect (de Bruin et al. 2005) or even adverse effect (Topiwala et al. 2017) on brain outcomes or cognition with moderate alcohol consumption. Study (Downer et al. 2014) reported moderate alcohol consumption could be associated with greater cognitive decline in learning and memory only among APOE-ε4 carriers, suggesting genetic risk factors may modify the effects of lifestyle choices.
Although numerous studies have explored the effects of diet on overall brain health and cognitive decline (Gu et al. 2015; Luciano et al. 2017; Croll et al. 2018; Jensen et al. 2021; Macpherson et al. 2021), there is limited research examining its effects on specific brain regions such as the visual and sensorimotor areas. In addition, other modifiable factors such as exercise, sleep quality, and education, also contribute to brain aging. Jointly analyzing the effects of modifiable and non-modifiable factors on brain structure is vital for understanding aging and brain health. However, most existing literature regarding the interactions among age, sex, genetic factors, and lifestyle only focused on cognitive functions (Lyall et al. 2019) or dementia incidence (Lourida et al. 2019), or limited brain structural traits (Mulugeta et al. 2022).
The purpose of this study is to conduct a comprehensive association analysis of genetic factors, lifestyle factors, SES, age, and sex with brain structure. To measure brain structure, we generated 101 brain regional volumes and 110 diffusion tensor imaging (DTI) measures of WM integrity based on the magnetic resonance imaging (MRI) data of >35,035 UKB subjects. We also extracted relevant risk factors of interest, including APOE genotypes, 16 PRSs, demographic information, lifestyle factors, and SES from these UKB subjects. Figure 1 depicts the study design, which integrates multimodal data sources. The large sample size of UKB enables association analysis with high statistical power and replicability. Findings may provide new insights into the nature and timing of dementia risks underlying various health disparity conditions.
Fig. 1.
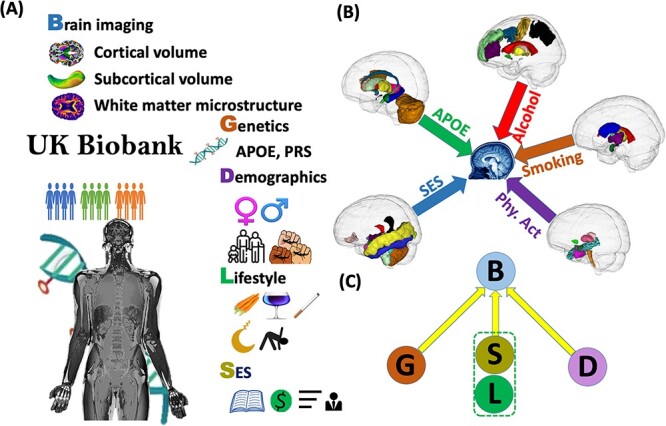
The study design. (A) Multi-omics data in the UKB. (B) Identified brain regions with structural changes associated with APOE, alcohol, smoking, physical activity (Phy. Act.), and SES. (C) A schematic diagram illustrating the associations we analyzed between genetics, demographics, lifestyle, SES factors, and brain structural traits in this study.
Methods
Study population
The UKB released a dataset of 500,000 participants, of which >40,000 had MRI data. Through data processing, we obtained DTI phenotype data of 37,123 subjects from UKB phase 1, 2, and 3, comprising both genetic and lifestyle information. Our analyses of WM were restricted to 35,035 unrelated individuals with DTI phenotypes, genetic, and lifestyle information available. Our primary discoveries were based on the 32,320 white British UKB subjects, which we validated on the remaining 2715 non-British ancestry subjects. For the study of brain volume, we obtained volumetric phenotype data for 39,216 UKB samples and restricted the brain volumetric analysis to 36,969 independent individuals with volumes for regions-of-interest (ROIs), genetic information, and lifestyle information available. Our primary discoveries were based on 34,097 white British subjects, which we validated on the remaining 2872 non-British ancestry subjects.
Imaging data processing
The T1 MRI and diffusion-weighted MRI datasets were processed using the procedures described in Zhao et al. (2019) and Zhao et al. (2021), respectively. Further details on image acquisition, preprocessing, and phenotype generation can be found in SI Appendix. We generated 101 brain global and regional volumetric phenotypes of 39,216 UKB individuals, including the WBV, WM, gray matter (GM), and cerebrospinal fluid (CSF), and 110 DTI phenotypes of 37,123 UKB individuals, including the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD), radial diffusivity (RD), and mode of diffusivity (MO) for 21 WM tracts. Subjects with any values greater than five times the median absolute deviation from the median value were considered as outliers and removed from the regression analyses.
APOE genotyping
The ε4 allele of the APOE gene is a well-established risk factor of AD. Two variants of single-nucleotide polymorphisms (SNPs), the rs429358 and rs7412, are used to determine the APOE genotype, resulting in three different alleles (APOE-ε2, APOE-ε3, and APOE-ε4) and six genotypes (APOE-ε3/APOE-ε3, APOE-ε3/APOE-ε4, APOE-ε2/APOE-ε3, APOE-ε4/APOE-ε4, APOE-ε2/APOE-ε4, and APOE-ε2/APOE-ε2) (Zhong et al. 2016). Unlike the APOE-ε4, the APOE-ε2 has been found to reduce the risk of AD risk and is the least common allele in the population, while the APOE-ε3 is the most common and considered to be risk-neutral (Ward et al. 2012). The APOE genotyping information was extracted from the imputed SNP data from the UKB resources. Among the 35,035 unrelated subjects used in the main discovery and validation of 110 DTI phenotypes, APOE genotypes are distributed as follows: 770 subjects (2.2%) are APOE-ε4 homozygous, with 420 male and 350 female; 202 subjects (0.58%) are APOE-ε2 homozygous, with 93 male and 109 female; 20,770 subjects (59.3%) are APOE-ε3 homozygous, with 9929 male and 10,841 female; 4330 subjects (12.4%) are APOE-ε2/APOE-ε3, with 2032 male and 2298 female; 833 subjects (2.4%) are APOE-ε2/APOE-ε4, with 374 male and 459 female; and 8130 subjects (23.2%) are APOE-ε3/APOE-ε4, with 3737 male and 4393 female. The allele frequencies of ε2, ε3, and ε4 are 7.9, 77.1, and 15.0%, respectively. Table 1 provides demographic information for each genotype.
Table 1.
Demographic info, lifestyle factors, and PRSs of the UKB subjects used in the association study on DTI phenotype.
| APOE-ε4 homozygote | APOE-ε3 homozygote | APOE-ε2 homozygote |
APOE-ε4/
APOE-ε3 |
APOE-ε2/
APOE-ε3 |
APOE-ε4/
APOE-ε2 |
Total | |
|---|---|---|---|---|---|---|---|
| Characteristicsa | (n = 770) | (n = 20,770) | (n = 202) | (n = 8130) | (n = 4330) | (n = 833) | (n = 35,035) |
| Age at baseline, mean(sd)/median(range) | |||||||
| 54.2 (7.26) | 54.9 (7.46) | 54.7 (7.67) | 54.5 (7.38) | 55.0 (7.41) | 54.8 (7.55) | 54.8 (7.44) | |
| 55.0 [40.0, 69.0] | 55.0 [40.0, 70.0] | 55.0 [40.0, 69.0] | 55.0 [40.0, 70.0] | 56.0 [40.0, 70.0] | 55.0 [40.0, 70.0] | 55.0 [40.0, 70.0] | |
| Age at brain imaging, mean(sd)/median(range) | |||||||
| 63.1 (7.30) | 63.8 (7.55) | 63.7 (7.88) | 63.3 (7.47) | 63.8 (7.50) | 63.7 (7.63) | 63.6 (7.53) | |
| 64.0 [47.0, 80.0] | 64.0 [45.0, 81.0] | 64.0 [46.0, 80.0] | 64.0 [46.0, 81.0] | 64.0 [45.0, 81.0] | 64.0 [47.0, 80.0] | 64.0 [45.0, 81.0] | |
| Ethnicity, number (%) | |||||||
| British | 725 (94.16) | 19,082 (91.87) | 187 (92.57) | 7535 (92.68) | 4002 (92.42) | 789 (94.72) | 32,320 (92.25) |
| Others | 45 (5.84) | 1688 (8.13) | 15 (7.43) | 595 (7.32) | 328 (7.58) | 44 (5.28) | 2715 (7.75) |
| Sex, number (%) | |||||||
| Male | 420 (54.55) | 9929 (47.8) | 93 (46.04) | 3737 (45.97) | 2032 (46.93) | 374 (44.9) | 16,515 (47.14) |
| Female | 350 (45.45) | 10,841 (52.2) | 109 (53.96) | 4393 (54.03) | 2298 (53.07) | 459 (55.1) | 18,520 (52.86) |
| Disease status, number (%) | |||||||
| Dementia | 0 (0) | 1 (0) | 1 (0.5) | 2 (0.02) | 2 (0.05) | 0 (0) | 6 (0.02) |
| Not dementia | 770 (100) | 20,769 (100) | 201 (99.5) | 8128 (99.98) | 4328 (99.95) | 833 (100) | 35,029 (99.98) |
| Family illness, number (%) | |||||||
| Father has AD | 128 (16.62) | 1764 (8.49) | 19 (9.41) | 1023 (12.58) | 365 (8.43) | 103 (12.36) | 3402 (9.71) |
| Mother has AD | 223 (28.96) | 3329 (16.03) | 25 (12.38) | 1855 (22.82) | 655 (15.13) | 189 (22.69) | 6276 (17.91) |
| Sibling has AD | 27 (3.51) | 261 (1.26) | 1 (0.5) | 191 (2.35) | 51 (1.18) | 20 (2.4) | 551 (1.57) |
| Marriage status, number (%)b | |||||||
| Living with a partner | 611 (79.35) | 16,077 (77.4) | 158 (78.22) | 6295 (77.43) | 3336 (77.04) | 624 (74.91) | 27,101 (77.35) |
| Not living with a partner | 65 (8.44) | 1521 (7.32) | 16 (7.92) | 629 (7.74) | 310 (7.16) | 74 (8.88) | 2615 (7.46) |
| Education, number (%) | |||||||
| College/university degree or above | 375 (48.7) | 9656 (46.49) | 88 (43.56) | 3750 (46.13) | 1978 (45.68) | 398 (47.78) | 16,245 (46.37) |
| No college/university degree or above | 350 (45.45) | 9636 (46.39) | 98 (48.51) | 3855 (47.42) | 2036 (47.02) | 374 (44.9) | 16,349 (46.66) |
| Social deprivation, number (%) | |||||||
| Above the Townsend deprivation index median | 307 (39.87) | 8750 (42.13) | 79 (39.11) | 3426 (42.14) | 1755 (40.53) | 334 (40.1) | 14,651 (41.82) |
| Below the Townsend deprivation index median | 463 (60.13) | 12,001 (57.78) | 123 (60.89) | 4697 (57.77) | 2568 (59.31) | 499 (59.9) | 20,351 (58.09) |
| Lifestyle factors, number (%) | |||||||
| Smoking status | |||||||
| Not current smoking | 732 (95.06) | 19,433 (93.56) | 192 (95.05) | 7603 (93.52) | 4061 (93.79) | 784 (94.12) | 32,805 (93.63) |
| Current smoking | 38 (4.94) | 1293 (6.23) | 10 (4.95) | 510 (6.27) | 263 (6.07) | 47 (5.64) | 2161 (6.17) |
| Doing regular physical activity | |||||||
| Yes | 586 (76.1) | 15,593 (75.07) | 153 (75.74) | 6075 (74.72) | 3265 (75.4) | 603 (72.39) | 26,275 (75) |
| No | 182 (23.64) | 5043 (24.28) | 49 (24.26) | 1997 (24.56) | 1043 (24.09) | 224 (26.89) | 8538 (24.37) |
| Healthy diet | |||||||
| Yes | 408 (52.99) | 10,133 (48.79) | 90 (44.55) | 4019 (49.43) | 2063 (47.64) | 398 (47.78) | 17,111 (48.84) |
| No | 362 (47.01) | 10,628 (51.17) | 112 (55.45) | 4105 (50.49) | 2265 (52.31) | 435 (52.22) | 17,907 (51.11) |
| Alcohol consumption | |||||||
| Excessive alcohol consumption | 203 (26.36) | 5842 (28.13) | 58 (28.71) | 2346 (28.86) | 1245 (28.75) | 216 (25.93) | 9910 (28.29) |
| Moderate alcohol consumption | 485 (62.99) | 12,666 (60.98) | 115 (56.93) | 4876 (59.98) | 2610 (60.28) | 529 (63.51) | 21,281 (60.74) |
| Not current drinking | 82 (10.65) | 2251 (10.84) | 29 (14.36) | 907 (11.16) | 474 (10.95) | 88 (10.56) | 3831 (10.93) |
| Sleeping between 6 and 8 h | |||||||
| Yes | 342 (44.42) | 9017 (43.41) | 89 (44.06) | 3645 (44.83) | 1810 (41.8) | 380 (45.62) | 15,283 (43.62) |
| No | 426 (55.32) | 11,706 (56.36) | 113 (55.94) | 4473 (55.02) | 2507 (57.9) | 451 (54.14) | 19,676 (56.16) |
aAll variables are measured at the baseline except the age at imaging.
bPercentiles do not add up to 1 due to missingness.
In our analyses, we included the number of APOE-ε4 allele and APOE-ε2 allele as two separate covariates in our models, to account for the additive effects of APOE-ε4 and APOE-ε2 alleles, respectively. This approach differs from some previous studies which categorized APOE-ε4 into two categories (APOE-ε4 homozygotes and all others), or considered APOE-ε2 homozygote and APOE-ε2/APOE-ε3 as APOE-ε2 carriers and the remaining four genotypes as controls. The results of models using such dichotomous categorization of APOE-ε4 and APOE-ε2 can be found in the SI Appendix.
Polygenic risk score
A wide range of heritable brain diseases and behavioral traits have been linked to variations in brain structure (Ranlund et al. 2018; Bittner et al. 2019; Kaufmann et al. 2019). In this study, we will focus on 10 diseases from the UNC PGC (https://pgc.unc.edu/for-researchers/download-results/), which include AD, ADHD, anxiety disorder, ASD, bipolar disorder, MDD, OCD, PD, SCH, and TS, as well as six behavioral factors, such as alcohol dependence, cognitive performance, education, opioid dependence, self-employment, and smoking. Each of these disorders and behavioral traits has been associated with common genetic variants through large-scale genome-wide association studies (GWAS) meta-analysis (Otowa et al. 2016; Stahl et al. 2019; Kunkle et al. 2019; Demontis et al. 2019; Grove et al. 2019; International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS) 2018; Forstner et al. 2021; Ripke et al. 2014; Yu et al. 2019; Walters et al. 2018; Benyamin et al. 2014; Lee et al. 2018; Polimanti et al. 2020; van der Loos et al. 2013; Liu et al. 2019), which can be combined to generate PRSs. A PRS represents an individual’s genetic susceptibility to a particular condition or behavior. We incorporated these PRSs in our models to investigate how genetic variations affect brain structure. Additional information regarding the generation and characterization of PRSs for each of the aforementioned clinical factors can be found in the SI Appendix.
Lifestyle and SES factors
Most of the lifestyle variables in our analysis were drawn following the same criterion as in the healthy lifestyle and dementia study (Lourida et al. 2019). Not being a current smoker, engaging in regular physical activity, and maintaining a healthy diet are well-established healthy lifestyle factors associated with a healthy brain. We categorized alcohol assumption into abstinence, moderate consumption, and excessive consumption due to contradicting evidence on moderate alcohol consumption (Sabia et al. 2018; Daviet et al. 2022). These four healthy lifestyle variables were collected through touchscreen questionnaires during the UKB baseline assessment. Smoking status was dichotomized into current-smokers and non-smokers. Following the American Heart Association guidelines, a minimum of 150 min or 5 d of moderate exercise per week, or a minimum of 75 min or at least 1 d of vigorous activity per week is considered regular physical activity. For alcohol, the maximum threshold of moderate alcohol consumption is 14 g/d for women and 28 g/d for men, and the abstinence includes people not currently drinking or drinking only on special occasions with an overall alcohol intake of 0 units per day. A healthy diet is defined as meeting the adequate intake of at least 4 out of the 7 dietary recommendations for cardiometabolic health (vegetables, fruits, fish, unprocessed meat, processed meat, whole grains, and refined grains) (Mozaffarian 2016). In addition, short and long sleep duration (sleeping <6 h or longer than 8 h) is also a risk factor for developing dementia (Palpatzis et al. 2022). Besides the above lifestyle factors, we also included education level and social deprivation factors in our analysis. Education level was divided into with or without a college or university degree or above. Social deprivation (SoDep) was measured by the Townsend deprivation index which corresponds to the postcode of each participant’s location. It was dichotomized into two groups, above the Townsend deprivation index median and below the median. Details with respect to how we produced these lifestyle and SES factors are provided in SI Appendix, Supplementary Text, and Table S1.
Covariates
In all the models, we adjusted for the participants’ partnership status (categorized into whether participants live with their partner), study phase (categorized into phase 1, phase 2, and phase 3), age at imaging, sex, and sex and age interaction. We adjusted for the WBV as a confounding covariate for analyses of all the other brain volumetric phenotypes.
Statistical analysis
Missing value imputations for covariates and imaging traits were included in the SI Appendix. We carried out four levels of multivariate linear regression models on the white British UKB subjects to answer the following questions: (i) Is there the APOE genotype and age interaction effect on DTI phenotypes and ROI volumes? (ii) Does the APOE-age interaction effect vary between males and females? (iii) Are there associations between DTI phenotypes or ROI volumes and healthy lifestyle factors, SES, and PRSs, and age interactions? (iv) Are there APOE genotype and environmental factor interaction effects on DTI phenotypes and ROI volumes?
To answer questions (i), we used the standardized 110 DTI measurements and 101 ROI volumes as the dependent variable and APOE-ε2, APOE-ε4 count, sex, age, PRSs, lifestyle factors, UKB study phase, marriage status, sex, and age as the predictor variables. We implemented Bonferroni correction for the P-values to adjust for multiple testing, with the threshold of significance for 211 independent testing set at approximately 2.37E-4 (0.05/211). To investigate the effect of APOE genotype and age interaction on DTI phenotypes and ROI volumes, we added these two-way interaction terms to our models. For identifying the difference of APOE genotype and age interaction effect on DTI phenotypes and ROI volumes in different sex groups in question (ii), the 3-way interaction term of age, sex, and APOE, and three 2-way interaction terms between age, sex, and APOE were included in the regression models, along with the full set of covariates. To address question (iii), two-way interaction terms between age and healthy lifestyle factors and SES were added separately. To address question (iv), we included APOE-environmental interactions and the age-environmental interaction, separately. Detailed statistical models we considered for the association between main covariates, two-way and three-way interactions and brain structure were displayed in SI Appendix, Supplementary text.
We verified our discovery on an independent set of non-British ancestry UKB subjects. The same association analyses were conducted in the non-British UKB populations using a confidence level of 0.05 as the cutoff. Due to the relatively small sample size of the validation set, we considered associations to be significant if they passed the Bonferroni corrected threshold of 2.37E-4 in our main discovery. The validation results serve to validate and demonstrate the robustness of findings. Throughout the paper, unless otherwise specified, we only report findings if they meet the following criteria: (i) they are significant among the white British population; (ii) They can be validated (showing the same direction of effects) in the non-British ancestry population. In addition, we discuss interaction effects with P < 0.01 among the white British population that can be validated by the non-British ancestry population. However, these effects will not be counted as significant findings unless their P-values pass the threshold of 2.37E-4. To interpret the interaction effect related with sex, the analyses were carried out separately for male and female groups (SI Appendix, Supplementary text).
Results
In this study, we included 36,969 unrelated participants aged 44–81 for brain volume analysis, of which 34,097 were white British. For WM diffusivity analyses, we included 35,035 participants, with 32,320 being white British. The sample of 35,035 participants comprised 16,515 (47%) males and 18,520 (53%) females. A total of 9191 (26%) individuals carried one APOE-ε4 allele, while 792 (2%) carried two. Demographic information is summarized in Table 1. Correlation plots for genetic factors, environmental (lifestyle and SES) factors, 101 ROI phenotypes, and 110 DTI traits (SI Appendix, Figs. S1–S3) indicated strong mutual correlations between brain structural traits, while correlations between environmental factors were much weaker (<0.1).
We conducted a 4-fold association analysis to study the effects of age, sex, and APOE two-way interactions, age-sex-APOE three-way interactions, APOE-environment interactions, and age-environment interactions on 110 DTI measurements and 101 ROI volumes. We considered PRSs, UKB study phase, marriage status, and WBV (for ROI volume only) as additional covariates to control for potential confounders (see Methods and SI Appendix, Supplementary Text).
Our primary findings were based on multiple linear regression models for each brain imaging trait using white British subjects, which were validated in non-British populations. Demographic information in white British and non-British populations were summarized in Supplementary Tables S3 and S4, respectively. Compared with the white British population, the non-British population was slightly younger. They had a lower proportion of individuals whose mothers had AD and who lived with a partner. Besides, they exhibited a higher proportion of individuals with advanced education, high Social Deprivation Index scores, current smoking habits, and excessive alcohol consumption. There were no significant differences in other demographic factors.
We identified a total of 331 significant associations for ROI volumes and 395 for DTI traits, including both main and interaction effects. The identified effects sizes (standardized coefficients) were reproducible, with correlations 0.87 (SI Appendix, Fig. S15(A)) for ROI volumes and 0.96 (Fig. S16(A)) for DTI traits between British and non-British populations. Our analysis revealed that education, alcohol, and smoking were the top three common environmental factors that influence most regions (SI Appendix, Figs S17 and S18).
Genetic associations with brain structural architecture
Our investigation into the relationships between brain structure, the APOE gene, age, sex, and PRSs yielded several intriguing discoveries.
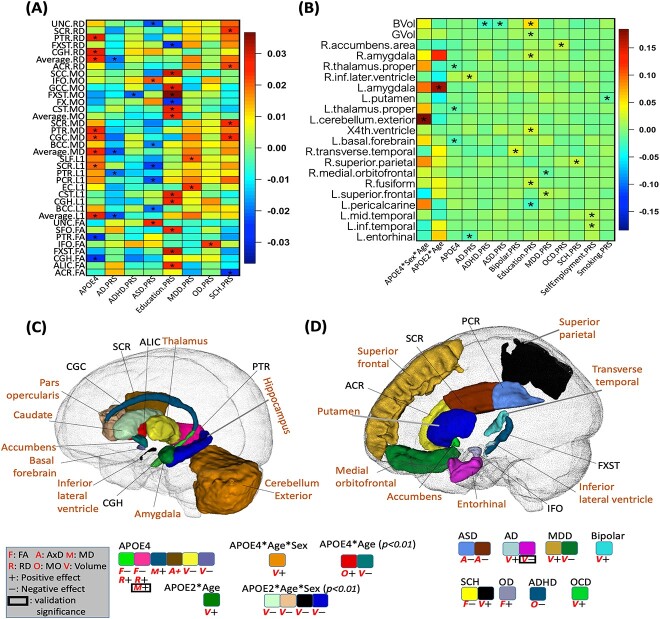
Of particular note, for the APOE-environment interaction, we identified a positive interaction (P = 2.22E-4) between APOE-ε4 and moderate alcohol consumption on the AxD of the superior fronto-occipital fasciculus (SFO) (SI Appendix, Fig. S20). This positive interaction suggests that the combination of the presence of the APOE-ε4 gene and moderate alcohol consumption may synergistically increase the risk of losing WM integrity. No significant APOE-environmental interaction effect was found on brain volumes (SI Appendix, Fig. S19).
A positive age-sex-APOE-ε4 interaction (P = 1.9E-04) was observed in relation to volumetric variations in the left cerebellum. Specifically, we observed that among APOE-ε4 carriers, the volume of the left cerebellar exterior decreases at a slower rate in males than in females (Fig. 2B). Furthermore, we found a negative age-APOE-ε4 interaction effect on the volume of amygdala only in females (SI Appendix, Fig. S5). These findings support prior studies (Altmann et al. 2014) suggesting that female carriers of APOE-ε4 have a heightened susceptibility to AD. In contrast, among APOE-ε4 non-carriers, negative age-sex interactions were widely observed on the volume of WM, GM, and the majority of cortical and subcortical regional volumes (SI Appendix, Fig. S7), suggesting accelerated brain volume reduction among males as opposed to females in the general population. This implied that the effect of sex diverges between APOE-ε4 carriers and non-carriers.
Fig. 2.
Results on genetic components. The heatmaps display standardized coefficients of the genetic (APOE, PRS) and age, sex, and gene-related interaction effects on WM diffusivity (A) and volume (B). An asterisk (*) denotes the 2.37E-4 significance level (the Bonferroni corrected significance level). The identified brain regions and signals on volume (shown with “V”) and diffusivity (shown with “F,” “A,” “M,” “R,” and “O” for FA, AxD, MD, RD, and MO, respectively) are shown for APOE-related effects (C) and PRS-related effects (D). In (C) and (D), we display the statistical association results with consistent directions between British and non-British ancestry populations. Imaging phenotypes at particular brain regions with P < 0.05 in non-British ancestry populations are marked by black box. Only columns with associations that pass the 2.37E-4 significance level are included in (A) and (B), while in (C) and (D) we also include additional interaction effects with P-values <0.01 but >2.37E-4. The direction of effects is denoted by “+” for positive and “−” for negative. PRS, polygenic risk score; FA, fractional anisotropy; AxD, axial diffusivity; MD, mean diffusivity; RD, radial diffusivity; MO, mode of diffusivity.
More interestingly, we found a positive age-APOE-ε2 interaction effect on left amygdala volume (P = 4.84E-06), while for right amygdala volume, such effects were only present in females (Fig. 2B and C and SI Appendix, Figs. S5, S11 and S12). This suggests that the directions of APOE-ε4 and APOE-ε2 interactions with age are opposite for amygdala volume, and both interactions are more pronounced in females.
In terms of well-established findings, our results corroborate the association of APOE-ε4 with reduced volumes of thalamus and basal forebrain, as well as the FA of the cingulum hippocampus (CGH) and posterior thalamic radiation (PTR). In addition, APOE-ε4 was positively associated with the RD, MD, and AxD of the CGH, PTR, superior corona radiata, and cingulum cingulate (CGC) (Fig. 2A–C). Such findings regarding the main effect of APOE-ε4 align well with previous studies (Operto et al. 2018; Novellino et al. 2019).
As for the PRS, we discovered that 15 PRSs were associated with brain structure (Fig. 2A, B, and D). Specifically, for the larger AD PRS, we observed an increase in ventricular volume (inferior lateral ventricle, P = 1.1E-4) and a decrease in the volume of the entorhinal cortex (P = 4.8E-5). This suggests that AD-related genetic information, beyond the APOE gene, also contributes to changes in brain structure.
Associations between smoking and brain structure
No interaction effects between smoking and age were observed, indicating that the impact of smoking on brain aging within this age range (44–81) may predominantly manifest as alterations to baseline brain structure values rather than influencing the rate of change over time.
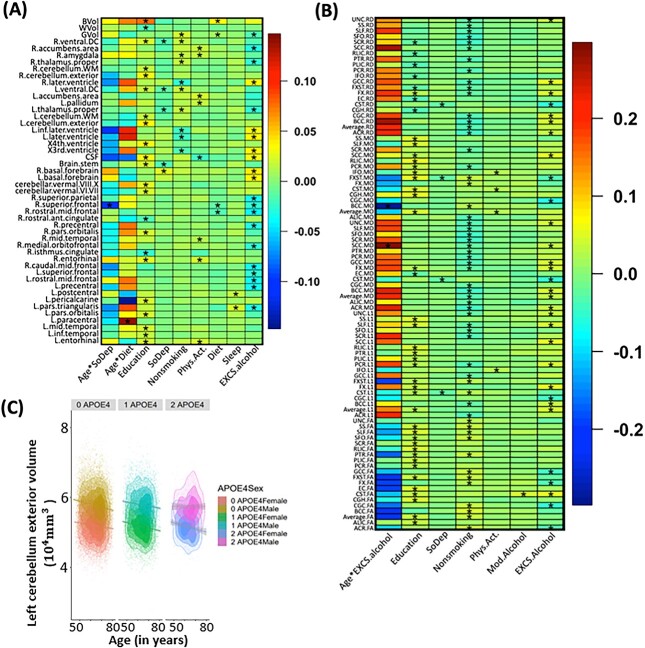
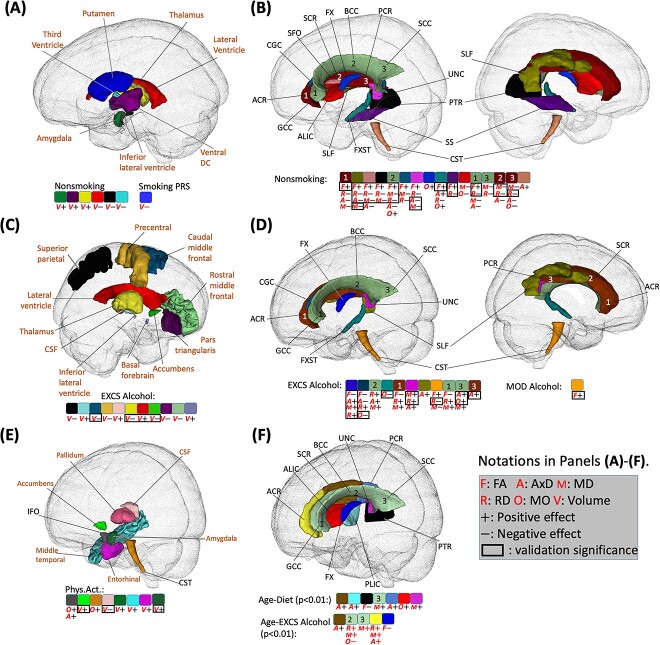
Smoking negatively affected overall GM volumes (P = 2.6E-22), and average FA (P = 4.5E-9), while increasing average RD (P = 7.5E-11) and MD (P = 2.0E-8). The identified associations included four subcortical regions (the thalamus, amygdala, putamen, and ventral diencephalon), three ventricular regions (the third ventricle, lateral ventricle, and inferior lateral ventricle), and 16 WM tracts (Figs 3A and 4B).
Fig. 3.
Heatmaps of lifestyle, SES effects and their interaction effects with age on brain volumes (A) and WM DTI measurements (B). (*) denotes the significant effect with P-value <2.37 × 10−4. (C) Scatterplots of left cerebellum exterior volume against age, from left to right are non-APOE-ε4, 1 APOE-ε4, and 2 APOE-ε4 carriers. Each subgraph is grouped by sex, the regression lines and 95% confidence intervals are also displayed.
Fig. 4.
Identified brain regions with the effects of lifestyle factors. (A) Regions with effects of non-smoking phenotype and smoking PRS score on volume (shown with “V”; directions of effects are denoted by “+” for positive and “−” for negative). (B) Regions with effects of non-smoking phenotype on WM diffusivity (shown with “F,” “A,” “M,” “R,” and “O” for FA, AxD, MD, RD, and MO, respectively). (C) Regions with effects of excessive alcohol (EXCS alcohol) on volume. (D) Regions with effects of excessive alcohol (EXCS alcohol) on diffusivity. (E) Regions with effects of physical activity (Phys.Act.) on volume and diffusivity. (F) Regions with effects of age-diet and age-EXCS alcohol interactions on diffusivity. In (A)-(E), only signals with P < 2.37E-4 in the British population that are validated by non-British ancestry populations (showing the same direction) are displayed. In (F), signals with P-values < 0.01 in the British population that are validated by non-British ancestry populations are displayed. Signals with P < 0.05 for non-British ancestry populations are enclosed with a black rectangle. PRS, polygenic risk score; FA, fractional anisotropy; AxD, axial diffusivity; MD, mean diffusivity; RD, radial diffusivity; MO, mode of diffusivity. Note that 0.01 and 0.05 are not Bonferroni corrected and 2.37E-4 is the Bonferroni corrected significance level.
The patterns of these effects were consistent across the imaging traits, including reduced subcortical volume, increased ventricular volume, decreased FA, and increased AxD, RD, and MD in WM tracts (Figs 3A, 3B, 4A, and 4B). These results align with previous research on the negative effects of smoking on brain structure (Gallinat et al. 2006; Zivadinov et al. 2009; Das et al. 2012; Sutherland et al. 2016; Vňuková et al. 2017), particularly for the GM, thalamus, cerebellum, putamen and lateral ventricle volumes, and diffusivity parameters (Lin et al. 2013; Savjani et al. 2014; Sexton et al. 2014; Umene-Nakano et al. 2014; Kristensen et al. 2021) on the corpus callosum (CC), anterior limb of internal capsule (ALIC), superior longitudinal fasciculus (SLF), and uncinate fasciculus (UNC).
The directions of these effects were consistent between white British and non-British ancestry racial groups. Specifically, the directions of signals were all (10 out of 10) consistent in terms of volume and 93% (53 out of 57) consistent in terms of WM diffusivity.
Associations between alcohol consumption and brain structure
We observed a positive interaction effect between age and excessive alcohol on the MD of the splenium of the CC (P = 2.0E-4; Figs. 3B and 4F, and SI Appendix, Fig. S14). Besides, although not reaching Bonferron significance level, there were other age interactions that passed P < 0.01, including positive effects on lateral ventricle volume, and AxD, RD, and MD of the CC and corona radiata (CR), and negative effects on the volumes of inferior temporal, precentral and caudate regions and FA of the fornix (FX) (Figs. 4F and 5C). These findings warrant attention as they were all (6 out of 6) validated by non-British ancestry populations with the same directions. The consistency supports the notion of increased brain aging with excessive alcohol consumption.
Fig. 5.
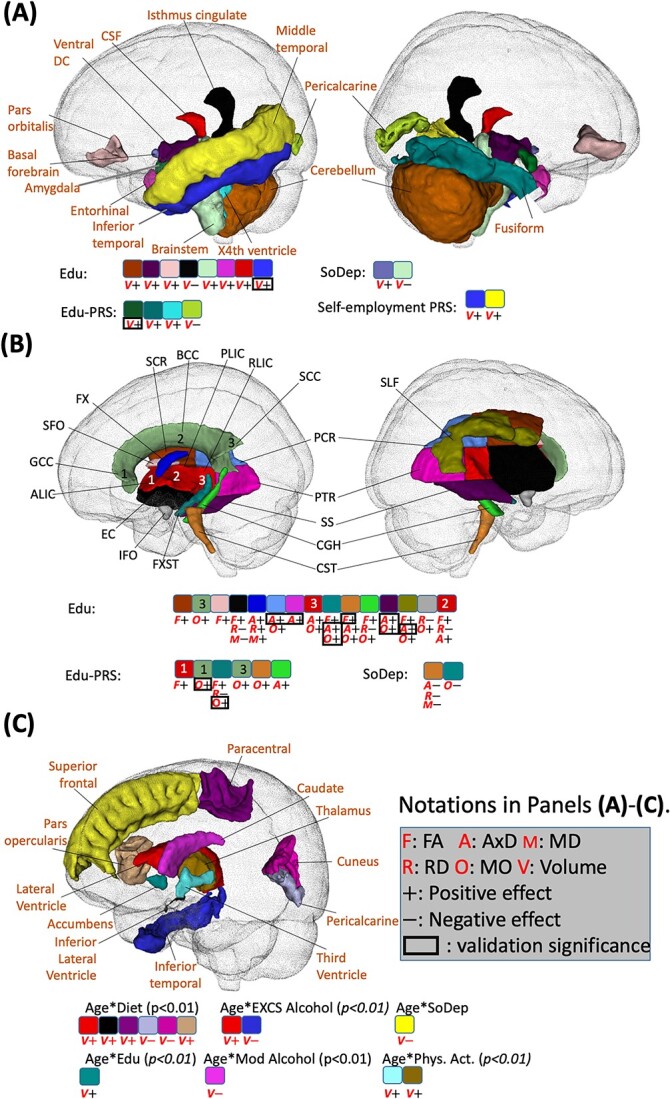
Identified brain regions with the effects of SES. (A) Regions with effects of SES phenotype on volume (shown with “V”; directions of effects are denoted by “+” for positive and “−” for negative). (B) Regions with effects of SES on WM diffusivity (shown with “F,” “A,” “M,” “R,” and “O” for FA, AxD, MD, RD, and MO, respectively). (C) Regions with effects of age-lifestyle and age-SES interactions on volume. In (A) and (B), signals with P < 2.37E-4 in the British ancestry population that are validated by non-British ancestry population (the same direction) are displayed. In (C), the statistical association results that are consistent between British and non-British ancestry populations are displayed. In addition, the imaging phenotypes at particular brain regions with P < 0.05 in non-British ancestry populations are marked by a black box. PRS, polygenic risk score; FA, fractional anisotropy; AxD, axial diffusivity; MD, mean diffusivity; RD, radial diffusivity; MO, mode of diffusivity. Note that 0.01 and 0.05 are not Bonferroni corrected and 2.37E-4 is the Bonferroni corrected significance level.
Regarding the main effects of alcohol consumption, excessive alcohol was associated with a reduction in WBV (P = 9.3E-15) and an increase in average AxD (P = 7.8E-7). The identified associations spanned various brain regions and WM tracts, with consistent directions of reduced cortical and subcortical volumes and inflated ventricular volume, decreased FA, and increased AxD, RD, and MD (Figs. 3, 4C and D). Furthermore, when comparing the directions of these main effects between white British and non-British ancestry populations, they were more consistent for WM diffusivity (30 out of 31) than brain volume (11 out of 21).
Associations between diet, physical activity, sleep, and brain structure
Our investigations revealed several findings related to age-diet interactions, physical activity, and sleep.
Regarding age-diet interactions, we observed positive effects on left paracentral volume (P = 2.0E-4). In addition, several age-diet interactions with P < 0.01 were identified and validated by non-British ancestry populations. In general, we found that some brain structural changes caused by diet were more evident among younger adults (ventricular and visual regions cuneus, pericalcarine) while some other regions were more affected among older adults (paracentral and pars opercularis) (Figs. 3A and 5C, and SI Appendix, Fig. S13). For the main effects, individuals with higher adherence to a healthy diet exhibited an increase in WBV (P = 9.3E-15).
No significant age-physical activity interactions were found. For the main effects, beneficial effects of physical activity were identified, including (Figs. 3A and 4E) positive associations with regional volume in temporal (middle temporal and entorhinal) and subcortical (amygdala, accumens, and pallium) regions, and negative associations with the CSF volume. These findings are in line with previous literature on the entorhinal region (Whiteman et al. 2016), nucleus accumbens (Yamamoto et al. 2017), and CSF (Scheewe et al. 2013).
Finally, we observed negative age-sleep interactions on the right superior frontal volume, suggesting that healthy sleep may benefit superior frontal regions more in young adults. In addition, healthy sleep demonstrated positive effects on the volume of the pars triangularis and postcentral regions (Fig. 3A). However, the directions of both those main and age-interaction effects could not be validated in non-British ancestry populations, highlighting the need for further investigations into minority populations to validate findings on sleep.
Associations between education and brain structure
We discovered inconsistencies in age-education interactions across racial groups. The directions of all identified age-interaction effects were 20.0% (1 out of 5) consistent for volume measures and 75.0% (6 out of 8) for WM diffusivity measures across racial groups (SI Appendix, Figs. S7 and S8). This might indicate race-dependent age-education interaction associations with brain volume or insufficiency of sample size for the non-British ancestry subjects.
Regarding the main effects, we observed 77.3% (17 out of 22) associations for ROI volumes and 79.5% (35 out of 44) for WM diffusivity measures that showed consistent directions when compared with non-British populations. They included brain temporal, cingulate, and ventricular regions, brainstem, cerebellum, and 15 WM tracts (Fig. 5A and B). Most associations followed the patterns of increased cortical, ventricular, subcortical, and cerebellar volumes, increased FA, AD, and MO, and decreased MD and RD with higher education levels.
Furthermore, our analysis revealed that directions of associations between the education PRS and brain structure were consistent with the education phenotype. These associations included increased volume of the amygdala, fusiform, and fourth ventricle, as well as increased FA, AD, RD, and MD (Fig. 5A and B). This suggests that the corresponding brain structural changes affected by education may be partially genetically driven.
Discussion
In this study, we performed a comprehensive multifactorial association analysis of brain structure with various risk factors using data from UKB subjects. Our findings underscore the impact of complex interactions between age, sex, genetics, and environmental exposures such as smoking, alcohol consumption, and education on brain structure.
A fine-grained underpinning of APOE effect on aging brains
We provided detailed association analyses of genetics, sex, and age with brain structure. As far as we know, our research is the first to systematically investigate age-sex-APOE-ε2/-ε4 three way interactions on brain structure while accounting for many confounders. Previous researches on interactions of APOE-ε2 are rare, limited by small sample sizes. Besides, the effects of APOE-ε2 are multifaceted and require careful examination. While APOE-ε2 has been demonstrated to have a protective effect against AD, it has also been associated with an increased risk of various other neurological disorders, such as post-traumatic stress disorder and argyrophilic grain disease (Kim et al. 2013; McKay et al. 2011; Li et al. 2020; Zhao et al. 2018; Ghebremedhin et al. 1998).
In our study, we found that APOE-ε4 accelerated the reduction of amygdala volume, whereas APOE-ε2 mitigated this decrease. These effects were more pronounced in females. In contrast, the brain structural changes in the general population (mostly APOE non-carriers) were more evident in males. These sex-disparate differences between APOE carriers and non-carriers could potentially be attributed to the greater hypometabolism and atrophy among female APOE-ε4 carriers (Sampedro et al. 2015) and protective influence of female sex hormones among APOE-ε4 non-carriers (Gur et al. 1991; Golomb et al. 1993; Cowell et al. 1994; Murphy et al. 1996; Raz et al. 1997).
Our study examined the APOE-alcohol interaction, contributing to the understanding of the complex relationship between alcohol consumption and brain health. Although some research suggests heavy alcohol consumption may be associated with a decline in brain volume (Pfefferbaum et al. 1992), other studies indicate that moderate alcohol consumption might have a protective effect (Gu et al. 2014). Findings on moderate alcohol consumption have been inconsistent, and the underlying mechanisms remain unclear (Gu et al. 2014; de Bruin et al. 2005; Downer et al. 2014; Davis et al. 2014). By using a larger sample size our study found associations between moderate alcohol consumption and diffusivity measures in the superior fronto-occipital fasciculus among APOE-ε4 carriers. This result aligned with a previous study (Downer et al. 2014), which demonstrated that moderate alcohol consumption could be harmful to learning and memory among APOE-ε4 carriers, but not in non-carriers. Additional investigation is warranted to better understand the underlying mechanisms for this association.
Environmental factors are extensively associated with brain structure changes
Smoking and excessive alcohol consumption, both well-established cardiovascular and brain structural risk factors, negatively affect the cardiovascular system and harm brain health (Messner and Bernhard 2014; Piano 2017). Conversely, healthy diet, education, and physical activity are three major factors extensively studied for their contributions to building brain reserve throughout life (Fratiglioni and Wang 2007; Gu et al. 2015). The effects of these lifestyle factors vary across the brain. Specifically, in our analyses, smoking primarily affected subcortical and ventricular regions, alcohol affected frontal, parietal, subcortical, and ventricular regions, and education influenced the temporal, inferior frontal, subcortical, and ventricular regions (Figs. 3–5).
Many of our findings are supported by previous literature. Smoking has been linked to reduction of subcortical volume (Das et al. 2012; Durazzo et al. 2017) and dysfunction of limbic network (Janes et al. 2012). Also based on the UKB data, (Linli et al. 2023) checked 166 regional brain GM volumes using 33,293 subjects and found that the small volume was most evident in thalamus. For WM microstructure, our findings were consistent with another study (Gray et al. 2020) based on 17,760 UKB subjects, suggesting reduced FA and increased MD with smoking. For alcohol, a recent study (Topiwala et al. 2022) identified that alcohol was even more associated with reduced GM volume than smoking, BMI, blood pressure, and cholesterol considered based on 25,378 UKB subjects. Specifically, our findings of alcohol were also validated on the volume of ventricle, frontal, parietal, accumbens and thalamus (Fein et al. 2009; Tomasi et al. 2021; Topiwala et al. 2022; Daviet et al. 2022), and CC, anterior corona radiata (ACR), posterior corona radiata (PCR), CGC, FX, ALIC, posterior limb of internal capsule (PLIC), and SLF (Yeh et al. 2009; Monnig et al. 2014; Topiwala et al. 2017). In addition, a previous study on physical activity discovered an association with the increase of GM volume among older adults (>60 years old) (Hamer et al. 2018) based on the 5272 participants from the UKB.
In our findings, the identified effects of excessive alcohol and smoking shared many similarities, including lower cortical and subcortical volumes, larger ventricular volume reduced WM integrity (as evidenced by smaller FA and enlarged AxD, RD, and MD). Actually a previous large-scale study (Cox et al. 2019) involving 9772 participants from the UKB has identified associations between vascular risk factors and smaller frontal, temporal cortical and subcortical volumes, reduced integrity of WM and thalamic pathways.
However, compared with smoking, excessive alcohol consumption appears to have more widespread detrimental effects on the brain, affecting both cortical and subcortical regions, and exhibiting stronger interaction effects with age. These differences may be attributed to the distinct mechanisms of brain damage caused by alcohol and smoking. Alcohol has direct neurotoxic effects on brain cells (Kruman et al. 2012) and neurotransmitter systems (Chastain 2006), leading to neuronal death and degeneration, while smoking exerts its effects on the brain indirectly through oxidative stress, vascular changes, and inflammation (Csiszar et al. 2009). In addition, older adults are more likely to be taking medications, and alcohol interacts with a wider range of medications, potentially causing further brain damage (Moore et al. 2007). Therefore the age interactions may differ between smoking and excessive alcohol consumption.
While there are studies focusing on the effects of diets on overall brain health or cognitive decline (Gu et al. 2015; Luciano et al. 2017; Croll et al. 2018; Jensen et al. 2021; Macpherson et al. 2021), research on specific brain regions during brain aging remain scarce, and rare existing literature addresses the age-related differences. A large study (Dekkers et al. 2019) looked into the association between obesity and brain volume and WM microstructure based on 12,087 participants from the UKB, but did not study the diet structure. A recent large-scale study specifically worked on the Mediterranean diet and the hippocampal and WM hyperintensity volumes based on 21,933 participants (Gregory et al. 2023). Another study (van Rooij et al. 2021) pointed out that the frontal, parietal, and temporal volumes might mediate the association between diet composition with behavioral disinhibition. We here discovered that diet might lead to ventricular and visual regional changes among younger adults affecting older adults more significantly among paracentral and inferior frontal regions. As the definition of a healthy diet was based on categories of fruits, vegetables, whole grains, refined grains, fish, unprocessed meat, and processed meat, further research is necessary to identify specific food categories that lead to the age-interaction patterns.
Although several studies explored the importance of healthy sleep for different age groups (Ohayon et al. 2004; Scullin and Bliwise 2015; Tarokh et al. 2016), they do not directly compare sleep’s impact on specific age groups. Our results, showing negative age-sleep interactions on the right superior frontal volume suggest that healthy sleep may have greater benefits for younger adults in superior frontal regions. This might be due to age-related declines in neural plasticity and differing sleep patterns in old adults, such as fragmented sleep, reduced slow-wave sleep, and earlier sleep timing. Aging is associated with natural structural and functional changes in the brain, which could make it less responsive to the positive effects of sleep. It is important to note that these explanations are speculative, and further research is needed to confirm these findings and better understand the underlying mechanisms behind this age-related difference.
Higher education level is considered a protective factor that benefits subcortical volumes and regional WM integrity. Previous research has shown that individuals with higher levels of education exhibit improved FA in fiber tracts of the parietal, temporal, and occipital lobes, including fusiform and parahippocampal gyrus (Teipel et al. 2009). In addition, higher education has been associated with larger volumes of frontal and temporal lobes (Kang et al. 2021), larger CSF volume (Kidron et al. 1997; Coffey et al. 1999), and improved functional connectivities between the anterior cingulate cortex and frontal, temporal, and parietal lobes (Arenaza-Urquijo et al. 2013).
However, the relationship between higher education and AxD is not well understood. Our observations suggest that higher education may be related to an increased AxD, which is different from the absence of smoking or excessive alcohol consumption. It is believed that higher education can increase brain plasticity and stimulate the formation of new connections between neurons, leading to an increase in AxD. This increase in AxD may indicate larger axons, which could result in more efficient signal transmission. In contrast, increases in AxD, RD, and MD due to smoking and excessive alcohol consumption suggest a less organized and less tightly packed WM network, as water molecules can move more freely. Further research is needed to fully understand differences of the relationship between AxD and education, smoking and alcohol on the WM network.
Limitations and future work
Our study differs from previous similar research in two ways. First, we investigated a wide range of modifiable and non-modifiable risk factors and explored their joint, conditional and interaction effects on brain structure. Second, we validated our findings from the white British population by using an independent non-British ancestry population as a test set. This ensured the robustness of our results against potential racial differences, outlier sensitivities, and sample size limitations.
There are several limitations in our current work that should be acknowledged. First, we observed some inconsistent effects across the white British and non-British ancestry populations. This may be due to several reasons, including differences in race, sample size, and outliers. It is unrealistic to further subdivide the non-British ancestry subjects into subgroups (Asian, African, or Hispanic) due to the limited sample size. Second, there may be measurement errors in some risk factors, as all questionnaire variables were measured only once. Third, since the UKB is a volunteer-based study, the participants may be healthier than the general population, which may lead to inconsistent conclusions when compared with previous community-based studies. Lastly, further analyses such as mediation and causal analyses would be needed to fully understand the underlying mechanisms of the direct and indirect main and interaction effects of risk factors considered in this study. Studies on causal pathways of nicotine dependence (Ye et al. 2021) using 23,624 subjects from UKB revealed that 272 SNP effects on smoking status were mediated by the FA, particularly at the right ALIC. In addition, another literature (Logtenberg et al. 2021) recognized the causal relationship between heavy alcohol use, smoking, and the reduced subcortical volume. As our future work, we will perform similar causal pathway analyses to further elucidate the relationships between risk factors and brain structure. Lastly, we categorized both smoking status and diet into binary classifications: current smokers versus non-smokers and healthy versus unhealthy diets. Future studies are warranted to delineate specific food categories contributing to the observed age-interaction patterns, and to comprehensively assess the correlation between smoking duration and brain structural changes.
Supplementary Material
Acknowledgments
We thank the individuals represented in the UK Biobank, studies for their participation and the research teams for their work in collecting, processing, and disseminating these datasets for analysis. We gratefully acknowledge all the studies and databases that made GWAS summary data available. This research has been conducted using the UK Biobank resource (application number 22783), subject to a data transfer agreement.
Contributor Information
Jie Chen, Department of Biostatistics, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill NC 27514, United States.
Tengfei Li, Department of Radiology, School of Medicine, University of North Carolina at Chapel Hill, 101 Manning Drive, Chapel Hill, NC 27514, United States; Biomedical Research Imaging Center, School of Medicine, University of North Carolina at Chapel Hill, 125 Mason Farm Road, Chapel Hill, NC 27599, United States.
Bingxin Zhao, Department of Statistics and Data Science, The Wharton School, University of Pennsylvania, 265 South 37th Street, 3rd & 4th Floors, Philadelphia, PA 19104-1686, United States.
Hui Chen, School of Public Health, Zhejiang University School of Medicine, 866 Yuhangtang Rd, Hangzhou 310058, China.
Changzheng Yuan, School of Public Health, Zhejiang University School of Medicine, 866 Yuhangtang Rd, Hangzhou 310058, China; Department of Nutrition, Harvard T H Chan School of Public Health, 665 Huntington Avenue Boston, MA, 02115, United States.
Gwenn A Garden, Department of Neurology, School of Medicine, University of North Carolina at Chapel Hill, 170 Manning Drive Chapel Hill, NC 27599-7025, United States.
Guorong Wu, Department of Psychiatry, School of Medicine, University of North Carolina at Chapel Hill, 101 Manning Drive, Chapel Hill, NC 27514, United States; Departments of Statistics and Operations Research, University of North Carolina at Chapel Hill, 318 E Cameron Ave #3260, Chapel Hill, NC 27599, United States; Departments of Computer Science, University of North Carolina at Chapel Hill, 201 South Columbia Street, Chapel Hill, NC 27599, United States; UNC Neuroscience Center, University of North Carolina at Chapel Hill, 116 Manning Dr, Chapel Hill, NC 27599, United States; Carolina Institute for Developmental Disabilities, 101 Renee Lynne Ct, Carrboro, NC 27510, United States.
Hongtu Zhu, Department of Biostatistics, University of North Carolina at Chapel Hill, 135 Dauer Drive, Chapel Hill NC 27514, United States; Biomedical Research Imaging Center, School of Medicine, University of North Carolina at Chapel Hill, 125 Mason Farm Road, Chapel Hill, NC 27599, United States; Departments of Statistics and Operations Research, University of North Carolina at Chapel Hill, 318 E Cameron Ave #3260, Chapel Hill, NC 27599, United States; Departments of Computer Science, University of North Carolina at Chapel Hill, 201 South Columbia Street, Chapel Hill, NC 27599, United States; Departments of Genetics, University of North Carolina at Chapel Hill, 120 Mason Farm Road, Chapel Hill, NC 27514, United States.
Author contributions
H. Z. and G. W. designed the study. J. C. and T. L. analyzed the data. T. L. and J. C. performed the data curation, image processing, and visualizations of results. J. C. and T. L. wrote the manuscript with feedback from all authors. G. W., H. Z.,B. Z. and G. G. reviewed and edited the paper. H. Z. and G. W. provided the funding support. H. C. and C. Y. contributed software and additional resources.
Funding
Research reported in this publication was partially supported by the National Institutes of Health (NIH) under Award Number MH116527 and NS110791, and the National Institute On Aging (NIA) of the NIH under Award Number RF1AG082938. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest statement: No conflict of interest was declared.
References
- Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative Investigators . Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014:75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004:43(10):1731–1737. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mézenge F, Perrotin A, Desgranges B, Bartrés-Faz D, Eustache F, Chételat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage. 2013:83(December):450–457. [DOI] [PubMed] [Google Scholar]
- Benyamin B, Pourcain B, Davis OS, Davies G, Hansell NK, MJA B, Kirkpatrick RM, et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol Psychiatry. 2014:19(2):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, et al. Brain charts for the human lifespan. Nature. 2022:604(7906):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner N, Jockwitz C, Mühleisen TW, Hoffstaedter F, Eickhoff SB, Moebus S, Bayen UJ, et al. Combining lifestyle risks to disentangle brain structure and functional connectivity differences in older adults. Nat Commun. 2019:10(1):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Jahanshad N, Stein JL, Barysheva M, McMahon KL, Zubicaray GI, Martin NG, et al. Common Alzheimer’s disease risk variant within the CLU gene affects white matter microstructure in young adults. J Neurosci. 2011:31(18):6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011:342(February):d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin EA, Hulshoff Pol HE, Bijl S, Schnack HG, Fluitman S, Böcker KBE, Kenemans JL, Kahn RS, Verbaten MN. Associations between alcohol intake and brain volumes in male and female moderate drinkers. Alcohol Clin Exp Res. 2005:29(4):656–663. [DOI] [PubMed] [Google Scholar]
- Chandler HL, Hodgetts CJ, Caseras X, Murphy K, Lancaster TM. Polygenic risk for Alzheimer’s disease shapes hippocampal scene-selectivity. Neuropsychopharmacology. 2020:45(7):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain G. Alcohol, neurotransmitter systems, and behavior. J Gen Psychol. 2006:133(4):329–335. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998:55(2):169–179. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999:53(1):189–196. [DOI] [PubMed] [Google Scholar]
- Cole JH. Multimodality neuroimaging brain-age in UK Biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging. 2020:92(August):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994:14(8):4748–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, Fawns-Ritchie C, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019:40(28):2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll PH, Trudy Voortman M, Ikram A, Franco OH, Schoufour JD, Bos D, Vernooij MW. Better diet quality relates to larger brain tissue volumes: the Rotterdam study. Neurology. 2018:90(24):e2166–e2173. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci (Landmark Ed). 2009:14(8):3128–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. 2012:17(4):817–825. [DOI] [PubMed] [Google Scholar]
- Daviet R, Aydogan G, Jagannathan K, Spilka N, Koellinger PD, Kranzler HR, Nave G, Wetherill RR. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. 2022:13(1):1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003:60(5):443. [DOI] [PubMed] [Google Scholar]
- Davis BJK, Vidal J-S, Garcia M, Aspelund T, Buchem MA, Jonsdottir MK, Sigurdsson S, et al. The alcohol paradox: light-to-moderate alcohol consumption, cognitive function, and brain volume. J Gerontol A. 2014:69(12):1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank study. Radiology. 2019:291(3):763–771. [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Baldursson G, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019:51(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004:328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer B, Zanjani F, Fardo DW. The relationship between midlife and late life alcohol consumption, APOE E4 and the decline in learning and memory among older adults. Alcohol Alcohol (Oxford, Oxfordshire). 2014:49(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend. 2017:177(August):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, Marchini J, Smith SM. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018:562(7726):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, Barakos J. Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res. 2009:33(10):1806–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, Salimi S, Sierra F, Cabo R. Measuring biological aging in humans: a quest. Aging Cell. 2020:19(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Cohen DM. Aging, metabolism, and Alzheimer disease: review and hypotheses. Exp Neurol. 1997:143(1):82–102. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010:21(3):187–221. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018:32(4):436–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner AJ, Awasthi S, Wolf C, Maron E, Erhardt A, Czamara D, Eriksson E, et al. Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Mol Psychiatry. 2021:26(8):4179–4190. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang H-X. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007:12(1):11–22. [DOI] [PubMed] [Google Scholar]
- Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, Lanham DC, Denckla MB, Kaufmann WE. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002:58(1):85–89. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006:24(6):1744–1750. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Giovannina Botez U, Rüb IS, Braak E, Braak H. Argyrophilic grain disease is associated with Apolipoprotein E Ε2 allele. Acta Neuropathol. 1998:96(3):222–224. [DOI] [PubMed] [Google Scholar]
- Golomb J, Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging: an association with recent memory impairment. Arch Neurol. 1993:50(9):967–973. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a Metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008:28(11):2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grama S, Willcocks I, Hubert JJ, Pardiñas AF, Legge SE, Bracher-Smith M, Menzies GE, et al. Polygenic risk for schizophrenia and subcortical brain anatomy in the UK Biobank cohort. Transl Psychiatry. 2020:10(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R. Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology. 2020:45(7):1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S, Pullen H, Ritchie CW, Shannon OM, Stevenson EJ, Muniz-Terrera G. Mediterranean diet and structural neuroimaging biomarkers of Alzheimer’s and cerebrovascular disease: a systematic review. Exp Gerontol. 2023:172(February):112065. [DOI] [PubMed] [Google Scholar]
- Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, Luman M, et al. Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls: smoking and white matter microstructure. Hum Brain Mapp. 2015:36(3):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Pallesen J, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019:51(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Scarmeas N, Short EE, Luchsinger JA, DeCarli C, Stern Y, Manly JJ, Schupf N, Mayeux R, Brickman AM. Alcohol intake and brain structure in a multiethnic elderly cohort. Clin Nutr (Edinburgh, Scotland). 2014:33(4):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, Scarmeas N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015:85(20):1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci. 1991:88(7):2845–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habes M, Janowitz D, Erus G, Toledo JB, Resnick SM, Doshi J, Van der Auwera S, et al. Advanced brain aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry. 2016:6(4):e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J Intern Med. 2018:284(4):439–443. [DOI] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager SR, Chung A, Hwang J, Daniels MA, Lee YS, Lyoo IK. Altered cingulate white matter connectivity in panic disorder patients. J Psychiatr Res. 2008:42(5):399–407. [DOI] [PubMed] [Google Scholar]
- Hueluer G, Dodge H. New developments in cognitive aging research. Innov Aging. 2018:2(suppl_1):382. [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci. 2014:111(2):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS) . Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018:23(5):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick B d B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012:125(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DEA, Leoni V, Klein-Flügge MC, Ebmeier KP, Suri S. Associations of dietary markers with brain volume and connectivity: a systematic review of MRI studies. Ageing Res Rev. 2021:70(September):101360. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999:96(2):291–302. [DOI] [PubMed] [Google Scholar]
- Jolly TAD, Cooper PS, Badwi SAWA, Phillips NA, Rennie JL, Levi CR, Drysdale KA, Parsons MW, Michie PT, Karayanidis F. Microstructural white matter changes mediate age-related cognitive decline on the Montreal Cognitive Assessment (MoCA). Psychophysiology. 2016:53(2):258–267. [DOI] [PubMed] [Google Scholar]
- Kang DW, Wang S-M, Na H-R, Kim N-Y, Lim HK, Lee CU. Differential impact of education on gray matter volume according to sex in cognitively normal older adults: whole brain surface-based morphometry. Front Psychiatry. 2021:12(March):644148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Meer D, Doan NT, Schwarz E, Lund MJ, Agartz I, Alnæs D, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci. 2019:22(10):1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidron D, Black SE, Stanchev P, Buck B, Szalai JP, Parker J, Szekely C, Bronskill MJ. Quantitative MR volumetry in Alzheimer’s disease. Topographic markers and the effects of sex and education. Neurology. 1997:49(6):1504–1512. [DOI] [PubMed] [Google Scholar]
- Kim TY, Chung HG, Shin H-S, Kim SJ, Choi JH, Chung MY, An SK, Choi TK, So HS, Cho H-S. Apolipoprotein E gene polymorphism, alcohol use, and their interactions in combat-related posttraumatic stress disorder. Depress Anxiety. 2013:30(12):1194–1201. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006:26(4):433–444. [DOI] [PubMed] [Google Scholar]
- Kristensen TD, Glenthøj LB, Ragahava JM, Syeda W, Mandl RCW, Wenneberg C, Krakauer K, et al. Changes in negative symptoms are linked to white matter changes in superior longitudinal fasciculus in individuals at ultra-high risk for psychosis. Schizophr Res. 2021:237(November):192–201. [DOI] [PubMed] [Google Scholar]
- Kruman II, Henderson GI, Bergeson SE. DNA damage and neurotoxicity of chronic alcohol abuse. Exp Biol Med. 2012:237(7):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019:51(3):414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C-L, Pilling LC, Atkins JL, Kuchel GA, Melzer D. ApoE E2 and aging-related outcomes in 379,000 UK Biobank participants. Aging (Albany NY). 2020:12(12):12222–12233 10.18632/aging.103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon H, Aydogan G, Dagher A, Bzdok D, Ruff CC, Nave G, Farah MJ, Koellinger PD. Human brain anatomy reflects separable genetic and environmental components of socioeconomic status. Sci Adv. 2022:8(20):eabm2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018:50(8):1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Shue F, Zhao N, Shinohara M, Guojun B. APOE2: protective mechanism and therapeutic implications for Alzheimer’s disease. Mol Neurodegener. 2020:15(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Guangyao W, Zhu L, Lei H. Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. 2013:129(1–2):82–87. [DOI] [PubMed] [Google Scholar]
- Linli Z, Rolls ET, Zhao W, Kang J, Feng J, Guo S. Smoking is associated with lower brain volume and cognitive differences: a large population analysis based on the UK Biobank. Prog Neuro-Psychopharmacol Biol Psychiatry. 2023:123(April):110698. [DOI] [PubMed] [Google Scholar]
- Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019:51(2):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logtenberg E, Overbeek MF, Pasman JA, Abdellaoui A, Luijten M, Holst RJ, Vink JM, et al. Investigating the causal nature of the relationship of subcortical brain volume with smoking and alcohol use. Br J Psychiatry J Ment Sci. 2022:221(1):377–385. [DOI] [PubMed] [Google Scholar]
- Loos MJHM, Rietveld CA, Eklund N, Koellinger PD, Rivadeneira F, Abecasis GR, Ankra-Badu GA, et al. The molecular genetic architecture of self-employment Edited by Stacey Cherny. PLoS One. 2013:8(4):e60542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuzma E, Llewellyn DJ. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019:322(5):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Corley J, Cox SR, Valdés MC, Hernández LCA, Craig DA, Dickie SK, et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017:88(5):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall DM, Celis-Morales C, Lyall LM, Graham C, Graham N, Mackay DF, Strawbridge RJ, et al. Assessing for interaction between APOE Ε4, sex, and lifestyle on cognitive abilities. Neurology. 2019:92(23):e2691–e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson H, McNaughton SA, Lamb KE, Milte CM. Associations of diet quality with midlife brain volume: findings from the UK Biobank cohort study. J Alzheimers Dis. 2021:84(1):79–90. [DOI] [PubMed] [Google Scholar]
- McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, Ho L, et al. Evidence of association of APOE with age-related macular degeneration - a pooled analysis of 15 studies. Hum Mutat. 2011:32(12):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwe C, Passchier R, Mufford M, Ramesar R, Dalvie S, Stein DJ. Polygenic risk for schizophrenia and associated brain structural changes: a systematic review. Compr Psychiatry. 2019:88(January):77–82. [DOI] [PubMed] [Google Scholar]
- Messner B, Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014:34(3):509–515. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Thayer RE, Caprihan A, Claus ED, Yeo RA, Calhoun VD, Hutchison KE. White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain Behav. 2014:4(2):158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Whiteman EJ, Ward KT. Risks of combined alcohol-medication use in older adults. Am J Geriatr Pharmacother. 2007:5(1):64–74. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4063202/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation. 2016:133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta A, Navale SS, Lumsden AL, Llewellyn DJ, Hyppönen E. Healthy lifestyle, genetic risk and brain health: a gene-environment interaction study in the UK Biobank. Nutrients. 2022:14(19):3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996:53(7):585–594. [DOI] [PubMed] [Google Scholar]
- Ning K, Zhao L, Matloff W, Sun F, Toga AW. Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci Rep. 2020:10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novellino F, López ME, Vaccaro MG, Miguel Y, Delgado ML, Maestu F. Association between hippocampus, thalamus, and caudate in mild cognitive impairment APOEε4 carriers: a structural covariance MRI study. Front Neurol. 2019:10:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Magnussen F, Lundquist A, Baaré W, Bartrés-Faz D, Bertram L, Boraxbekk CJ, Brandmaier AM, Drevon CA, Ebmeier K, et al. Educational attainment does not influence brain aging. Proc Natl Acad Sci. 2021:118(18):e2101644118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health. J Am Coll Cardiol. 2007:50(11):1009–1014. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004:27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- Operto G, Cacciaglia R, Grau-Rivera O, Falcon C, Brugulat-Serrat A, Ródenas P, Ramos R, et al. White matter microstructure is altered in cognitively normal middle-aged APOE-Ε4 homozygotes. Alzheimers Res Ther. 2018:10(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operto G, Molinuevo JL, Cacciaglia R, Falcon C, Brugulat-Serrat A, Suárez-Calvet M, Grau-Rivera O, et al. Interactive effect of age and APOE-Ε4 allele load on white matter myelin content in cognitively normal middle-aged subjects. NeuroImage Clinical. 2019:24:101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, Bigdeli T, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016:21(10):1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpatzis E, Bass N, Jones R, Mukadam N. Longitudinal association of apolipoprotein E and sleep with incident dementia. Alzheimers Dement. 2022:18(5):888–898. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992:16(6):1078–1089. [DOI] [PubMed] [Google Scholar]
- Piano MR. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017:38(2):219–241. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513687/. [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Walters RK, Johnson EC, McClintick JN, Adkins AE, Adkins DE, Bacanu S-A, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the psychiatric genomics consortium. Mol Psychiatry. 2020:25(8):1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004:61(7):720. [DOI] [PubMed] [Google Scholar]
- Ranlund S, Rosa MJ, Jong S, Cole JH, Kyriakopoulos M, Fu CHY, Mehta MA, Dima D. Associations between polygenic risk scores for four psychiatric illnesses and brain structure using multivariate pattern recognition. NeuroImage Clin. 2018:20:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997:7(3):268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage. 2010:51(2):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régy M, Dugravot A, Sabia S, Fayosse A, Mangin J-F, Chupin M, Fischer C, et al. Association of APOE Ε4 with cerebral gray matter volumes in non-demented older adults: the MEMENTO cohort study. NeuroImage. 2022:250(April):118966. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999:319(7224):1523–1528. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC28294/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, Lee P, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014:511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011:342(February):d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooij D, Schweren L, Shi H, Hartman CA, Buitelaar JK. Cortical and subcortical brain volumes partially mediate the association between dietary composition and behavioral disinhibition: a UK Biobank study. Nutrients. 2021:13(10):3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, Kivimäki M, Singh-Manoux A. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018:362(August):k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro F, Vilaplana E, Leon MJ, Alcolea D, Pegueroles J, Montal V, Carmona-Iragui M, et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015:6(29):26663–26674. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4694943/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani RR, Velasquez KM, Thompson-Lake DGY, Baldwin PR, Eagleman DM, De La Garza RII, Salas R. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend. 2014:145(December):134–142. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Backx FJG, Takken T, Jörg F, Strater ACP, Kroes AG, Kahn RS, Cahn W. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand. 2013:127(6):464–473. [DOI] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015:10(1):97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014:34(46):15425–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller R-A. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry. 2011:52(3):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. NeuroImage. 2019:200(October):528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, Mattheisen M, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019:51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer E-M, Bethlehem RAI, Warrier V, Murray GK, Romero-Garcia R, Seidlitz J, Bullmore ET. Grey and white matter microstructure is associated with polygenic risk for schizophrenia. Mol Psychiatry. 2021:26(12):7709–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999:56(3):254. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, Laird AR. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016:12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F. Successful aging: multiple trajectories and population heterogeneity. Int J Soc Sci Stud. 2014:2(3):12–22. https://econpapers.repec.org/article/rfajournl/v_3a2_3ay_3a2014_3ai_3a3_3ap_3a12-22.htm. [Google Scholar]
- Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: physiology, cognition and mental health. Neurosci Biobehav Rev. 2016:70(November):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Bürger K, Reiser MF, Herpertz S, Möller H-J, Hampel H. White matter microstructure in relation to education in aging and Alzheimer’s disease. J Alzheimers Dis. 2009:17(3):571–583. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, Erp T, Poutanen V-P, Huttunen M, Lönnqvist J, et al. Genetic influences on brain structure. Nat Neurosci. 2001:4(12):1253–1258. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wiers CE, Manza P, Shokri-Kojori E, Michele-Vera Y, Zhang R, Kroll D, et al. Accelerated aging of the amygdala in alcohol use disorders: relevance to the dark side of addiction. Cereb Cortex. 2021:31(7):3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, Mahmood A, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ (Clinical Research Ed). 2017:357(June):j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala A, Ebmeier KP, Maullin-Sapey T, Nichols TE. Alcohol consumption and MRI markers of brain structure and function: Cohort study of 25,378 UK Biobank participants. NeuroImage: Clinical. 2022:35(January):103066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene-Nakano W, Yoshimura R, Kakeda S, Watanabe K, Hayashi K, Nishimura J, Takahashi H, et al. Abnormal white matter integrity in the corpus callosum among smokers: tract-based spatial statistics. PLoS One. 2014:9(2):e87890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasic N, Wolf ND, Grön G, Sosic-Vasic Z, Connemann BJ, Sambataro F, Strombeck A, et al. Baseline brain perfusion and brain structure in patients with major depression: a multimodal magnetic resonance imaging study. J Psychiatry Neurosci. 2015:40(6):412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Arfan Ikram M, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MMB. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009:66(5):545–553. [DOI] [PubMed] [Google Scholar]
- Vňuková M, Ptáček R, Raboch J, Stefano GB. Decreased central nervous system Grey Matter Volume (GMV) in smokers affects cognitive abilities: a systematic review. Med Sci Monit. 2017:23(April):1907–1915. 10.12659/MSM.901870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, Aliev F, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018:21(12):1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Qian S, Liu K, Li B, Li M, Xin K, Sun G. Reduced white matter integrity and its correlation with clinical symptom in first-episode, treatment-naive generalized anxiety disorder. Behav Brain Res. 2016:314(November):159–164. [DOI] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Michael Arrighi H. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE E4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012:38(1):1–17. [DOI] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: a voxel-based morphometry study. NeuroImage. 2016:126(February):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Wada-Isoe K, Yamashita F, Nakashita S, Kishi M, Tanaka K, Yamawaki M, Nakashima K. Association between exercise habits and subcortical gray matter volumes in healthy elderly people: a population-based study in Japan. ENeurologicalSci. 2017:7(March):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mo C, Liu S, Hatch KS, Gao S, Yizhou Ma L, Hong E, et al. White matter integrity and nicotine dependence: evaluating vertical and horizontal pleiotropy. Front Neurosci. 2021:15(October):738037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P-H, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009:173(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, Illmann C, et al. Interrogating the genetic determinants of Tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry. 2019:176(3):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Liu C-C, Van Ingelgom AJ, Linares C, Kurti A, Knight JA, Heckman MG, et al. APOE Ε2 is associated with increased tau pathology in primary tauopathy. Nat Commun. 2018:9(1):4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Luo T, Li T, Li Y, Zhang J, Shan Y, Wang X, et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat Genet. 2019:51(11):1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li T, Yang Y, Wang X, Luo T, Shan Y, Zhu Z, et al. Common genetic variation influencing human white matter microstructure. Science. 2021:372(6548):eabf3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li T, Fan Z, Yang Y, Shu J, Yang X, Wang X, et al. Heart-brain connections: phenotypic and genetic insights from magnetic resonance images. Science. 2023:380(6648):abn6598. [DOI] [PubMed] [Google Scholar]
- Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. Edited by Keith Laws. PLOS ONE. 2015:10(3):e0118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Xie Y-Z, Cao T-T, Wang Z, Wang T, Li X, Shen R-C, Huaxi X, Guojun B, Chen X-F. A rapid and cost-effective method for genotyping apolipoprotein E gene polymorphism. Mol Neurodegener. 2016:11(January):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Weinstock-Guttman B, Hashmi K, Abdelrahman N, Stosic M, Dwyer M, Hussein S, Durfee J, Ramanathan M. Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology. 2009:73(7):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.